Abstract
Rhizobia are the common bacterial symbionts that form nitrogen-fixing root nodules in legumes. However, recently other bacteria have been shown to nodulate and fix nitrogen symbiotically with these plants. Neptunia natans is an aquatic legume indigenous to tropical and subtropical regions and in African soils is nodulated by Allorhizobium undicola. This legume develops an unusual root-nodule symbiosis on floating stems in aquatic environments through a unique infection process. Here, we analyzed the low-molecular-weight RNA and 16S ribosomal DNA (rDNA) sequence of the same fast-growing isolates from India that were previously used to define the developmental morphology of the unique infection process in this symbiosis with N. natans and found that they are phylogenetically located in the genus Devosia, not Allorhizobium or Rhizobium. The 16S rDNA sequences of these two Neptunia-nodulating Devosia strains differ from the only species currently described in that genus, Devosia riboflavina. From the same isolated colonies, we also located their nodD and nifH genes involved in nodulation and nitrogen fixation on a plasmid of approximately 170 kb. Sequence analysis showed that their nodD and nifH genes are most closely related to nodD and nifH of Rhizobium tropici, suggesting that this newly described Neptunia-nodulating Devosia species may have acquired these symbiotic genes by horizontal transfer.
Neptunia natans (L.f.) Druce is an aquatic legume native to several continents of the humid tropics and is used for both human consumption and as green manure for rice cultivation in Asiatic countries. This legume is unusual in that it normally develops buoyant floating stems that grow profusely on the surface of freshwater ponds, and in this aquatic environment it develops many stem-associated nitrogen-fixing nodules.
The developmental morphology of the infection process leading to formation of nitrogen-fixing nodules in N. natans has been examined under strict gnotobiotically controlled conditions, and several unique aspects of this specialized, aquatic plant-bacterium symbiosis distinguish it from other legume root-nodule symbioses (22). After colonizing the root and floating stem surfaces, the aquatic bacterium symbiont enters the primary root cortex and stem interior through natural wounds caused by splitting of the epidermis and emergence of young lateral and adventitious roots, respectively, and then stimulates early development of nodules in the cortex at the base of these roots primordia, but not in the stem itself (22). Following crack entry through the nodule periphery, the bacteria penetrate internal nodule host cells; induce formation of bona fide tubular infection threads that disseminate them further intracellularly; and then release the bacteria into infection droplets, where they multiply. The endosymbiotic bacteria transform into nitrogen-fixing bacteroids within symbiosomes that eventually become filled with an unusual fibrillar matrix towards the end of their active nitrogen-fixing cycle in the aquatic environment. This symbiotic developmental process was the same for both bacterial isolates used in this previous study, named strains J1 and J2, isolated from surface-sterilized N. natans nodules collected in India. Those two strains exhibited a host preference for N. natans and were unable to nodulate several other legumes tested. Because these two isolates nodulated Neptunia roots, symbiotically fixed nitrogen, and were fast growing on standard Rhizobium culture media, they were provisionally called “Rhizobium neptunii.”
The genera Burkholderia (13) and Ralstonia (3) are members of the β-subclass of the Proteobacteria (β-proteobacteria), recently reported to contain species that nodulate legumes, but most bacterial genera that form nitrogen-fixing nodules on legumes, including Methylobacterium (23), are in several families of α-proteobacteria. Recently, several fast-growing Neptunia-nodulating bacteria isolated from Senegal (Africa) were assigned to a new rhizobial genus, Allorhizobium (10). This proposed bacterial genus included only one species, Allorhizobium undicola, belonging to the family Rhizobiaceae. We wondered whether all the bacteria that develop nitrogen-fixing root-nodule symbioses with N. natans are Allorhizobium species or whether they might include other taxa as well. Therefore, the primary objective of this work was to examine the phylogenetic relationship of the original isolates from India used in the previous study that documented the infection process during symbiotic root-nodule development in N. natans (22) and, by so doing, to determine if the taxon A. undicola as described by de Lajudie et al. (5) and Kuykendall and Dazzo (10) fully encompasses the unique group of diazotrophic nodule-inducing endosymbionts of this unusual aquatic legume.
MATERIALS AND METHODS
Bacterial strains and culture media.
Strains J1 and J2 isolated from root nodules of N. natans in India (22) and the type strain of Devosia riboflavina, LMG 2277T, were cultivated in YMA medium (1) at 28°C.
LMW RNA analysis.
Low-molecular-weight (LMW) RNA extraction was accomplished following the phenol-chloroform method described by Höfle (8). The following commercial molecules from Boehringer Mannheim (Mannheim, Germany) and Sigma (St. Louis, Mo.) were used as reference: 5S rRNA from Escherichia coli MRE 600 (120 and 115 nucleotides) (2) and E. coli tRNAs specific for tyrosine (85 nucleotides) and valine (77 nucleotides) (21). Samples containing 5 μg of LMW RNA were added to 5 μl of loading solution (sucrose [300 mg/ml], urea [460 mg/ml], 20% sodium dodecyl sulfate [10 μl/ml], xylene cyanol [1 mg/ml]) and, after 10 min of heating at 70°C, applied to each well. LMW RNA profiles were obtained using staircase electrophoresis in gels (400 by 360 by 0.4 mm) using a vertical slab unit (Poker Face SE 1500 sequencer; Hoeffer Scientific Instruments, San Francisco, Calif.). The separating gel contained 14% acrylamide-N,N-methylene bisacrylamide (29:1 [wt/wt]) and 7 M urea in TBE buffer (100 mM Tris, 83 mM boric acid, 1 mM EDTA [pH 8.5]). The system was stabilized at 50°C, and then a preelectrophoresis run of 30 min at 100 V was performed. The running buffer (1.2× TBE) was recycled at a flow rate of 300 ml/min with a peristaltic pump (MasterFlex; Cole Parmer Instruments, Chicago, Ill.). After electrophoresis, the gels were silver stained according to the method of Haas et al. (6).
DNA extraction and 16S ribosomal DNA (rDNA) sequence analysis of nodD and nifH genes.
DNA was extracted as described previously (17) from a single colony of each strain. The PCR amplification of whole 16S rDNA of J1 and J2 strains was carried out using primers 8F (5′-AGAGTTTGATCTGGCTCAG-3′) and 1522R (5′-AAGGAGGTGATCCANCCRCA-3′) under the following conditions: preheating at 95°C for 9 min; 35 cycles of denaturing at 95°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 2 min; and a final extension at 72°C for 7 min. The sequence reaction was performed on an ABI377 sequencer (Applied Biosystems Inc.) using a BigDye terminator (version 3.0) cycle sequencing kit as supplied by the manufacturer. The following primers were used: 5′-AACGCTGGCGGCRKGCYTAA-3′, 5′-ACTCCTACGGGAGGCAGCAG-3′, 5′-CTGCTGCCTCCCGTAGGAGT-3′, 5′-CGTGCCAGCAGCCGCGGTAA-3′, 5′-CAGGATTAGATACCCTGGTAG-3′, and 5′-GAGGAAGGTGGGGATGACGTC-3′, which correspond to E. coli small-subunit rDNA sequence positions 32 to 52, 336 to 356, 356 to 336, 512 to 532, 782 to 803, and 1173 to 1194, respectively. The PCR amplifications of nodD and nifH genes were carried out with the DNA extracted from the same isolated colonies using the primer pair 5′-CTCGTCGCGCTCGACGCATTGA-3′ and 5′-TGCCCCATGGACATGTA-3′ (nodD) and the primer pair 5′-GTCTCCTATGACGTGCTCGG-3′ and 5′-GCTTCCATGGTGATCGGGGT-3′ (nifH) under the following conditions: preheating at 95°C for 9 min; 35 cycles of denaturing at 95°C for 1 min, annealing at 60°C for 2 min, and extension at 72°C for 2 min; and a final extension at 72°C for 7 min. The sequence reaction was performed the same way as for the 16S rDNA gene. The same primers for amplification were used in the gene sequencing.
16S rDNA analysis.
The sequences obtained were compared with those from the GenBank using the FASTA program (15). Sequences were aligned using the Clustal W software (24). The distances were calculated according to Kimura's two-parameter method (9). Phylogenetic trees were inferred using the neighbor-joining method (19). Bootstrap analysis was based on 1,000 resamplings. The PHYLIP package (Phylogenetic inference package, version 3.5c; University of Washington, Seattle) was used for all analyses. The phylogenetic trees were rooted using Bradyrhizobium japonicum as the outgroup.
Plasmid profile analysis.
The isolated strains were subjected to plasmid profile analysis according to the method of Plazinski et al. (16) except that electrophoresis was done at 2 V cm−1 for 90 min, followed by 3 V cm−1 for 60 min and finally at 6 V cm−1 for 3 h. The 175- and 205-kb plasmids of Sinorhizobium meliloti GR4 (25) were used as size markers. Plasmid DNA was capillary transferred to a nylon membrane according to the method of Southern (20) and immobilized by baking at 80°C for 2 h.
Oligonucleotide primers and preparation of DNA probe.
Oligonucleotide primers were designed to amplify a fragment of the nodD and nifH genes conserved among members of the family Rhizobiaceae. These primers were nodD1F (5′-CTCGTCGCGCTCGACGCATTGA-3′) and nodD1R (5′-TGCCCCATGGACATGTA-3′) (positions 31 to 53 and 585 to 568; S. meliloti nodD gene, AE007238) and primers nifH1F (5′-GTCTCCTATGACGTGCTCGG-3′) and nifH1R (5′-GCTTCCATGGTGATCGGGGT-3′) (positions 367 to 389 and 794 to 774; S. meliloti nifH gene, AE007235).
The PCR-amplified fragments of nifH and nodD were digoxigenin labeled with the DIG DNA labeling kit (Roche Diagnostics Corp.) following manufacturer instructions and used as probes. Hybridization was detected with the DIG nucleic acid detection kit (Boehringer Mannheim, USA), using 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium as substrates for alkaline phosphatase, according to the manufacturer instructions.
RESULTS AND DISCUSSION
LMW RNA analysis.
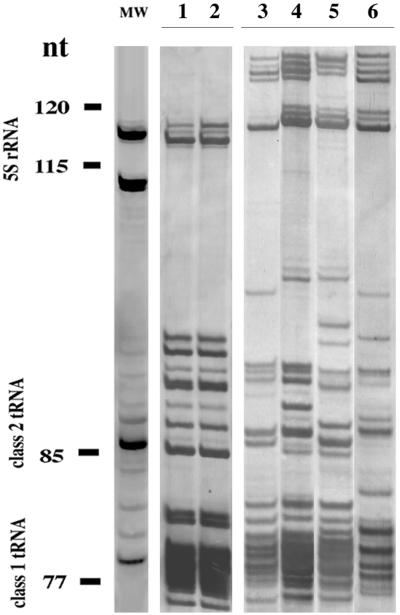
Staircase electrophoresis typically separates LMW RNA molecules into three resolved zones: 5S rRNA and class 1 and class 2 tRNA (4). We have already demonstrated that this powerful electrophoretic profiling technique can rapidly differentiate many bacterial genera on the basis of their 5S rRNA zone (26, 27, 28, 29) and many species (from the same or different genera) according to their tRNA profiles. Therefore, these profiles provide an excellent way to detect new microbial genera and species. For this reason we analyzed the LMW RNA profile of the Neptunia-nodulating isolates J1 and J2. Figure 1 (lanes 1 and 2) shows that the J1 and J2 strains are closely related in that they display identical LMW RNA profiles. However, the 5S rRNA zone of the profiles for both strains J1 and J2 is very different from the corresponding 5S rRNA zone of the type strain of A. undicola (Fig. 1, lane3), which also nodulates N. natans. The 5S rRNA zone of strains J1 and J2 was also different from the corresponding zone in the LMW RNA profile of the other fast-growing rhizobia, Rhizobium leguminosarum (lane 4) and S. meliloti (lane 5), and from those of Mesorhizobium loti (lane 6). Therefore, staircase electrophoresis of LMW RNA profiling provided early, clear evidence that the Neptunia-nodulating strains J1 and J2 are unrelated to any of the type strains of fast-growing rhizobial genera already described. The profile of the 5S rRNA zones of strains J1 and J2 is different from those of the genus Allorhizobium, which nodulates Neptunia, and the other genera of fast-growing rhizobia, Rhizobium and Sinorhizobium, indicating that these two Neptunia-nodulating isolates do not belong to these genera.
FIG. 1.
LMW RNA profiles of type species of fast-growing genera of bacteria from the family Rhizobiaceae that nodulate various legumes and of strains that nodulate N. natans: strain J1 (lane 1), strain J2 (lane 2), A. undicola LMG 11875T (lane 3), R. leguminosarum ATCC 10004T (lane 4), S. meliloti ATCC 3390T (lane 5), and M. loti ATCC 3669T (lane 6).
16S rDNA sequencing and analysis.
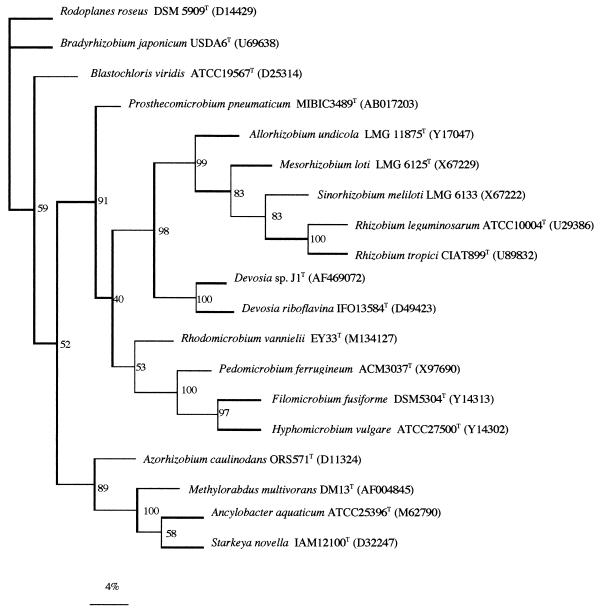
As the accepted technique to identify bacteria at genus level is 16S rDNA sequencing, we have obtained the complete 16S rDNA sequences of strains J1 and J2. These sequences were compared with those from databanks using the FASTA program (15). Both sequences showed a 100% similarity to each other, and therefore only strain J1 (accession number AF469072) was included in developing the phylogenetic tree. Unexpectedly, the phylogenetic analysis of its 16S rDNA sequence (Fig. 2) showed that isolate J1 was most closely related to the genus Devosia in the family Hyphomicrobiaceae within the order Rhizobiales (α-proteobacteria), which also contains the rhizobial species Azorhizobium caulinodans. The genus Devosia was proposed by Nakagawa et al. (14), and until now, this genus did not contain any nitrogen-fixing legume endosymbionts. Devosia currently includes only one known species, Devosia riboflavina (formerly Pseudomonas riboflavina) isolated from soil. This species is a gram-negative, aerobic, nonsporulated motile rod that contains long-chain 3-hydroxy fatty acids. Phylogenetic analysis of the 16S rDNA sequence of strain J1 indicates that its closest known species is D. riboflavina (95.9% similarity), but the difference of 30 nucleotide positions in the 16S rDNA sequences of both Neptunia-nodulating strains J1 and J2 strongly suggest that they are not D. riboflavina, but rather should belong to a new species of Devosia.
FIG. 2.
Comparative sequence analysis of 16S rDNA from strain J1 and representative related strains from GenBank. The significance of each branch is indicated by a bootstrap value calculated for 1,000 subsets. Bar, 4 nt substitutions per 100 nt.
Amplification and sequencing of nodD and nifH genes in strain J1.
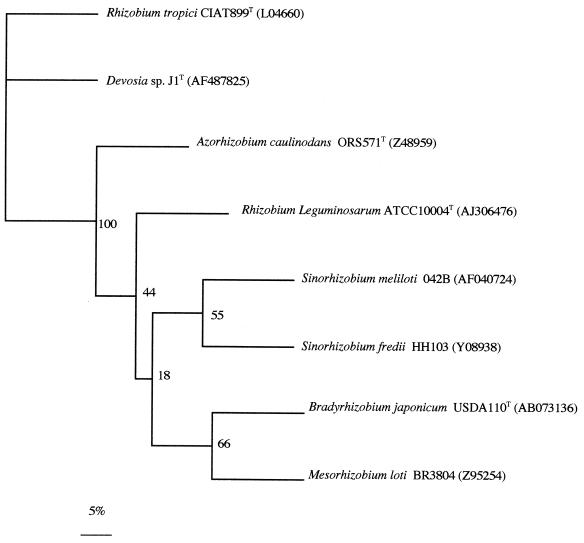
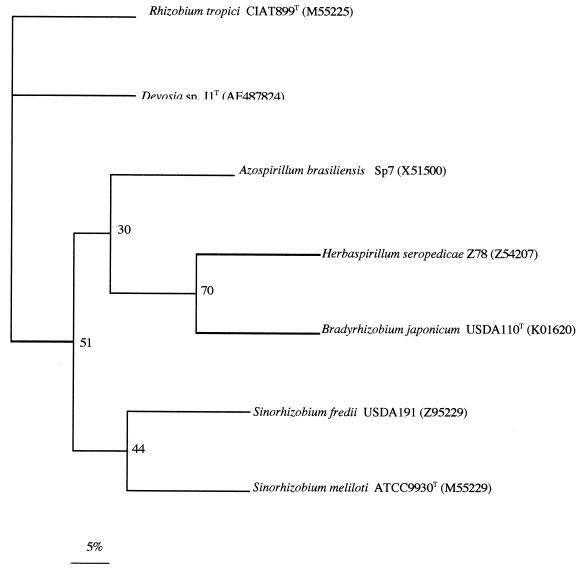
The characteristics of legume nodulation and symbiotic nitrogen fixation in rhizobia are codified in nod and nif genes, respectively. Among these, the nodD and nifH genes are highly conserved among rhizobia. To design adequate primers for amplification of rhizobial nodD and nifH genes, we analyzed their sequences from several rhizobial and nonrhizobial species in GenBank. Based on highly homologous sequences, we designed the primers used in this work to amplify the nodD and nifH genes. Using total DNA from the same isolated colony of strain J1 that had also been used to analyze its 16S rDNA sequence, we have amplified a band corresponding to these nodD and nifH genes (accession numbers AF487825 and AF487824, respectively), and their sequences were obtained and compared with those of rhizobia (Fig. 3 and 4). As shown in Fig. 3 and 4, the sequences of nodD and nifH from strain J1 are closely related to the nodD and nifH genes of Rhizobium tropici CIAT 899T, respectively. CIAT 899 is a well-known, highly competitive and effective strain used in many studies of the bean-rhizobium root-nodule symbiosis in Central and South America.
FIG. 3.
Comparative sequence analysis of nodD gene sequences from strain J1 and representative related strains from GenBank. The significance of each branch is indicated by a bootstrap value calculated for 1,000 subsets. Bar, 5 nt substitutions per 100 nt.
FIG. 4.
Comparative sequence analysis of nifH gene sequences from strain J1 and representative related strains from GenBank. The significance of each branch is indicated by a bootstrap value calculated for 1,000 subsets. Bar, 5 nt substitutions per 100 nt.
Plasmid profile and location of nodD and nifH genes in strains J1 and J2.
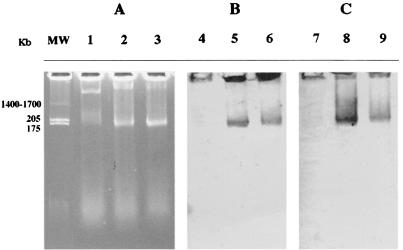
The symbiotic genes that codify nodulation and nitrogen fixation are located in plasmids in fast-growing rhizobia and on chromosomes in slow-growing rhizobia. These symbiotic plasmids in fast-growing rhizobia can be transferred among species in the rhizosphere. We analyzed the plasmid profiles of J1 and J2 to test if their nodD and nifH genes are located in extrachromosomal DNA elements. This technique revealed two plasmids in both strains J1 and J2 (Fig. 5A, lanes 2 and 3) and a single plasmid in the type strain of D. riboflavina (Fig. 5A, lane 1). Specific probes detected both nodD and nifH in the same plasmid of ca. 170 kb in the plasmid profiles of both J1 and J2 isolates (Fig. 5B, lanes 5 and 6, and Fig. 5C, lanes 8 and 9, respectively), and neither of these two symbiotic genes was detected in the type strain of D. riboflavina (Fig. 5B and C, lanes 4 and 7, respectively).
FIG. 5.
(A) Plasmid profile in horizontal 0.7% agarose gel. Lanes: 1, D. riboflavina LMG2277T; 2, strain J1; 3, strain J2; MW, S. meliloti GR4. (B) Southern blot hybridization with the digoxigenin-labeled nodD probe. Lanes: 4, D. riboflavina LMG2277T; 5, strain J1; 6, strain J2. (C) Southern blot hybridization with the digoxigenin-labeled nifH probe. Lanes: 7, D. riboflavina LMG2277T; 8, strain J1; 9, strain J2.
Based on these results, we conclude that natural isolates belonging to a new species in the genus Devosia are able to establish a nitrogen-fixing root-nodule symbiosis with the aquatic legume N. natans (L.f.) Druce. The aquatic growth habit and developmental morphology displayed in this beneficial plant-bacterium symbiosis has been thoroughly described and represents a unique ecology and infection process (22). These isolates of Devosia described here are phylogenetically unrelated to R. tropici (90% similarity in 16S rDNA sequences) and A. undicola, representing the only other bacterial species currently reported to form root nodules on the same aquatic legume host. These data add to the extension of legume-nodulating bacterial symbionts, e.g., the genera Methylobacterium, Burkholderia, and Ralstonia (3, 13, 23), that are away from the traditional rhizobia and bradyrhizobia, but because only about 50 of the 750 known legume genera have been studied, it is quite likely that more species of diazotrophic endosymbionts of legume root nodules unrelated to rhizobia will continue to be discovered by characterizing the bacteria nodulating unexplored legumes or geographical locations.
The nod and nif genes of fast-growing rhizobia required for nodulation and symbiotic nitrogen fixation in legumes commonly reside in plasmids, called pSym, that in some cases are autoconjugative. Consistent with this, we found that both nodD and nifH genes in strains J1 and J2 are located in a ca. 170-kb plasmid. The very similar sequences of the nodD and nifH of strains J1 and J2 with the corresponding genes of R. tropici suggest that these two Neptunia-nodulating Devosia isolates from India may have acquired these symbiotic genes by horizontal transference from rhizobia, possibly R. tropici that has a broad host range (7, 12) and carries Sym plasmids prone for horizontal transfer to different bacterial backgrounds (11, 18).
We are currently performing a polyphasic analysis to thoroughly describe this new species of Devosia (tentatively named “Devosia neptuniae”) and are studying several additional nodule isolates from Neptunia from Mesoamerica to compare the diversity of populations that nodulate this unique legume in several continents.
Acknowledgments
This work was supported by Junta de Castilla y León and DGCYT of Spain.
REFERENCES
- 1.Bergersen, F. J. 1961. The growth of Rhizobium in synthetic media. Austr. J. Biol. 14:349-360. [Google Scholar]
- 2.Bidle, K. D., and M. Fletcher. 1995. Comparison of free-living and particle-associated bacterial communities in the Chesapeake Bay by stable low-molecular-weight RNA analysis. Appl. Environ. Microbiol. 61:944-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, W. M., S. Laevens, T. M. Lee, T. Coenye, P. de Vos, M. Mergeay, and P. Vandamme. 2001. Ralstonia taiwanensis sp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. Int. J. Syst. E vol. Microbiol. 51:1729-1735. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Sánchez, J. M., E. Velázquez, P. Mateos, and E. Martínez-Molina. 1997. Enhancement of resolution of low molecular weight RNA profiles by staircase electrophoresis. Electrophoresis 18:1909-1911. [DOI] [PubMed] [Google Scholar]
- 5.de Lajudie, P., E. Laurent-Fullele, A. Willems, U. Torck, R. Coopman, M. D. Collins, K. Kersters, B. Dreyfus, and M. Gillis. 1998. Allorhizobium undicola gen. nov., sp. nov., nitrogen-fixing bacteria that efficiently nodulate Neptunia natans in Senegal. Int. J. Syst. Bacteriol. 48:1277-1290. [DOI] [PubMed] [Google Scholar]
- 6.Haas, H., B. Budowle, and G. Weiler. 1994. Horizontal polyacrylamide gel electrophoresis for the separation of DNA fragments. Electrophoresis 15:153-158. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Lucas, I., L. Segovia, E. Martínez-Romero, and S. G. Pueppke. 1995. Phylogenetic relationships and host range of Rhizobium spp. that nodulate Phaseolus vulgaris L. Appl. Environ. Microbiol. 61:2775-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Höfle, M. G. 1988. Identification of bacteria by low molecular weight RNA profiles: a new chemotaxonomic approach. J. Microbiol. Methods 8:235-248. [Google Scholar]
- 9.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 10.Kuykendall, L. D., and F. B. Dazzo. Allorhizobium De Lajudie, Laurent-Fulele, Willems, Torck, Coopman, Collins, Kersters, Dreyfus & Gillis, 1998. Allorhizobium. In D. Brenner, N. Krieg, J. Staley, and G. Garrity (ed.), The Proteobacteria. Bergey's manual of systematic bacteriology, 2nd ed., vol. 2, in press. Springer-Verlag, New York, N.Y.
- 11.Martínez, E., R. Palacios, and F. Sánchez. 1987. Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J. Bacteriol. 169:2828-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Romero, E., L. Segovia, F. M. Mercante, A. A. Franco, P. Graham, and M. A. Pardo. 1991. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int. J. Syst. Bacteriol. 41:417-426. [DOI] [PubMed] [Google Scholar]
- 13.Moulin, L., A. Munive, B. Dreyfus, and C. Boivin-Masson. 2001. Nodulation of legumes by members of β-subclass of Proteobacteria. Nature 411:948-950. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa, Y., T. Sakani, and A. Yokota. 1996. Transfer of “Pseudomonas riboflavina” (Foster 1944), a gram-negative, motile rod with long-chain 3-hydroxy fatty acids, to Devosia riboflavina gen. nov., sp. nov., nom. rev. Int. J. Syst. Bacteriol. 46:16-22. [DOI] [PubMed] [Google Scholar]
- 15.Pearson, W. R., and D. J. Lipman. 1988. Search for DNA homologies was performed with the FASTA program. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plazinski, J., Y. H. Chen, and B. G. Rolfe. 1985. General method for the identification of plasmid species in fast-growing soil microorganisms. Appl. Environ. Microbiol. 48:1001-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivas, R., E. Velázquez, A. Valverde, P. F., Mateos, and E. Martínez-Molina. 2001. A two primers random amplified polymorphic DNA procedure to obtain polymerase chain reaction fingerprints of bacterial species. Electrophoresis 22:1086-1089. [DOI] [PubMed] [Google Scholar]
- 18.Rogel, M. A., I. Hernández-Lucas, L. D. Kuykendall, D. L. Balkwill, and E. Martínez-Romero. 2001. Nitrogen-fixing nodules with Ensifer adhaerens harboring Rhizobium tropici symbiotic plasmids. Appl. Environ. Microbiol. 67:3264-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitou, N., and M. Nei. 1987. A neighbour-joining method: a new method for reconstructing phylogenetics trees. Mol. Biol. Evol. 44:406-425. [DOI] [PubMed] [Google Scholar]
- 20.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 21.Sprinzl, M., J. Moll, F. Meissner, and T. Hartmann. 1985. Compilation of tRNA sequences. Nucleic Acids Res. 13:1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subba-Rao, N. S., P. F. Mateos, D. Baker, H. Pankrazt, J. Palma, J., F. B. Dazzo, and J. I. Sprent. 1995. The unique root-nodule symbiosis between Rhizobium and the aquatic legume Neptunia natans (L.f.) Druce. Planta 196:311-320. [Google Scholar]
- 23.Sy, A., E. Giraud, P. Jourand, N. García, A. Willems, A., P. de Lajudie, Y. Prin, M. Neyra, M. Gillis, C. Boivin-Masson, and B. Dreyfus. 2001. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J. Bacteriol. 183:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The clustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toro, N., and J. Olivares. 1986. Characterization of a large plasmid of Rhizobium meliloti involved in enhancing nodulation. Mol. Gen. Genet. 202:331-335. [Google Scholar]
- 26.Velázquez, E., E. Cervantes, J. M. Igual, A. Peix, P. F. Mateos, S. Benamar, A. Moiroud, C. T. Wheeler, J. Dawson, D. Labeda, C. Rodríguez-Barrueco, and E. Martínez-Molina. 1998. Analysis of LMW RNA profiles of Frankia strains by staircase electrophoresis. Syst. Appl. Microbiol. 21:539-545. [DOI] [PubMed] [Google Scholar]
- 27.Velázquez, E., J. M. Igual, A. Willems, M. P. Fernández, E. Muñoz, P. F. Mateos, A. Abril, N. Toro, P. Normand, E. Cervantes, M. Gillis, and E. Martínez-Molina. 2001. Description of Mesorhizobium chacoense sp. nov. that nodulates Prosopis alba in the Chaco Arido region (Argentina). Int. J. Syst. E vol. Microbiol. 51:1011-1021. [DOI] [PubMed] [Google Scholar]
- 28.Velázquez, E., E. Martínez-Romero, D. N. Rodríguez-Navarro, M. E. Trujillo, A. Daza, P. F. Mateos, E. Martinez-Molina, and P. Van Berkum. 2001. Characterization of rhizobial isolates of Phaseolus vulgaris by staircase electrophoresis of low-molecular-weight RNA. Appl. Environ. Microbiol. 67:1008-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velázquez, E., M. E. Trujillo, A. Peix, J. L. Palomo, P. García-Benavides, P. F. Mateos, A. Ventosa, and E. Martínez-Molina. 2001. Stable low molecular weight RNA analyzed by staircase electrophoresis, a molecular signature for both prokaryotic and eukaryotic microorganisms. Syst. Appl. Microbiol. 24:490-499. [DOI] [PubMed] [Google Scholar]