Abstract
Cellular localization of organelles, protein complexes and single mRNAs depends on the directed transport along microtubule tracks, a process mediated by ATP-driven molecular motor proteins of the dynein and kinesin superfamilies. Kinesin II is a heterotrimeric protein complex composed of two motor subunits and a unique nonmotor Kinesin-associated protein (Kap). Kap was shown to transport both particulate cargo, as axoneme components in rafts, and membrane-bounded organelles such as melanosomes. Drosophila Kinesin II was shown to be essential for the axonal transport of choline acetyltransferase in a specific set of neurons. We have generated Kap mutants and show that gene activity is not only required for neuronal function but also for separation of follicles during early oogenesis. The data suggest that Kap participates in the transport of signalling components required for instructive interactions between germline and soma cells.
Keywords: Drosophila, Kinesin II, oogenesis, signalling, cellular transport
Introduction
Asymmetric localization of cellular components, such as organelles, vesicles and mitochondria, depends on active transport along microtubule tracks by ATP-driven molecular motors of the dynein and kinesin protein superfamilies (reviewed in Goldstein & Yang, 2000). Members of the Kinesin I and the Kinesin II/Kif3 families function in the transport of organelles as well as soluble cellular components, and have a major role in axonal transport from neuronal cell bodies to nerve termini in vertebrates, Caenorhabditis elegans and Drosophila (reviewed in Goldstein & Yang, 2000; Hirokawa, 2000).
In Drosophila, Kinesin II forms a heterotrimeric complex (reviewed in Cole, 1999; Hirokawa 2000) composed of two motor subunits, KLP68D and KLP64D, and a singular nonmotor protein, the Kinesin-associated protein 3 (Kap3), which acts as adaptor and regulator of the complex (Ray et al, 1999). Recent studies on KLP64D mutants suggest that Kinesin II is specifically required for the axonal transport of choline acetyltransferase in a subgroup of neurons (Ray et al, 1999). A similar phenotype was reported for Kap mutants (Sarpal et al, 2003), suggesting that Kap/Kinesin II complexes are only required for neural function. However, in mice, humans and rats as well as in sea urchins and algae, Kinesin II was shown to be necessary for the formation and maintenance of cilia/axoneme structures (reviewed in Rosenbaum et al, 1999). In some organisms, a third motor protein, Kif3C, has been identified. Kif3C was proposed to be part of distinct Kinesin II complexes, which act in a spatiotemporal and/or cellspecific fashion (reviewed in Cole, 1999). We identified a Kif3C homologue in the Drosophila genome (CG17461; FlyBase, 2002; see supplementary information online), suggesting that the previous studies may not have revealed all aspects of Kinesin II-dependent Kap function in the fly (Ray et al, 1999; Sarpal et al, 2003). To test this possibility, we generated Kap lack-of-function mutants. Here we report that in addition to the recently described neural function, Kap activity is also required in germ cells for proper follicle separation during oogenesis. The results suggest that Kap participates in signalling necessary for the establishment of follicleseparating stalk cells.
Results And Discussion
Generation and molecular analysis of Kap mutants
To assess Kinesin II requirement, we generated mutations affecting the single non-motor component Kap of Drosophila (Sarpal & Ray, 2002; own observation). The organization of the gene (Fig 1) and the conserved domain structure of the protein as revealed by comparison of Kap with human, mouse and sea urchin homologues are shown (supplementary Fig 1 online). We recovered a semilethal mutation, l(1)G0396, in which a P{lacW} element (Peter et al, 2002) was inserted in the first intron of the Kap gene (Fig 1A). In all, 95% of hemizygous l(1)G0396 males develop into pupae and die as pharate adults; about 5% hatch and show a paralytic phenotype as described for KLP64D (Ray et al, 1999). Precise excisions of the P-element (Bellen et al, 1989) resulted in wild-type flies, indicating that the P insertion is the cause of the mutation. We also obtained imprecise excisions (Bellen et al, 1989), which represent hypomorphic Kap alleles, which have small internal deletions in the protein coding sequence. In addition, we obtained alleles such as Kap89, which lack the promoter and the first exon of the gene (Fig 1A). Homozygous Kap89 mutants fail to express Kap as revealed by in situ hybridization. In addition, their phenotype was as strong as the phenotype of transheterozygous Kap89/Df(1)v-N48 mutant individuals (data not shown). These findings indicate that Kap89 is a null mutation. Kap89 and the hypomorphic mutants could be rescued by Kap activity that was derived from an Actin5C enhancer-driven Kap cDNA transgene, confirming that the mutations affect only the Kap gene (supplementary Fig 2 online).
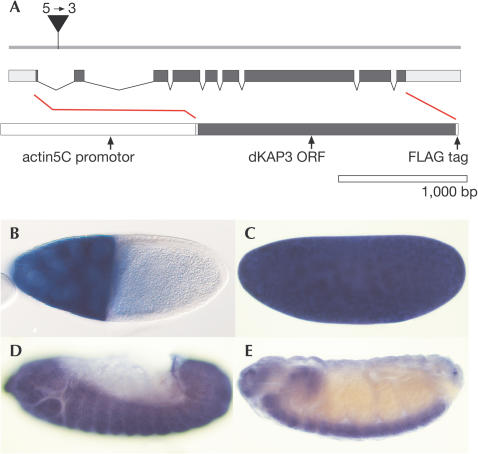
Figure 1.
Structure and expression of the Kap (CG11759) transcription unit (region 10B of X chromosome; FlyBase, 2002; Sarpal & Ray, 2002). (A) Genomic organization. The Kap transcript contains nine exons (black bars: coding region; white boxes: untranslated sequences). Position of the L(1)G0396 P-element insertion is marked. The sequence of Kap is shown in supplementary Fig 1 online. (B–E) Expression patterns in a Drosophila follicle (B) and embryos (C–E) as revealed by whole-mount in situ hybridization of a Kap-specific RNA probe; orientation: anterior left and dorsal up. Note strong Kap expression in nurse cells (stage 9) (B), a ubiquitous distribution of maternal transcripts in a stage 2 embryo (C), zygotic expression in ectodermal and mesodermal derivatives (stage 12 embryo) (D) and the enrichment of transcripts in the central and peripheral nervous systems in older embryos (E; details in Sarpal & Ray, 2002).
To determine the sites of Kap expression in the organism, we performed RNA in situ hybridization on staged ovaries and embryos. During oogenesis, Kap expression is observed in nurse cells from where transcripts are transported into the growing oocyte (Fig 1B). The transcripts remain ubiquitously distributed in eggs and embryos until the blastoderm stage (Fig 1C). Zygotic Kap expression is initiated during gastrulation in both ectoderm and mesoderm (Fig 1D) and is subsequently enriched in neurons (Fig 1E). Based on the strong maternal expression of the gene, we asked whether Kap also has a role during oogenesis in addition to its recently reported function in the nervous system (Sarpal et al, 2003).
Lack of Kap activity affects oogenesis
To assess the function of maternal Kap activity (Fig 1C), we generated homozygous Kap mutant germline clones using the FLP/ovoD1 system (Chou & Perrimon, 1992). Females with homozygous Kap89 mutant germ lines are sterile, as germline mutant follicles degenerate after they reached stage 6 of oogenesis (100%; n>1,000; staging according to Spradling, 1993). Up to this stage, mutant follicles lacking Kap contain more cells than wild-type follicles. The supernumerary cells are either only nurse cells (type I follicles) or both nurse cells and oocytes (type II follicles). Type II follicles have multiple oocytes and a corresponding number (ratio 1:15) of extra nurse cells. Of all follicles scored (n>1,000; more than 300 individuals examined), 50% showed mixed type I/II follicles. Of the remaining follicles, few (⩽5%) showed either type I or type II follicles only.
The supernumerary cells in type I follicles (Fig 2C–G) suggest that the mutant germline cells have undergone more than the normal four rounds of mitotic divisions. To confirm this proposal, we inspected the number of ring channels and the fusome of the germline mutant ovaries. Both structures derive from an incomplete separation of daughter cells after the division, resulting in a maximum of four ring channels in the case of the wild-type oocyte (reviewed in Spradling, 1993) and in a branched fusome structure that interconnects the germ cells of a follicle (de Cuevas & Spradling, 1998). We found Kap mutant germ cells that have more than four ring channels (Fig 2C,G) and fusomes connecting up to about 50 cells (supplementary Fig 3 and movie online). This indicates that at least one extra round of mitotic divisions occurs in Kap mutant follicles. The use of oocyte markers, such as gurken (grk) and oskar (osk) mRNA (reviewed in Riechmann & Ephrussi, 2001), identified either one oocyte or multiple oocytes in ectopic locations within the follicles (Fig 3C–F). Collectively, these findings indicate that the determination and the initial stages of oocyte differentiation do not depend on Kap activity, whereas the cellular processes underlying the formation of individual follicles and regulation of germ cell proliferation do.
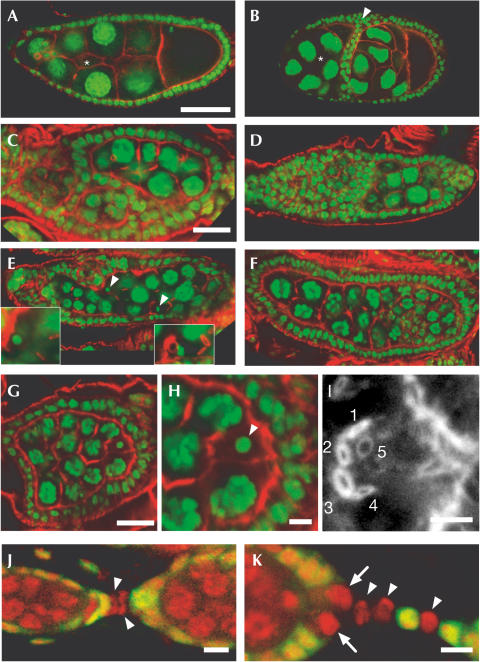
Figure 2.
Morphological analysis of Kap mutant germline clones showing actin in red and DNA in green (A–I). (A) Wild-type follicle (stage 9A) showing highly polyploid nurse cells and the single-layered follicle epithelium. At this stage, the epithelial cells move towards posterior (right side). The position of border cells (out of focal plane; asterisk) is indicated. (B) Hypomorphic mutant showing two fused follicles with a double row of follicle cells (arrowhead) between the germ cell clusters. Follicle cells behave otherwise as in one individual follicle; both the surrounding epithelium and border cells (other focal plane, asterisk) move normally. Note two prospective oocytes as identified by nuclear morphology, massive subcortical actin and yolk accumulation. (C,D) Two compound ovarioles of lack-of-function mutant ovaries. Note again the follicle fusion. (E,F) Examples of follicles with supernumerary germ cells in a single layer of follicle cells. Note two oocyte nuclei (arrowheads in E) and an incorrect number of ring channels (insets: examples with three and five instead of four ring channels, respectively). Note also the normal morphology of the anterior most nurse cells shown in (F) and the altered size and shape in more posterior positions; also, no oocyte was found. (G–J) Mutant follicle surrounded by a single layer of follicle cells. Single plane overview shows 31 nurse cells (G; enlarged in H) and a single oocyte nucleus (arrowhead in H). This oocyte has five ring channels (1–5) as revealed by an overlay of nine successive focal planes, indicating at least five mitotic divisions of the germ cells. (J,K) Clonal analysis showing stalk formation by Kap mutant cells (marked by the absence of GFP staining in green; DNA staining: red). (J) Early stalk development (stage 1 connected to stage 3 follicle; Kap-deficient cells are marked by an arrowhead). (K) A mature stalk where differentiated stalk cells (arrowheads) and the polar cells (arrows) lack Kap. Scale bar in (A) (representative for A,B,D–F), 50 μm. Other scale bars are 20 μm (C,G) and 5 μm (H–K). See text for further details.
Figure 3.
Characterization of Kap germline mutant ovaries. (A) Anti-Orb antibody staining of wild-type ovaries. Note initial Orb expression in wild-type germ cells (germarium region 2), the subsequent accumulation in the two pre-oocytes (region 2A; arrow) in the adjacent germ cell cluster and the final concentration in one posterior cell of the next cell cluster (region 2B; asterisk in A). (B) Anti-Orb antibody staining of Kap mutant ovaries. Note that initial Orb expression and accumulation (arrow) is normal, but is not maintained in the adjacent and older germ cell cluster (asterisk). (C,D) In situ hybridization showing that grk RNA is expressed in one (C) or two (D) germline cells, which correspond to young oocytes in Kap mutant ovaries. Older follicles show no detectable grk transcripts (C; arrow). (E,F) osk RNA appears in region 2 of dKap mutants (E), but fades out (F; arrow). (G,H) A101lacZ expression in wild-type (G) and Kap mutant follicles (H). (I,J) unpaired expression in the polar cells (J; wild type) cannot be detected in Kap mutants (arrow; K).
Kinesin I has previously been shown to participate in the transport of distinct mRNA species and associated protein to the posterior pole of the Drosophila oocyte (Brendza et al, 2000; Cha et al, 2002). To assess the integrity of this type of microtubule-dependent transport in Kap89 mutant follicles, we examined the expression and localization of the oo18 RNA-binding protein (Orb). Orb is necessary for the directed transport and localized translation of grk and osk mRNA, the axial polarity determinants of the growing oocyte (Chang et al, 1999, 2001). We found that the expression of Orb is initiated normally, although it is not maintained subsequently (Fig 3B). The oocyte determination factor Bic-D (Wharton & Struhl, 1989) and β-tubulin are expressed normally, and oskar RNA is normally expressed and transported in Kap89 mutant follicles (data not shown). Thus, cytoskeletal structures and the transport of oskar mRNA into the growing oocyte appear not to be directly affected by the lack of Kap activity.
Lack of Kap activity affects follicle separation
Separation of follicles during early oogenesis involves the formation of a characteristic intervening stalk structure (Spradling, 1993). This structure derives from a small group of precursor cells (located in germarium region 2B), which develop into polar follicle cells and the stalk cells (Tworoger et al, 1999; Besse & Pret, 2003). Their proper development depends on cell–cell communication events between the germline and soma cells as shown by Notch/Delta, JAK/STAT and Hedgehog signalling mutants, which develop fused follicles and lack morphologically distinct stalks (Grammont & Irvine, 2001; Lopezschier & St Johnston, 2001; Besse et al, 2002; McGregor et al, 2002). As such a phenotype was also observed with the Kap mutant ovaries (Fig 2C), we asked whether polar follicle cells, which can be visualized by A101-lacZ expression (Fig 3G; Ruohola et al, 1991), and stalk cells are formed. In Kap mutant follicles, supernumerary A101-lacZ expressing (Fig 3H) polar follicle cells were observed, whereas follicleseparating stalk cells were absent. Identical results were obtained by anti-FasIII antibody staining, a different marker for polar follicle cells (Ruohola et al, 1991; not shown). These observations suggest that stalk precursor cells fail to differentiate, remain in the follicle epithelium and express the molecular characteristics of follicle cells as has been observed in Notch mutants (Grammont & Irvine, 2001). Clonal analysis showed that stalk cells are formed properly in the absence of Kap activity in the somatic epithelial cells (Fig 2J,K). Thus, Kap activity has a cell autonomous effect on germline cell proliferation, and the absence of Kap in germ cells also affects somatic precursor cells in a non-cell autonomous manner.
The non-autonomous Kap effect on follicle cell determination could be explained by a requirement for Kap for the transport and/or localization of components that are needed to signal cell fate to the surrounding somatic precursor cells. We therefore examined Kap mutant ovaries for the expression of Delta, which activates Notch signalling (Portin, 2002), hedgehog (Forbes et al, 1996) and the JAK/STAT-activating ligand unpaired (Harrison et al, 1998). Delta and hedgehog expressions were not affected (data not shown), whereas unpaired failed to be expressed in polar follicle cells of Kap germline mutant ovaries (Fig 3I,J). This observation suggests that lack of Kap activity interferes with the signalling-mediated crosstalk between germline and somatic cells.
Recent studies on Kap/Kinesin II complexes showed that Kap is required for neural function (Ray et al, 1999; Sarpal et al, 2003). Our results provide evidence that Kap activity in germline cells is required for the proper differentiation of a distinct group of epithelial cells, the stalk cells, which separate individual follicles during early oogenesis. We found that the absence of Kap in germ cells prevents somatic target cells from differentiating into proper polar follicle cells, because they fail to express the ligand Unpaired. Unpaired activates JAK/STAT signalling (Harrison et al, 1998) and thereby induces stalk cell fate in the respective precursor cells (McGregor et al, 2002). We are currently investigating the details of the germline cell/polar follicle cell/stalk cell signalling cascade to address the cellular mechanism of these interactions.
Methods
Stocks and genetics. Wild-type strain Oregon R and mutant strains FM6y1w1dm+B/Dp(1;Y)y+, Df(1)v-N48,f*/C(1)DXy1f1/Dp(1;Y)y+v+#3, P{ry+t7.2=lArB}neurA101ry506/TM3ryRKSb1Ser1, y1w*v24P{w+mW.hs=FRT(whs)}FRT101, y1w1118P{w+mC=Ubi-GFP.nls}X1P{w+mW.hs=FRT(whs)}9-2; KrIf-1/CyO and w*ovoD1v24P{w+mW.hs =FRT(whs)}FRT101/C(1)DX/Y;P{ryt7.2=hsFLP}38 were obtained from the Bloomington stock centre; l(1)G0396 was derived from a lethal P-element screen (Peter et al, 2002). A total of 13 Kap mutants were generated by mobilizing the X chromosomal l(1)G0396 insertion (Bellen et al, 1989). Homozygous cell clones were generated by mitotic recombination using the FLP-ovoD1 system (Chou & Perrimon, 1992). F1 larvae of the genotype Kapmut/ovoD1; FLP38/+ were heat shocked twice (38°C; 1 h; second and third instar stages) to induce recombination. To distinguish wild-type (green) and mutant cells, we used Kapmut/ubiGFPnls; FLP38/+ individuals. Ovaries were isolated and examined after staining with different markers (see below); the phenotypes (see text) obtained with ovoD1 and GFP marked germlines were identical. For gene symbols and alleles, see Lindsley & Zimm (1992).
Transgenic flies. Sequences corresponding to the open reading frame of Kap were PCR amplified (cDNA clone LP01387; FlyBase 2002) using the Kap-ATG-EcoRI (5′-TAGTCGAATTCCGACAGC AGCAGCAACAACAACTG-OH) and Kap-FLAGsTOP-XbaI (5′-TT TCTAGATTACTTGTCATCGTCATCCTTGTAATCAGCCATC AGC AGCTCCTCG-OH) primers. The PCR product was digested (EcoRI and XbaI) and cloned into the matching sites of pAc5.1-V5-His-B (Invitrogen). The SmaI–XbaI fragment was subcloned into SmaI/XbaI-digested pC4-hs43-AUGβGal (pC4-Ac5.1-Kap-FLAG) and transformed into Drosophila (Rubin & Spradling, 1982).
Staining of ovaries and embryos. Embryos were fixed and devitellinized (Patel, 1994), and ovaries were prepared according to Theurkauf et al (1992). Rabbit anti-β-gal (1:5,000; Cappel), rabbit anti-GFP (Lot 71B1-3; 1:600; Molecular Probes) and mouse anti-Orb 4H8 and 6G4 (1:20, DSHB; Lantz et al, 1994) were used as primary antibodies; goat anti-mouse and anti-rabbit (Alexa 488 or 546; 1:500; Molecular Probes) were used as secondary antibodies. Filamentous actin was stained by adding 1.5 U of Alexa 488- or 633-coupled phalloidin (Molecular Probes, Leiden) during secondary antibody incubation. For DNA detection, samples were extensively treated with RNaseA (Sigma; 20 μg/ml in all buffers) and stained with 0.1 μM Sytox Green or TOTO-3 (Molecular Probes; ⩾1 h), also added to the embedding medium (0.05 μM).
Whole-mount in situ hybridizations were performed according to Hauptmann & Gerster (2000). Digoxigenin- or fluorescein- (DIG and FITC labelling mix; Roche) labelled probes and horse-radish-peroxidase-or alkaline-phosphatase-coupled anti-DIG and anti-FITC antibodies (Roche) were used in combination with nitro blue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (Sigma) or Tyramide Signal Amplification System Plus-Cyanine 3 (Perkin-Elmer), respectively. Images were taken with Zeiss Axiophot and LSM microscopes.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v5/n5/extref/7400141s1.pdf).
Supplementary Material
Supplementary Figures
Supplementary Movie
Acknowledgments
We thank our colleagues in the lab for their various contributions and critical discussions, G. Dowe for sequencing and H. Taubert for transformations. This work was supported by the Max-Planck-Society and the Fonds der Chemischen Industrie (H.J.).
References
- Bellen HJ, O'Kane CJ, Wilson C, Grossniklaus U, Pearson RK, Gehring WJ (1989) P-element mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev 3: 1288–1300 [DOI] [PubMed] [Google Scholar]
- Besse F, Pret AM (2003) Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 130: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Besse F, Busson D, Pret AM (2002) Fused-dependent Hedgehog signal transduction is required for somatic cell differentiation during Drosophila egg chamber formation. Development 129: 4111–4124 [DOI] [PubMed] [Google Scholar]
- Brendza RP, Serbus LR, Duffy JB, Saxton WM (2000) A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science 289: 2120–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha BJ, Serbus LR, Koppetsch BS, Theurkauf WE (2002) Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat Cell Biol 4: 592–598 [DOI] [PubMed] [Google Scholar]
- Chang JS, Tan L, Schedl P (1999) The Drosophila CPEB homolog, orb, is required for oskar protein expression in oocytes. Dev Biol 215: 91–106 [DOI] [PubMed] [Google Scholar]
- Chang JS, Tan L, Wolf MR, Schedl P (2001) Functioning of the Drosophila orb gene in gurken mRNA localization and translation. Development 128: 3169–3177 [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N (1992) Use of a yeast sitespecific recombinase to produce female germline chimeras in Drosophila. Genetics 131: 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG (1999) Kinesin-II, the heteromeric kinesin. Cell Mol Life Sci 56: 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC (1998) Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development 125: 2781–2789 [DOI] [PubMed] [Google Scholar]
- FlyBase Consortium (2002) The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res 30: 106–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC (1996) hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122: 1125–1135 [DOI] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z (2000) Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci 23: 39–71 [DOI] [PubMed] [Google Scholar]
- Grammont M, Irvine KD (2001) Fringe and Notch specify polar cell fate during Drosophila oogenesis. Development 128: 2243–2253 [DOI] [PubMed] [Google Scholar]
- Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N (1998) Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev 12: 3252–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T (2000) Multicolor whole-mount in situ hybridization. Methods Mol Biol 137: 139–148 [DOI] [PubMed] [Google Scholar]
- Hirokawa N (2000) Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1: 29–34 [DOI] [PubMed] [Google Scholar]
- Lantz V, Chang JS, Horabain JI, Bopp D, Schedl P (1994) The Drosophila orb RNA binding protein is required for the formation of the egg chamber and establishment of polarity. Genes Dev 8: 598–613 [DOI] [PubMed] [Google Scholar]
- Lindsley D, Zimm G (1992) The Genome of Drosophila melanogaster. San Diego, CA: Academic Press [Google Scholar]
- Lopezschier H, St Johnston D (2001) Delta signaling from the germline controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev 15: 1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor JR, Xi R, Harrison DA (2002) JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development 129: 705–717 [DOI] [PubMed] [Google Scholar]
- Patel NH (1994) Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol 44: 445–487 [DOI] [PubMed] [Google Scholar]
- Peter A et al. (2002) Mapping and identification of essential gene functions on the X chromosome of Drosophila. EMBO Rep 3: 34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portin P (2002) General outlines of the molecular genetics of the Notch signaling pathway in Drosophila melanogaster: a review. Hereditas 136: 89–96 [DOI] [PubMed] [Google Scholar]
- Ray K, Perez SE, Yang Z, Xu J, Ritchings BW, Steller H, Goldstein LS (1999) Kinesin-II is required for axonal transport of choline acetyltransferase in Drosophila. J Cell Biol 147: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A (2001) Axis formation during Drosophila oogenesis. Curr Opin Genet Dev 11: 374–383 [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Cole DG, Diener DR (1999) Intraflagellar transport: the eyes have it. J Cell Biol 144: 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353 [DOI] [PubMed] [Google Scholar]
- Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN (1991) Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66: 433–450 [DOI] [PubMed] [Google Scholar]
- Sarpal R, Ray K (2002) Dynamic expression pattern of kinesin accessory protein in Drosophila. J Biosci 27: 479–487 [DOI] [PubMed] [Google Scholar]
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF (2003) Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol 13: 1687–1696 [DOI] [PubMed] [Google Scholar]
- Spradling AC (1993) Developmental genetics of oogenesis. In The Development of Drosophila melanogaster, Bate M, Martinez-Arias A (eds) pp 1–70. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM (1992) Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115: 923–936 [DOI] [PubMed] [Google Scholar]
- Tworoger M, Larkin MK, Bryant Z, Ruohola-Baker H (1999) Mosaic analysis in the Drosophila ovary reveals a common hedgehog-inducible precursor stage for stalk and polar cells. Genetics 151: 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton RP, Struhl G (1989) Structure of the Drosophila BicaudalD protein and its role in localizing the posterior determinant nanos. Cell 59: 881–892 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Movie