Abstract
Both heterotypic and homotypic fusion events are required to deliver endocytosed macromolecules to lysosomes and remodel late endocytic organelles. A trans-SNARE complex consisting of Q-SNAREs syntaxin 7, Vti1b and syntaxin 8 and the R-SNARE VAMP8 has been shown by others to be responsible for homotypic fusion of late endosomes. Using antibody inhibition experiments in rat liver cell-free systems, we confirmed this result, but found that the same Q-SNAREs can combine with an alternative R-SNARE, namely VAMP7, for heterotypic fusion between late endosomes and lysosomes. Co-immunoprecipitation demonstrated separate syntaxin 7 complexes with either VAMP7 or VAMP8 in solubilized rat liver membranes. Additionally, overexpression of the N-terminal domain of VAMP7, in cultured fibroblastic cells, inhibited the mixing of a preloaded lysosomal content marker with a marker delivered to late endosomes. These data show that combinatorial interactions of SNAREs determine whether late endosomes undergo homotypic or heterotypic fusion events.
Keywords: lysosome, endosome, endocytosis
Introduction
The delivery of endocytosed macromolecules from late endosomes to lysosomes probably involves ‘kiss and run' and complete fusion events, the latter resulting in formation of hybrid organelles from which lysosomes may be re-formed (reviewed in Mullins & Bonifacino, 2001; Luzio et al, 2003). Late endosomal fusion events conform to the tenets of the SNARE hypothesis (reviewed in Chen & Scheller, 2001). At the core of each fusion event is the formation of a specific tetrameric transsNARE complex consisting of interacting coiled coils from a t- (target membrane) SNARE made up of a heavy chain and two light chains (Fukuda et al, 2000) plus a v- (vesicle membrane) SNARE or, using an alternative nomenclature (defined in Bock et al, 2001), Qa-, Qb- and QcsNARE plus an R-SNARE. There is good evidence that the Qa-SNARE syntaxin 7 is required for both homotypic late endosome fusion (Antonin et al, 2000) and heterotypic fusion with lysosomes (Mullock et al, 2000; Ward et al, 2000). In the former case, the other SNAREs required have been identified, using antibody inhibition of cell-free assays, as Vti1b, syntaxin 8 and VAMP8. The structure of the four-helix SNARE protein bundle (syntaxin 7, Vti1b, syntaxin 8 and VAMP8) has been solved (Antonin et al, 2002). Uncertainty remains over the SNAREs involved in heterotypic fusion of late endosomes with lysosomes. Immunoprecipitated syntaxin 7 complexes have been found to contain a number of different SNAREs: Vti1b, syntaxin 6, VAMP7 and VAMP8 (Wade et al, 2001). Antibodies against VAMP7 have been shown to inhibit both ligand delivery to lysosomes in permeabilized cells (Advani et al, 1999) and homotypic lysosome fusion in vitro (Ward et al, 2000). In contrast, we have previously observed no inhibition of cell-free late endosome–lysosome fusion with antibodies against VAMP8 (Mullock et al, 2000).
Results and Discussion
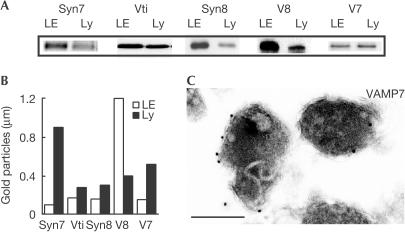
Candidate SNAREs for a specific fusion event must be present in the membranes of vesicles or organelles that fuse. We found that syntaxin 7, Vti1b, syntaxin 8, VAMP7 and VAMP8 could all be detected by immunoblotting, in late endosome- and lysosome-enriched rat liver membrane fractions prepared for cell-free fusion assays (Fig 1A). Moreover, immunogold labelling of the SNAREs in specific organelles in these fractions showed the presence of Vti1b, syntaxin 8 and VAMP7, in addition to syntaxin 7 and VAMP8, on the limiting membrane of late endosomes and lysosomes (Fig 1B,C). All except VAMP8 were more enriched on the limiting membrane of lysosomes than on late endosomes.
Figure 1.
SNAREs in the late endocytic pathway. (A) Immunoblots of SDS–PAGEseparated SNAREs from late endosome- and lysosome-enriched membrane fractions from the rat liver. 20 μg protein/track. (B) Quantification of gold particles per micrometre limiting membrane of late endosomes and lysosomes after immunogold labelling with anti-SNARE antibodies. Data for Syn7 and V8 from Mullock et al (2000) and the same EM blocks were used to localize the other SNAREs. (C) Immunoelectron micrograph showing labelling of VAMP7 on lysosomes. Gold particles, 10 nm; scale bar, 0.5 μm. Syn7, syntaxin 7; Vti, Vti1b; Syn8, syntaxin 8; V8, VAMP8; V7, VAMP7; LE, late endosome; Ly, lysosome.
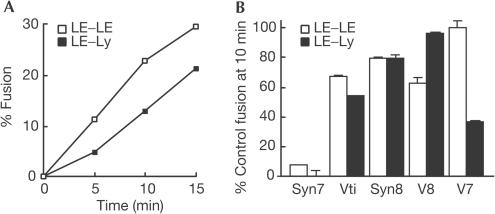
We next established a cell-free homotypic late endosome fusion assay using late endosome-enriched fractions from rat liver to compare with our previously established heterotypic lysosome–late endosome fusion assays (Mullock et al, 1998; Pryor et al, 2000). The assays showed similar initial time courses and extent of fusion (Fig 2A). We then added affinity-purified antisNARE antibodies to fusion reactions, which were run for 10 min to ensure that we were in the linear range of the fusion assays shown in Fig 2A. All antibodies, when added at 100 μg/ml, showed inhibition of fusion in one or both of the assays, with some antibodies being more effective than others (Fig 2B). We have previously shown that different antibodies against syntaxin 7 inhibit the heterotypic fusion assay to different extents (Mullock et al, 2000). In the present experiments, we found that antibodies against the cytosolic domains of syntaxin 7, Vti1b and syntaxin 8 inhibited to a similar extent both late endosome homotypic fusion and late endosome–lysosome fusion (Fig 2B). In contrast, antibodies against VAMP8 inhibited only the homotypic fusion, and antibodies against VAMP7 only the heterotypic fusion (Fig 2B). Another syntaxin 7-interacting SNARE is syntaxin 6 (Wade et al, 2001). However, antibodies against syntaxin 6 that inhibit immature secretory granule fusion (Wendler et al, 2001) had no effect on our assays. The inhibition data on homotypic late endosome fusion are entirely consistent with those reported by Antonin et al (2000), using organelles from PC12 cells. The results of the antibody inhibition experiments on heterotypic fusion of late endosomes and lysosomes suggest a requirement for a transsNARE complex consisting of syntaxin 7, Vti1b, syntaxin 8 and VAMP7, consistent with the Qa-, Qb-, Qc- and R-SNARE core complex model proposed by Bock et al (2001).
Figure 2.
Cell-free fusion assays. (A) Time courses of content mixing assays for late endosome–lysosome fusion and homotypic late endosome fusion using membrane fractions from the rat liver. (B) Inhibition of fusion by affinity-purified antibodies against SNAREs. Standard fusion assays were run for 10 min at 37°C in the presence of 100 μg/ml affinity-purified antisNARE antibodies. Representative experiments (means±range of pairs) are shown in which the same affinity-purified antibody preparations were used in each of the two assays on the same day with the same late endosome preparations containing avidin–ASF.
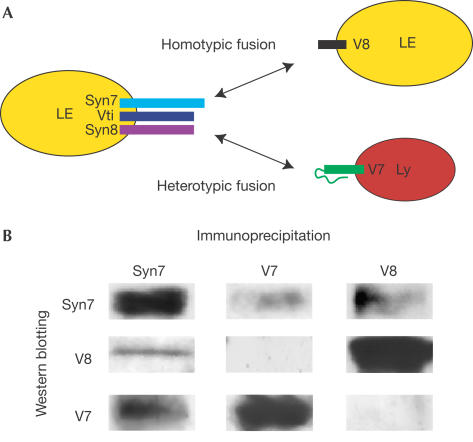
As modelled in Fig 3A, our data suggest that two combinatorial SNARE complexes are required for homotypic and heterotypic fusion events involving late endosomes such that a different v-(R-)SNARE interacts with the same t-(Qa,b,c)SNARE. Combinatorial SNARE complexes have been demonstrated previously, based on in vitro binding studies and genetic/biochemical studies of yeast SNAREs (Tsui et al, 2001; Parlati et al, 2002). Additional data to support our model were obtained by immunoprecipitation of detergentsolubilized rat liver membranes. Both VAMP7 and VAMP8 co-immunoprecipitated with syntaxin 7, and syntaxin 7 co-immunoprecipitated with antibodies against either R-SNARE (Fig 3B). However, immunoprecipitation of VAMP7 did not co-precipitate VAMP8 or vice versa. Although our model for heterotypic fusion (Fig 3A) shows the functional v-(R-)SNARE VAMP7 on lysosomes and the functional t-(Qa,b,c)SNARE on late endosomes, we have no data to exclude the reverse model, as all of the relevant SNAREs were found on both organelles. Preliminary experiments in which late endosome and lysosome fractions were pretreated separately with anti-VAMP7 antibodies before re-isolation, washing and addition to the cell-free fusion assay were consistent with functional VAMP7 being present on both organelles because partial inhibition was observed in either case (data not shown). Nevertheless, we have not observed inhibition of late endosome homotypic fusion with antibodies against VAMP7.
Figure 3.
Combinatorial SNARE complexes. (A) Schematic representation of combinatorial SNARE interactions late in the endocytic pathway with the same t-(Q-)SNARE complex interacting with different v-(R-)SNAREs (V8 and V7) to allow, respectively, homotypic fusion of late endosomes or heterotypic fusion with lysosomes. Although not shown, individual SNAREs are present, and may be functional, on either membrane. (B) Immunoprecipitation of SNAREs from solubilized liver membrane fractions followed by SDS–PAGE and immunoblotting.
VAMP7 is the first component of the late endocytic fusion machinery shown to be differentially required for heterotypic fusion of late endosomes and lysosomes rather than homotypic fusion events. VAMP7 (also known as toxin-insensitive VAMP, TI-VAMP or SYBL1) is, along with yeast and mammalian orthologues of Sec22p (Gonzalez et al, 2001) and Ykt6p (Tochio et al, 2001), a member of the longin subfamily of RsNAREs (Filippini et al, 2001). All longins are distinguished from brevins by the presence of an additional ∼110–140 amino-acid N-terminal domain. The structure of this longin domain has been solved for both mammalian Sec22b (Gonzalez et al, 2001) and Ykt6p (Tochio et al, 2001), showing it to resemble a circular permutation of the overall fold of the actin regulatory protein, profilin, the σsubunit of the clathrin adaptor AP2, the N-terminal domain of the μ-subunit of AP2 and the N-terminal domain of the SRα subunit of the signal recognition particle receptor (Schwartz & Blobel, 2003). The N-terminal longin domains have been suggested to regulate transsNARE complex formation either by folding over the R-SNARE motif and/or by an unknown mechanism possibly involving interaction with non-SNARE proteins (Gonzalez et al, 2001; Tochio et al, 2001; Martinez-Arca et al, 2000, 2003).
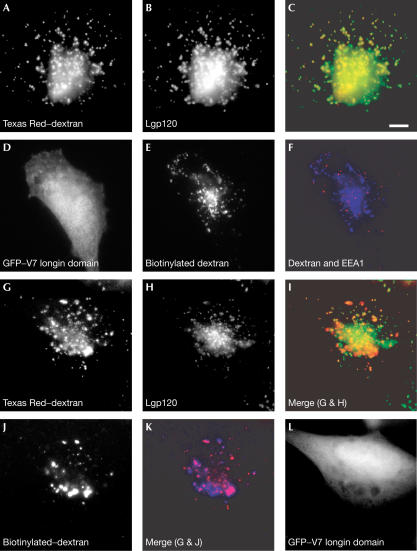
To demonstrate that VAMP7 is required for fusions of late endosomes with lysosomes in living cells, we took advantage of the observation that expression of a chimaera consisting of green fluorescent protein (GFP) fused to the longin domain of VAMP7 could inhibit neurite outgrowth in PC12 cells (Martinez-Arca et al, 2000). This process requires VAMP7 in the formation of ‘apical' SNARE complexes, which mediate fusion of intracellular vesicles with the plasma membrane. We tested whether expression of the same GFP–longin domain chimaera could inhibit mixing between endocytosed markers in lysosomes and late endosomes of cultured fibroblastic cells. The markers were taken up by fluid-phase endocytosis and examined either by fluorescence or EM. When fluorescent-labelled dextran was taken up into cultured normal rat kidney (NRK) cells for 4 h followed by a 20 h chase, it was, as expected, all delivered to compartments, which could be stained with antibodies against the lysosomal membrane protein lgp120 (Fig 4A–C). When cells were preloaded with Texas Red–dextran (4 h pulse, 20 h chase), then transfected with the plasmid encoding GFP–VAMP7 longin domain (24 h), there was reduced delivery of subsequently endocytosed biotinylated dextran (4 h pulse, 20 h chase) to lgp120-positive compartments (Fig 4G–L). Consistent with this, there was incomplete mixing of the biotinylated dextran with the Texas Red–dextran (Fig 4G,J,K). By the end of this experiment (72 h), fewer of the lgp120-containing organelles were positive for Texas Red–dextran (Fig 4G–I) than after 24 h (Fig 4A–C), possibly as a result of inactivation of the Texas Red fluor. The endocytosed biotinylated dextran had travelled beyond early endosomes in the transfected cells because it did not colocalize with EEA1 (Fig 4D–F). To confirm the effect of the VAMP7 longin domain on inhibiting delivery to lysosomes, we monitored the traffic of endocytosed gold particles to lysosomes previously marked with a different size colloidal gold. We had earlier shown that when bovine serum albumin (BSA)–gold is taken up into NRK cells for 4 h and chased for 20 h, >85% is delivered to dense core lysosomes with all of the gold having aggregated as a result of passing through compartments in which BSA is hydrolysed (Bright et al, 1997). In the cells expressing the GFP–VAMP7 longin domain, identified by green fluorescence before fixation and embedding, there was significant inhibition of mixing of the two sizes of gold, and inhibition of delivery of the second pulse of gold to dense core lysosomes, when compared with the surrounding nontransfected cells or cells expressing GFP-tagged cytosolic domain of VAMP8 (Fig 5A–D). In agreement with earlier experiments on NRK cells (Bright et al, 1997), almost all of the second pulse of gold was delivered to dense core lysosomes containing the first pulse of gold in the nontransfected and GFP–VAMP8-expressing cells (Fig 5A–C). Taken together, our microscopy experiments show that overexpression of the longin domain of VAMP7 has no effect on the passage of fluid-phase markers through early endosomes but prevents mixing of markers that are in late endosomes and dense core lysosomes. This is consistent with the ability of bacterially expressed, purified VAMP7 longin domain to inhibit cell-free lysosome–late endosome content mixing (Fig 5E) and with the proposed function of VAMP7 in heterotypic fusion of late endosomes and lysosomes.
Figure 4.
Effect of overexpressing the longin domain of VAMP7 on traffic through the endocytic pathway of NRK cells. (A) Distribution of Texas Red–dextran after uptake for 4 h followed by 20 h chase. (B) Immunofluorescence localization of lgp120 in the same cell as in (A). (C) Colocalization (yellow) of Texas red–dextran (A, red) and lgp120 (B, green). (D) Expression of GFP–V7 longin domain (24 h) in a cell subsequently pulse-chased (4 h pulse, 20 h chase) with biotinylated dextran (E). (F) Immunofluorescence localization of EEA1 (red) in the same cell shown in (E) containing biotinylated dextran (blue). (G–L) Distribution of Texas Red–dextran (G), lgp120 (H) and biotinylated dextran (J) in a cell preloaded with Texas Red–dextran (4 h pulse, 20 h chase), transfected with a plasmid encoding GFP–longin domain of VAMP7 (L), and after 24 h, incubated with biotinylated dextran (4 h uptake, 20 h chase). (I) Colocalization (yellow) of Texas Red–dextran (G, red) and lgp120 (H, green). (K) Colocalization (magenta) between biotinylated dextran (J, blue) and Texas Red–dextran (G, red). Scale bar, 10 μm.
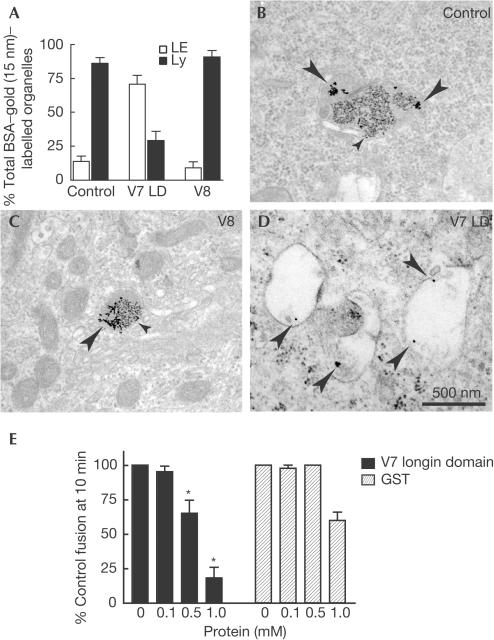
Figure 5.
Inhibition of delivery to lysosomes by the VAMP7 longin domain. NRK cells were preloaded with BSA–5 nm gold (4 h pulse, 20 h chase). They were then transiently transfected with a plasmid encoding GFP–longin domain of VAMP7 (V7 LD), or GFP–VAMP8 (V8), and after 24 h allowed to take up BSA–15 nm gold (4 h pulse, 20 h chase). (A) After EM, the percentage of organelles containing BSA–15 nm gold that were electron dense/contained (closed bars), or electron lucent/did not contain (open bars), BSA–5 nm gold measured in cells expressing V7 LD or V8, or surrounding cells not expressing GFP (control). The data shown are mean±s.e.m. from single sections of six control and six V7 LD-transfected cells from two separate experiments and four V8-transfected cells from a different experiment (surrounding nontransfected cells had a distribution of gold particles not significantly different from the control shown). (B–D) Representative images of main organelles containing 15 nm gold in transfected and control cells (large arrows, 15 nm gold; small arrows, 5 nm gold). (E) Cell-free late endosome–lysosome fusion after addition of bacterially expressed his6-tagged V7 longin domain or GST as a control (*P<0.03 versus GST control). Data are means±s.e.m., n=4.
In our present study, we have defined the SNAREs required for fusion of late endosomes with lysosomes in mammalian cells and shown that the R-SNARE VAMP7 determines heterotypic fusion in contrast to the R-SNARE VAMP8 required for homotypic fusion of late endosomes. Recent work on SNARE complexes required for fusion events in the macropinocytic pathway of the slime mould Dictyostelium discoideum has shown the formation and function of a SNARE complex made up of syntaxin 7, Vti1, syntaxin 8 and VAMP7 (Bogdanovic et al, 2002), consistent with our present observations being relevant beyond mammals. As VAMP7 is such a key player in heterotypic fusion events involving lysosomes, there is now a need to understand its biology more fully. Little is known about how late endocytic SNAREs are localized or recycle. Recently, it has been shown that VAMP7 is targeted to late endocytic compartments by interaction of its longin domain with the adaptor protein AP-3 (Martinez-Arca et al, 2003). VAMP7 has also been localized to small vesicles leaving cholesterol-containing organelles late in the endocytic pathway (Ko et al, 2001), which may reflect its recycling route. Further work is clearly required to understand how the longin domain of VAMP7 regulates heterotypic fusion and whether any interacting proteins are important to this regulation.
Methods
Antibodies against SNAREs
Polyclonal antibodies against syntaxin 6 were a gift from Dr Sharon Tooze (CRUK, London) and polyclonal antibodies against syntaxin 7 and VAMP8 were as described (Mullock et al, 2000). Polyclonal antibodies against cytosolic domains of other SNAREs were raised against GST fusion proteins and affinity-purified as described previously (Mullock et al, 2000), using mouse syntaxin 8 (amino acids (aa) 1–215, GenBank™ accession number AB040054), VAMP7 (aa 1–188, XP135737) and Vti1b (aa 1–207, O88384; cDNA a gift from Dr Gabriele Fischer von Mollard).
Immunoblotting and immunoprecipitation
Preparation of fractions, immunoprecipitations of solubilized rat liver membranes, SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting were as described previously (Mullock et al, 2000; Wade et al, 2001; Poupon et al, 2003).
Cell-free fusion assays
The late endosome–lysosome content mixing assay was carried out as described previously using rat liver organelle fractions loaded respectively with avidin–asialo-fetuin (ASF) and iodinated, biotinylated polymeric IgA (Mullock et al, 1998; Pryor et al, 2000).
The late endosome–late endosome homotypic fusion assay was established as follows. ASF was biotinylated and iodinated as described for polymeric IgA (Mullock et al, 1994). A late endosome-enriched fraction (dense endosomes) was prepared from the liver of a rat that had received an intravenous injection of ∼0.03 nmol biotinylated, iodinated ASF, 6 min before killing. The dense endosome peak from a Ficoll gradient was diluted and sedimented, then resuspended in pig brain cytosol and mixed with dense endosomes that had been loaded with avidin–ASF and stored in liquid N2 (Mullock et al, 1998). Content mixing between the two sets of endosomes was assayed exactly as for endosome–lysosome content mixing.
The effect of anti-SNARE antibodies and bacterially expressed proteins on fusion was determined as described by Mullock et al (2000). The VAMP7 longin domain (aa 1–120) was cloned into the plasmid pMWH6, expressed as His6-tagged protein in BL21(DE3) pLysS cells and purified using nickel-nitrilotriacetic acid–agarose. Glutathione S-transferase (GST) was expressed in BL21(DE3) pLysS cells, using plasmid pGEX-4T1, and purified using glutathione–Sepharose 4B.
Microscopy
NRK cells were incubated with labelled dextrans (Molecular Probes Inc., Eugene, OR) at 10 mg/ml, fixed and stained with antibodies against EEA1 (BD Biosciences, Lexington, KY) and lgp120 as described previously (Poupon et al, 2003). Biotinylated dextran was observed by staining fixed cells with 100 μg/ml NeutrAvidin-Alexa Fluor 350 (Molecular Probes Inc., Eugene, OR, USA). Fluorescence was observed with a Zeiss Axioplan microscope equipped with a CCD camera. Multiple fluors were visualized using a single stationary (multiband) beam splitter and appropriate filters fitted to filter wheels. We used the 86000: Sedat Quad filter set with single-band excitation and emission filters, details of which can be found at http://www.chroma.com/products/products/byfilterset_seriesdetail.cfm?ID=86000. Uptake of BSA–gold, ultrastructural analysis using transmission EM with 70 nm sections observed in a Philips CM100 EM and quantification of gold mixing were as described previously (Bright et al, 1997). In experiments using transiently transfected cells, transfection was as described by Poupon et al (2003) using a plasmid encoding GFP–VAMP7 longin (N-terminal) domain (aa 1–120) constructed as described by Martinez-Arca et al (2000), or the same plasmid encoding GFP–VAMP8 (aa 1–215). ImmunoEM and quantification of SNAREs on organelle membranes were as described by Mullock et al (2000).
Acknowledgments
This work was funded by an MRC programme grant to J.P.L., and an HFSP grant to R.C.P., J.P.L. and D.E.J. S.C.W.R. was supported by AHA postdoctoral fellowship grants 0120475Z and 0325605Z. C.I.M.R. is in receipt of a strategic award from the Wellcome Trust. Polyclonal antibodies against syntaxin 6 were a gift from Dr Sharon Tooze.
References
- Advani RJ, Yang B, Prekeris R, Lee KC, Klumperman J, Scheller RH (1999) VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol 146: 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R (2000) A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J 19: 6453–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR (2002) Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol 9: 107–111 [DOI] [PubMed] [Google Scholar]
- Bock J, Matern HT, Peden AA, Scheller RH (2001) A genomic perspective on membrane compartment organization. Nature 409: 839–841 [DOI] [PubMed] [Google Scholar]
- Bogdanovic A, Bennett N, Kieffer S, Louwagie M, Morio T, Garin J, Satre M, Bruckert F (2002) Syntaxin 7, syntaxin 8, Vti1 and VAMP 7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem J 368: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Reaves BJ, Mullock BM, Luzio JP (1997) Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J Cell Sci 110: 2027–2040 [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH (2001) SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 1–9 [DOI] [PubMed] [Google Scholar]
- Filippini F, Rossi V, Galli T, Budillon A, D'Urso M, D'Esposito M (2001) Longins: a new evolutionarily conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci 26: 407–409 [DOI] [PubMed] [Google Scholar]
- Fukuda R, McNew JA, Weber T, Parlati F, Engel T, Nickel W, Rothman JE, Sollner TH (2000) Functional architecture of an intracellular membrane tsNARE. Nature 407: 198–202 [DOI] [PubMed] [Google Scholar]
- Gonzalez LC, Weis WI, Scheller RH (2001) A novel SNARE N-terminal domain revealed by the crystal structure of Sec 22b. J Biol Chem 276: 24203–24211 [DOI] [PubMed] [Google Scholar]
- Ko DC, Gordon MD, Jin JY, Scott MP (2001) Dynamic movements of organelles containing Niemann-Pick CI protein: NPC1 involvement in late endocytic events. Mol Biol Cell 12: 601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Poupon V, Lindsay MR, Mullock BM, Piper RC, Pryor PR (2003) Membrane dynamics and the biogenesis of lysosomes (Review). Mol Memb Biol 20: 141–154 [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T (2000) Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol 149: 889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S et al. (2003) A dual mechanism controlling the localization and function of exocytic vsNAREs. Proc Natl Acad Sci USA 100: 9011–9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins C, Bonifacino JS (2001) The molecular machinery for lysosome biogenesis. BioEssays 23: 333–343 [DOI] [PubMed] [Google Scholar]
- Mullock BM, Perez JH, Kuwana T, Gray S, Luzio JP (1994) Lysosomes can fuse with a late endosomal compartment in a cell-free system from rat liver. J Cell Biol 126: 1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP (1998) Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol 140: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock BM et al. (2000) Syntaxin 7 is localized to late endosome compartments, associates with VAMP 8, and is required for endosome–lysosome fusion. Mol Biol Cell 11: 3137–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, Varlamov O, Paz K, McNew JA, Hurtado D, Sollner TH, Rothman JE (2002) Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc Natl Acad Sci USA 99: 5424–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon V, Stewart A, Gray SR, Piper RC, Luzio JP (2003) The role of mVps18p in clustering, fusion and intracellular localization of late endocytic organelles. Mol Biol Cell 14: 4015–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP (2000) The role of intra-organellar Ca2+ in late endosome–lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol 149: 1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T, Blobel G (2003) Structural basis for the function of the β subunit of the eukaryotic signal recognition particle receptor. Cell 112: 793–803 [DOI] [PubMed] [Google Scholar]
- Tochio H, Tsui MMK, Banfield DK, Zhang M (2001) An autoinhibitory mechanism for nonsyntaxin SNARE proteins revealed by the structure of Ykt6p. Science 293: 698–702 [DOI] [PubMed] [Google Scholar]
- Tsui MMK, Tai WCS, Banfield DK (2001) Selective formation of Sed5p-containing SNARE complexes is mediated by combinatorial binding interactions. Mol Biol Cell 12: 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade N, Bryant NJ, Connolly LM, Simpson RJ, Luzio JP, Piper RC, James DE (2001) Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP 7 in B16 melanoma cells. J Biol Chem 276: 19820–19827 [DOI] [PubMed] [Google Scholar]
- Ward DM, Pevsner J, Scullion MA, Vaughn M, Kaplan J (2000) Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimidesensitive factor attachment protein receptors required for late endosome–lysosome and homotypic lysosome fusion in alveolar macrophages. Mol Biol Cell 11: 2327–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler F, Page L, Urbe S, Tooze S (2001) Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol Biol Cell 12: 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]