Abstract
DNA is packed together with histone proteins in cell nuclei to form a compact structure called chromatin. Chromatin represents a scaffold for many genetic events and shows varying degrees of condensation, including a relatively open form (euchromatin) and a highly condensed form (heterochromatin). Enzymes such as histone acetyltransferases (HATs) and methylases covalently label the amino-termini of histones, thereby creating a 'histone code' of modifications that is interpreted by the recruitment of other proteins through recognition domains. Ultimately, this network of interacting proteins is thought to control the degree of chromatin condensation so that DNA is available when it is required for genomic processes. Reviewed here are the structures of HAT and SET domains, which mediate the acetylation and methylation of histones, respectively, and bromodomains and chromodomains, which recognize the modified histones. How these structures have increased our understanding of DNA regulation is also discussed.
Keywords: bromodomain, chromatin, chromodomain, HAT domain, histone, SET domain
Introduction
A major target for chromatin structure regulation is the nucleosome, which comprises a core of two copies of each of the histone proteins H2A, H2B, H3 and H4, with 146 base pairs of DNA wrapped around the core (Luger et al, 1997; Fig 1A). The positioning of nucleosomes in chromatin is a reversible adenosine triphosphate (ATP)-dependent process. Chromatin can therefore be considered as a variably compact, dynamic and yet stable construction.
Figure 1.
The wide range of histone modifications. (A) Chromatin is formed by nucleosome subunits comprising an octameric core of histones around which ∼1.8 superhelical turns of DNA are wrapped. DNA binds to the positively charged histone surfaces through H-bonds and electrostatic interactions. In the 11-nm chromatin fibre, successive nucleosomes are separated by 10–80 base pairs of linker DNA. Histone H1, which binds to nucleosomes and adjacent linker DNA, can mediate further condensation into the 30-nm chromatin fibre. (B) The amino-termini of histones H3 and H4 protrude from the nucleosome core and contain dense clusters of modifiable residues. Residues are coloured on well-documented modification sites, with red indicating where acetylation or phosphorylation increase acidity and blue indicating methylation. Note that H3 Lys 9 (pink) can be either acetylated or methylated. Pale green shading highlights putative 'modification cassettes' and boxes indicate potential 'methyl–phos binary switches'. Further possible modifications are listed elsewhere (Felsenfeld & Groudine, 2003; Fischle et al, 2003a). (C) Histone modifications create new chemical environments. For example, lysine acetylation (red) neutralizes the positive charge of the Nζ group and introduces the carbonyl oxygen, which is a potential H-bond acceptor. Lysine methylation (blue) increases both hydrophobicity and the cationic nature of the Nζ group. Depending on the modifying enzyme, lysines can be mono-, di- or tri-methylated.
Histones are conserved from yeast to man and regulate many cellular events, including transcription, DNA replication, recombination and repair. Histones primarily act either by promoting DNA packaging into repressive heterochromatin in which DNA is largely inaccessible, or by relaxing chromatin into the less compact euchromatin. Variations in the generally repressive euchromatin structure aid bioprocesses by enhancing the accessibility of the DNA. The various ways that such changes occur have been reviewed recently (Felsenfeld & Groudine, 2003) and include ATP-dependent chromatin remodelling, the incorporation of histone variants and covalent modifications to the histones, which were first described more than 30 years ago (Allfrey et al, 1964).
The most studied covalent modifications are the acetylation and deacetylation of histone lysine residues, which usually correlate with transcriptional activation and repression, respectively (Eberharter & Becker, 2002). A simplistic model for activation is that acetylation cancels positive charges on histone lysine side chains, which facilitates the release of DNA to enhance its accessibility to transcription factors. By contrast, histone deacetylation reverses this effect, rendering DNA less available. In addition to acetylation, phosphorylation and methylation also occur in the histone amino (N)-termini, and the interplay of modifications directs a host of genetic responses, as given in 'the histone code' (Strahl & Allis, 2000; Fig 1). A framework for understanding the basis of signalling by several simultaneous histone modifications was recently proposed, in which neighbouring modifications act together as 'binary switches'. Furthermore, it has recently been proposed that numerous residues in linear strings of densely clustered modifiable sites might form 'modification cassettes' to create a large array of readouts through many local permutations (Fischle et al, 2003a).
Chromatin can also be regulated by larger-scale covalent attachments, including ubiquitylation and sumoylation. Histone ubiquitylation is implicated in transcriptional regulation by priming other histone modifications; for example, the ubiquitylation of yeast H2B Lys 123 is required before the methylation of H3, resulting in a trans-histone regulatory mechanism that induces telomeric gene silencing (Zhang, 2003). Although histone ubiquitylation has long been known, the Bre1 RING domain has only recently been identified as the ubiquitin ligase for H2B (Hwang et al, 2003; Wood et al, 2003), and a ligase for H2A remains elusive. The conjugation of the small ubiquitin-related modifier (SUMO) to H4 has also been observed and ascribed a role in transcriptional repression (Shiio & Eisenman, 2003). Because the structural understanding of histone ubiquitylation and sumoylation is relatively poor, this area is not discussed further. Rather, to illustrate the molecular basis of chromatin regulation by histone modifications, this review focuses first on histone acetyltransferase (HAT) domains that mediate histone acetylation and bromodomains that recognize the acetylated histones; and second on the recently emerging SET domains that mediate histone methylation and chromodomains that recognize the methylated histones. Although some aspects of this topic have been reviewed previously (Jenuwein & Allis, 2001; Marmorstein, 2001a), the wealth of recent structures has prompted a reappraisal of this growing field.
Histone acetylation, deacetylation and bromodomains
Histone acetylation and deacetylation are mediated by opposing enzyme families: the HATs and the histone deacetylases (HDACs), respectively. HATs associate with many cofactors in complexes that allow either gene-specific or genome-wide activity. HDACs also occur in complexes, often with co-repressors, through which DNA-binding proteins might direct local transcriptional repression by deacetyl-ation. So, the roles of HAT and HDAC can be regulated through their availability to interact with cofactors, or through their activity or concentration (Legube & Trouche, 2003). In structural terms, the HDACs are relatively poorly characterized, whereas several structural analyses have revealed many details of HAT target specificity and catalysis.
HATs comprise several families, with high sequence similarity within families and low similarity between families. Whereas the GCN5 family HATs activate a distinct subset of genes, the p300/CBP family HATs act as global transcriptional activators. By contrast, some members of the MYST family confer gene silencing. The functional diversity of HAT families is not readily explained by their in vitro histone specificities (Sterner & Berger, 2000), which indicates that the readout of acetylation depends on a local histone code of modifications. Some HATs also acetylate specific or general transcription factors, thereby promoting DNA binding and transcription. The HDACs can deacetylate acetyl-Lys residues on histones or transcription factors such as p53 (Vaziri et al, 2001).
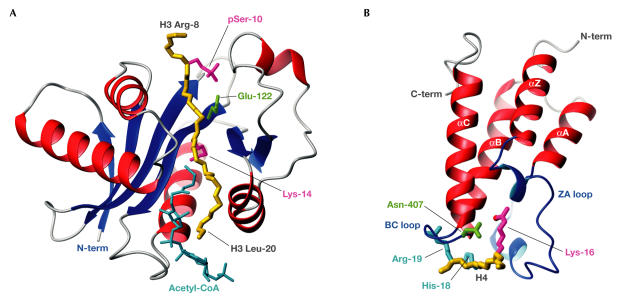
Some conserved structural features of the catalytic domain within HATs have emerged (Marmorstein, 2001b). The HAT-domain core comprises a threestranded antiparallel β-sheet followed by an α-helix, and is completed by a loop–β-strand motif (Fig 2A). The core scaffold has flanking regions that are more variable but do contain a structurally conserved N-terminal α-helix–loop motif and a carboxy (C)-terminal loop–α-helix motif. The HAT core binds to the acetyl-CoA cofactor in a similar manner in each structure, whereas the N- and C-terminal flanking regions bind to histones and the variability within these regions allows target discrimination. The structure of Tetrahymena GCN5 (tGCN5) complexed with acetyl-CoA and a histone H3 peptide phosphorylated on serine 10 (pSer 10) shows the cofactor in a surface pocket of the HAT core close to the H3 Lys 14 substrate (Clements et al, 2003). A proximal tGCN5 glutamate (Glu 122) is thought to catalyse acetyl transfer to Lys 14, which is a modification that is linked to transcriptional activation. Although this Glu is conserved in the GCN5 family, it is not present in all HATs, which indicates that the fold might support diverse catalytic mechanisms. Illustrating the synergy of the histone code, several pSer–HAT interactions stabilize binding compared with the non-phosphorylated substrate, so enhancing the acetylation of Lys 14 (Cheung et al, 2000).
Figure 2.
Acetyl-Lys modification and recognition. (A) The tGCN5 HAT domain with bound acetyl-CoA (cyan) and an H3 peptide spanning Arg 8 to Leu 20 (backbone in gold; Protein Data Bank (PDB) code ). Also shown are side chains of the catalytic Glu 122 (green) and H3 pSer 10, and target Lys 14 (magenta). (B) The GCN5 bromodomain bundle (red), bound to an H4 peptide spanning Ala 15 to Arg 19 (backbone in gold; PDB code ). The acetyl-Lys 16 (magenta) of H4 reaches far into the pocket that is formed by the bromodomain loops ZA and BC (blue). An H-bond forms between the acetyl oxygen atom (red) of Lys 16 and Asn 407 (green). The H4 residues His 18 and Arg 19 (cyan), in positions acetyl-Lys +2 and +3, make interactions that are specific for GCN5.
Acetylated histones are targets for the bromodomains of many HATs and other chromatin-associated proteins. Bromodomains bind specifically to acetylated lysines, thereby interpreting HAT activity and the histone code. They can also bind to acetylated non-histone proteins, such as the transcription factor p53 and the HIV-1 Tat protein. Bromodomains that occur in HAT proteins might enhance the off-rate of the catalytic domain whilst tethering HAT activity to chromatin to enhance local acetylation.
Bromodomains were linked to chromatin regulation when the p300/CBP-associated factor (PCAF) bromodomain structure was solved and shown by nuclear magnetic resonance spectroscopy to bind to histone-derived peptides containing acetyl-Lys (Dhalluin et al, 1999). Extending these in vitro studies, it has recently been shown by fluorescence resonance energy transfer assays that bromodomain proteins also show selective recognition of acetylated histones in vivo (Kanno et al, 2004). Studies of the yeast GCN5 bromodomain bound to an acetylated histone H4 peptide revealed the basis for acetyl-Lys specificity (Owen et al, 2000). Bromodomains adopt a left-hand twisted four-helical bundle, with two loops at one end of the bundle forming a hydrophobic lysine-binding pocket that selects acetyl-Lys rather than the charged unmodified lysine. The methyl and methylene groups of acetyl-Lys make many hydrophobic contacts with conserved bromodomain residues. Moreover, an asparagine that is found in most bromodomains makes an H-bond with the acetyl-Lys side-chain oxygen (Fig 2B).
Whereas HAT-mediated lysine acetylation activates binding, neighbouring residues determine bromodomain specificity. Surprisingly, a recent study showed that key residues for recognition by the bromodomain can be on either side of the acetyl-Lys; that is, the PCAF bromodomain contacts acetyl-Lys +2 and +3 residues in histone H4, but uses the same hydrophobic pocket to contact the acetyl-Lys −3 residue of HIV-1 Tat (Mujtaba et al, 2002). Recently, the structure of the CBP bromodomain bound to a Lys-acetylated p53 peptide revealed a specific interaction that is crucial for the activity of p53 in response to ultraviolet-induced DNA damage (Mujtaba et al, 2004). Collectively, these bromodomain structures reveal the hydrophobic pocket as a conserved recognition device, whereas subtle differences in the bound peptides also highlight the adaptability of the bromodomain as a ligand-binding scaffold.
Histone methylation: SET proteins and chromodomains
Despite past interest in histone acetylation and deacetylation, the most active area of chromatin-orientated research at present concerns histone methylation. Compared with acetylation, signalling by methylation seems to be more complex because lysines can be mono-, di- or tri-methylated, arginines can be mono- or di-methylated (symmetrically or asymmetrically), and both site specificity and the number of methyl groups modulate the epigenetic signal (Fig 1). Histone methylation can mediate gene silencing and heterochromatin formation, as dictated by H3 Lys 9 di-methylation or H3 Lys 27 tri-methylation (for a review, see Fischle et al, 2003b). As heterochromatin can pass through the cell cycle into daughter cells, a repressive epigenetic role for methylation has emerged. So far, histone demethylases have not been identified, which might explain the durable effects of methylation in contrast to acetylation. However, the potential for the methyl-Lys signals to be modulated by the phosphorylation of proximal residues, as encompassed by the 'binary switch' model (Fischle et al, 2003a), could render methylation-based signalling more dynamic than it first seems.
The histone-methylation story took another turn when certain active chromatin sites were found to harbour methylated histone H3 (Strahl et al, 1999). Although several inactive genes were shown to be di-methylated on H3 Lys 4, transcriptional competence was found to correlate with tri-methyl Lys 4 (Santos-Rosa et al, 2002). The growing number of responses that are governed by distinct methylations, which act together with other local modifications, reiterates the interplay that is central to the histone code.
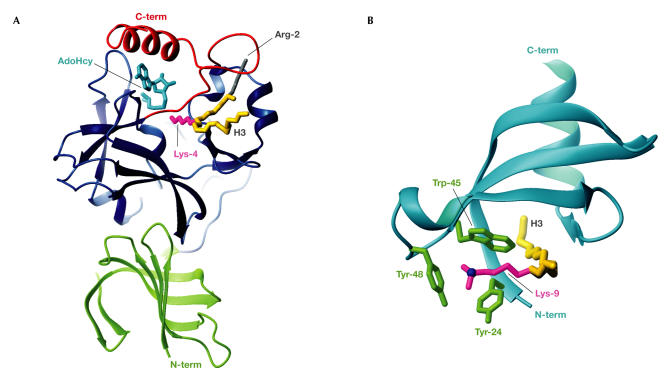
Lysine methylation is mediated by SET domains, which are named after the proteins in which they were found: Drosophila Su(var)3-9, Ez and Trithorax. The recent determination of several SET domain structures has shed light on this field. The SET domain core of ∼130 residues comprises two terminal regions, SET-N and SET-C, with a variable intervening SET-I region (Fig 3A). SET-C has a conserved knot-like structure that hosts the catalytic cofactor S-adenosylmethionine (AdoMet), which is used to transfer a methyl group to the target lysine. The remarkable catalytic mechanism of SET domains was revealed by the structure of the SET7/9 protein complexed with the cofactor product (S-adenosylhomocysteine (AdoHcy)) and a histone H3 methyl-Lys 4 substrate (Xiao et al, 2003). The AdoHcy and histone bind to opposite faces of the SET domain, requiring the target lysine side chain to approach the methyl-donating cofactor through a conserved trans-enzyme pore: the lysine-access channel. This structure indicates that a histone can remain bound to a SET domain while cofactor molecules cycle until the required level of mono-, di- or tri-methylation of lysine is achieved.
Figure 3.
Methyl-Lys modification and recognition. (A) The SET7/9 domain complexed with AdoHcy (cyan) and an H3 peptide spanning Arg 2 to Lys 9 (backbone in gold; Protein Data Bank (PDB) code ). The H3 methyl-Lys 4 side chain (magenta) projects from the front face, through the lysine-access channel, to reach AdoHcy at the back. The SET domain (blue) contains the conserved SET-N and SET-C domains, each of three or four β-strands, a short helix and several loops, plus the variable SET-I region. The N-terminal SET7/9 domain (green) makes hydrophobic interactions with the SET domain. The SET-C flanking region (red) packs against the SET domain to form the lysine-access channel, the histone-binding groove that selects H3 through interactions with Arg 2 (grey) and hydrophobic interactions with AdoHcy, which collectively explain its importance for SET activity. (B) The HP1β chromodomain (cyan) complexed with an H3 peptide (Gln 5 to Ser 10; backbone in gold) sandwiched between strands β4 and the histone-induced strand β1 (PDB code ). The H3 di-methyl-Lys 9 side chain (magenta) is surrounded along its length by hydrophobic residues that are conserved in many chromodomains. The Lys-Nζ atom (blue) sits centrally in the three-walled aromatic cage that is formed by Tyr 24, Trp 45 and Tyr 48 (green), which can accommodate di- or tri-methyl-Lys.
The notable features of the SET7/9 complex structure were that it included not only the histone substrate but also the SET-C flanking region (Fig 3A). The catalytic mechanism was discerned from the orientation of numerous SET tyrosine side chains, water molecules and the cofactor. The cofactor is orientated so that its methyl group just enters the lysine-access channel, which is lined by hydrophobic residues, in order to select the long lysine side chain. Steric features of the active site dictate that wild-type SET7/9 cannot accommodate di- or tri-methyl-Lys, which reflects its mono-methyltransferase activity. However, in an impressive example of molecular redesign, a SET7/9 point mutant was created with an enlarged substrate/cofactor-binding pocket that converted the enzyme into a di- and tri-methyltransferase (Xiao et al, 2003). A subsequent structure of the DIM-5 SET domain complexed with AdoHcy and an H3 peptide containing its Lys 9 target site yielded further functional insight. A DIM-5 point mutant was successfully designed to change it from a tri- into a mono-methyltransferase (Zhang et al, 2003). In short, SET-domain product specificity is determined by aromatic residues that dictate the accommodation or exclusion of methyl groups that are attached to the Lys-Nζ atom in the active site: high steric hindrance in SET7/9 restricts productivity to mono-methylation, whereas greater freedom in DIM-5 allows tri-methylation.
Similar to the fact that HAT activity produces acetylated histones that can recruit bromodomains, SET proteins produce methylated histones that can recruit other proteins through their chromodomains. Chromodomains are linked to transcriptional repression through the formation of heterochromatin or heterochromatin-like complexes and might ultimately signal epigenetic gene repression (Jones et al, 2000).
A major advance in understanding chromatin regulation by methylation came from studies on the chromodomain of heterochromatin protein 1β (HP1β) bound to histone H3 methylated on Lys 9 (Jacobs & Khorasanizadeh, 2002; Nielsen et al, 2002), which is a product of SET protein activity that confers gene repression. Methyl-histone binding induces N-terminal chromodomain residues to form a βstrand that contacts the H3 peptide and creates a β-sandwich. Chromodomains select for methyl-Lys targets, as the methylated side chain is more hydrophobic and its Nζ has increased cationic potential to enhance interactions with negative ligands. Accordingly, the HP1β structures have a hydrophobic pocket that can bind mono-, di- or tri-methyl-Lys 9 with increasing relative affinity (dissociation constant (KD) ∼2 μM for tri-methyl-Lys). The pocket is formed by conserved side chains that fold on histone binding to form an open cage with three aromatic mutually orthogonal walls (Fig 3B). The histone Lys-Nζ occupies the centre of the cage for maximal interactions with the aromatic group π-electrons. Specificity for lysine is achieved as shorter side chains would not extend far enough to reach the aromatic cage, which is reminiscent of the bromodomainselection mechanism for acetyl-Lys. Selection of the target methyl-Lys 9 is largely owing to interactions between the chromodomain and Thr 6/Ala 7 of H3. The HP1β complex structures also indicate that phosphorylation of Ser 10 would inhibit binding to the methylated histone, which is consistent with the 'methyl–phos binary switch' concept in which local phosphorylation could regulate binding of an effector protein (Fischle et al, 2003a). Although phosphorylation might also regulate acetylation, there are many more phosphorylatable residues adjacent to known methylation sites than to acetylation sites.
Recent work also shows that many structural features of HP1β are conserved in the Drosophila Polycomb (Pc) chromodomain bound to an H3 peptide containing tri-methyl-Lys 27, as found in repressed chromatin (Fischle et al, 2003c; Min et al, 2003). Interestingly, the Pc chromodomain seems to be able to form a dimer in which the bound histones interact through conserved residues, which indicates that histone–histone interactions might be important for Pc function. As HP1β also dimerizes in solution, an appealing model emerges in which chromodomain-enhanced histone–histone interactions might lock nucleosomes into the compact structures of repressive heterochromatin.
Despite many conserved features in the HP1β and Pc proteins, the cage-forming residues are not found in all chromodomains, which indicates a functional versatility of the fold. Indeed, some more distantly related chromodomains do not bind to histones but instead bind to RNA or DNA (Akhtar et al, 2000; Bouazoune et al, 2002). Moreover, one report revealed homology to chromodomains within the Tudor and Agenet domains. Agenet domains are plantspecific homologues of Tudor domains, which often occur in RNA- or DNA-associated proteins (Maurerstroh et al, 2003). The related Tudor and chromodomain folds have structurally coincident aromatic pockets that bind to peptides that contain methyl-Arg or methyl-Lys, respectively (Sprangers et al, 2003). It is possible that some Tudor domains regulate chromatin analogously to chromodomains because arginine methylation in histones is linked to gene activation (Bauer et al, 2002).
Histone phosphorylation
In this review, phosphorylation has only been discussed as a modulator of signalling by acetylation or methylation, because few structural details of histone phosphorylation are known. However, phosphorylation has long been observed in diverse chromatin contexts (Cheung et al, 2000). For example, H3 phosphorylation by the mitogen- and stress-activated kinases (MSK)-1 and MSK-2 accompanies acetylation and gene activation in the immediate-early response (Soloaga et al, 2003). Localized phosphorylation by MSKs might inhibit chromodomain binding and then synergize with histone acetylation, as discussed above. Unlike local phosphorylation, genome-wide H3 phosphorylation that coincides with chromatin condensation before mitosis is mediated by Aurora kinases (Crosio et al, 2002). Clarifying the molecular basis of histone phosphorylation and its readout is an important goal. As a widespread regulator of many signal-transduction pathways, which often ultimately activate transcription factors, it would not be surprising to find that phosphorylation is intimately involved in chromatin regulation. As phosphorylation is explored, it will also be interesting to see if there is a phosphohistone-recognition domain.
Perspectives: emerging chromatin-regulating domains
Many other chromatin-linked domains are now emerging, including the SAND, PWWP, PHD, MYND and SANT domains. The first SANT domain structure, which was within the nucleosome-recognition module of the remodelling factor ISWI, was recently solved (Grune et al, 2003). Other SANT domains are also implicated in chromatin regulation, either as histone-interacting modules or through binding to HDACs in repressor complexes (Yu et al, 2003). Similarly, the MYND domain, which is at present an unknown structure, recruits HDACs and repressor activity (Zhang et al, 2001). The PHD finger is structurally related to RING domains and therefore might be a ubiquitin ligase (Capili et al, 2001; Uchida et al, 2004). However, a general function for PHD fingers has not yet emerged, with reports also showing that the ING2 PHD finger binds phosphoinositides (Gozani et al, 2003), whereas the p300 PHD finger binds nucleosomes (Ragvin et al, 2004). The SAND domain is found in several chromatin-regulating proteins and often occurs alongside PHD or MYND domains (Bottomley et al, 2001). The PWWP domain is structurally similar to the chromodomain and shows 19% identity to the Tudor-domain core, which indicates a common ancestry. Whereas the Tudor domain binds to methyl-peptides, PWWP is a DNA-binding domain that is found in DNA methyltransferases, which might imply a shared epigenetic role (Qiu et al, 2002). These domains all modulate chromatin-regulating complexes and future studies should aim to define their roles in this molecular network. In addition, still more histone modifications might be identified, with the promise of exciting new structures to reveal their molecular functions.

Acknowledgments
I thank M. Sattler, A. Akhtar and P. Lo Surdo for their helpful comments on the manuscript. I apologize to those authors who are not cited owing to limitations in space. I am grateful for a Marie Curie Fellowship.
References
- Akhtar A, Zink D, Becker PB (2000) Chromodomains are protein–RNA interaction modules. Nature 407: 405–409 [DOI] [PubMed] [Google Scholar]
- Allfrey VG, Faulkner R, Mirsky AE (1964) Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA 51: 786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer UM, Daujat S, Nielsen SJ, Nightingale K, Kouzarides T (2002) Methylation at arginine 17 of histone H3 is linked to gene activation. EMBO Rep 3: 39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley MJ, Collard MW, Huggenvik JI, Liu Z, Gibson TJ, Sattler M (2001) The SAND domain structure defines a novel DNA-binding fold in transcriptional regulation. Nat Struct Biol 8: 626–633 [DOI] [PubMed] [Google Scholar]
- Bouazoune K, Mitterweger A, Langst G, Imhof A, Akhtar A, Becker PB, Brehm A (2002) The dMi-2 chromodomains are DNA binding modules important for ATP-dependent nucleosome mobilization. EMBO J 21: 2430–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capili AD, Schultz DC, Rauscher IF, Borden KL (2001) Solution structure of the PHD domain from the KAP-1 corepressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J 20: 165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P (2000) Signaling to chromatin through histone modifications. Cell 103: 263–271 [DOI] [PubMed] [Google Scholar]
- Clements A, Poux AN, Lo WS, Pillus L, Berger SL, Marmorstein R (2003) Structural basis for histone and phosphohistone binding by the GCN5 histone acetyltransferase. Mol Cell 12: 461–473 [DOI] [PubMed] [Google Scholar]
- Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P (2002) Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol 22: 874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature 399: 491–496 [DOI] [PubMed] [Google Scholar]
- Eberharter A, Becker PB (2002) Histone acetylation: a switch between repressive and permissive chromatin. EMBO Rep 3: 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M (2003) Controlling the double helix. Nature 421: 448–453 [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD (2003a) Binary switches and modification cassettes in histone biology and beyond. Nature 425: 475–479 [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD (2003b) Histone and chromatin cross-talk. Curr Opin Cell Biol 15: 172–183 [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S (2003c) Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev 17: 1870–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozani O et al. (2003) The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114: 99–111 [DOI] [PubMed] [Google Scholar]
- Grune T, Brzeski J, Eberharter A, Clapier CR, Corona DF, Becker PB, Muller CW (2003) Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol Cell 12: 449–460 [DOI] [PubMed] [Google Scholar]
- Hwang WW, Venkatasubrahmanyam S, Ianculescu AG, Tong A, Boone C, Madhani HD (2003) A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol Cell 11: 261–266 [DOI] [PubMed] [Google Scholar]
- Jacobs SA, Khorasanizadeh S (2002) Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295: 2080–2083 [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Jones DO, Cowell IG, Singh PB (2000) Mammalian chromodomain proteins: their role in genome organisation and expression. Bioessays 22: 124–137 [DOI] [PubMed] [Google Scholar]
- Kanno T, Kanno Y, Siegel RM, Jang MK, Lenardo MJ, Ozato K (2004) Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol Cell 13: 33–43 [DOI] [PubMed] [Google Scholar]
- Legube G, Trouche D (2003) Regulating histone acetyltransferases and deacetylases. EMBO Rep 4: 944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260 [DOI] [PubMed] [Google Scholar]
- Marmorstein R (2001a) Protein modules that manipulate histone tails for chromatin regulation. Nat Rev Mol Cell Biol 2: 422–432 [DOI] [PubMed] [Google Scholar]
- Marmorstein R (2001b) Structure of histone acetyltransferases. J Mol Biol 311: 433–444 [DOI] [PubMed] [Google Scholar]
- Maurerstroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP (2003) The Tudor domain 'Royal Family': Tudor, plant Agenet, Chromo, PWWP and MBT domains. Trends Biochem Sci 28: 69–74 [DOI] [PubMed] [Google Scholar]
- Min J, Zhang Y, Xu RM (2003) Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev 17: 1823–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba S, He Y, Zeng L, Farooq A, Carlson JE, Ott M, Verdin E, Zhou MM (2002) Structural basis of lysine-acetylated HIV-1 Tat recognition by PCAF bromodomain. Mol Cell 9: 575–586 [DOI] [PubMed] [Google Scholar]
- Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik-Le NJ, Ronai Z, Zhou MM (2004) Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell 13: 251–263 [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Mott HR, Callaghan J, Bannister A, Kouzarides T, Murzin AG, Murzina NV, Laue ED (2002) Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 416: 103–107 [DOI] [PubMed] [Google Scholar]
- Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA (2000) The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J 19: 6141–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Sawada K, Zhang X, Cheng X (2002) The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nat Struct Biol 9: 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragvin A et al. (2004) Nucleosome binding by the bromodomain and PHD finger of the transcriptional cofactor p300. J Mol Biol 337: 773–788 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T (2002) Active genes are tri-methylated at K4 of histone H3. Nature 419: 407–411 [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN (2003) Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci USA 100: 13225–13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS (2003) MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J 22: 2788–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers R, Groves MR, Sinning I, Sattler M (2003) High-resolution X-ray and NMR structures of the SMN Tudor domain: conformational variation in the binding site for symmetrically dimethylated arginine residues. J Mol Biol 327: 507–520 [DOI] [PubMed] [Google Scholar]
- Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64: 435–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403: 41–45 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Ohba R, Cook RG, Allis CD (1999) Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena. Proc Natl Acad Sci USA 96: 14967–14972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D et al. (2004) AIRE functions as an E3 ubiquitin ligase. J Exp Med 199: 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159 [DOI] [PubMed] [Google Scholar]
- Wood A et al. (2003) Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol Cell 11: 267–274 [DOI] [PubMed] [Google Scholar]
- Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ (2003) Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature 421: 652–656 [DOI] [PubMed] [Google Scholar]
- Yu J, Li Y, Ishizuka T, Guenther MG, Lazar MA (2003) A SANT motif in the SMRT corepressor interprets the histone code and promotes histone deacetylation. EMBO J 22: 3403–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hug BA, Huang EY, Chen CW, Gelmetti V, Maccarana M, Minucci S, Pelicci PG, Lazar MA (2001) Oligomerization of ETO is obligatory for corepressor interaction. Mol Cell Biol 21: 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang Z, Khan SI, Horton JR, Tamaru H, Selker EU, Cheng X (2003) Structural basis for the product specificity of histone lysine methyltransferases. Mol Cell 12: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y (2003) Transcriptional regulation by histone ubiquitination and deubiquitination. Genes Dev 17: 2733–2740 [DOI] [PubMed] [Google Scholar]