Abstract
Cholera toxin (CT) follows a glycolipid-dependent entry pathway from the plasma membrane through the trans-Golgi network (TGN) to the endoplasmic reticulum (ER) where it is retro-translocated into the cytosol to induce toxicity. Whether access to the Golgi apparatus is necessary for transport to the ER is not known. Exo2 is a small chemical that rapidly blocks anterograde traffic from the ER to the Golgi and selectively disrupts the Golgi apparatus but not the TGN. Here we use Exo2 to determine the role of the Golgi apparatus in CT trafficking. We find that under the condition of complete Golgi ablation by Exo2, CT reaches the TGN and moves efficiently into the ER without loss in toxicity. We propose that even in the absence of Exo2 the glycolipid pathway that carries the toxin from plasma membrane into the ER bypasses the Golgi apparatus entirely.
Keywords: chemical genetics, membrane traffic, phenotypic screen, toxin
Introduction
Cholera toxin (CT) induces the massive diarrhoea caused by infection with Vibrio cholerae. The toxin is composed of a pentameric B-subunit and an enzymatically active A-subunit that contains an endoplasmic reticulum (ER)-targeting KDEL motif (Sixma et al, 1993). The Bsubunit co-opts ganglioside GM1, a plasma membrane (PM) glycolipid, that carries the toxin all the way from the PM into the ER of host cells (Fujinaga et al, 2003; Lencer & Tsai, 2003). In the ER, the Asubunit hijacks the machinery for retro-translocation of misfolded proteins to enter the cytosol where it activates adenylyl cyclase to induce disease (Tsai et al, 2001; Tsai & Rapoport, 2002). Although it is well established that CT enters the cell and reaches the Golgi apparatus, it is less clear as to how it then moves to the ER. It is known that the KDEL motif is not required for toxicity, suggesting that COPI-mediated retrograde traffic from Golgi to ER is not required (Lencer et al, 1995). Indeed, we have recently found that mutation or removal of the KDEL motif does not hinder access to the ER (Fujinaga et al, 2003). An example of another toxin that follows a similar pathway and lacks a KDEL motif is Shiga toxin, which moves to the ER via a Rab6-dependent and COPI-independent pathway (Sandvig et al, 1992; Johannes et al, 1997; White et al, 1999). These observations raise the possibility that the toxins may bypass the Golgi apparatus altogether, trafficking directly from the trans-Golgi network (TGN) to the ER.
Evidence that transport through the Golgi apparatus is required for toxin action was obtained using the fungal toxin brefeldin A (Lencer & Tsai, 2003). Brefeldin A primarily disrupts the Golgi apparatus and the TGN by inhibiting ARF-GEF activity (Donaldson et al, 1992). As a consequence of the massive disappearance of ARF from the Golgi and TGN membranes, these organelles tubulate and collapse to the ER and endosomal compartments, respectively (Lippincottschwartz et al, 1989, 1991). Owing to this pleiotropic effect, it is not possible to distinguish whether CT requires the Golgi apparatus to reach the ER or whether it can access the ER directly from the TGN.
Here, we address the problem of how the toxins move backwards from the PM to ER by using the chemical compound Exo2 (4-hydroxy-3-methoxy-(5,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidin-4-yl)hydrazone benzaldehyde). Exo2 is a small molecule that we recently discovered in the course of an image-based phenotypic screen for inhibitors of the secretory pathway (Feng et al, 2003; Yarrow et al, 2003). Exo2 prevents anterograde movement of the viral glycoprotein VSVG from the ER to the Golgi but has little effect on the endocytic pathway. In this study, we find that Exo2 completely disrupts the Golgi apparatus but has minimal effect on the integrity of the TGN. Nevertheless, Exo2 does not prevent retrograde traffic of CT from PM to TGN and ER. Thus, an alternative pathway for traffic from the TGN to ER bypassing the Golgi apparatus must exist in cells treated with Exo2.
Results
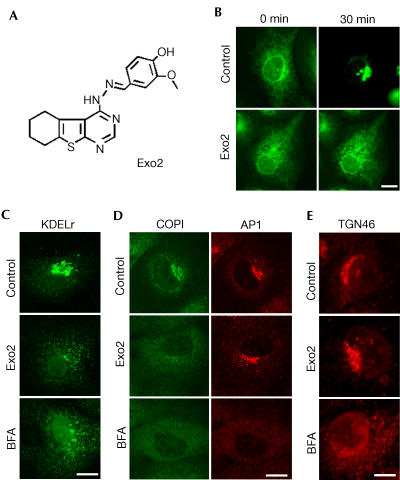
Using a phenotypic imaging-based screen approach (Feng et al, 2003; Yarrow et al, 2003), we searched for compounds interfering with the secretory pathway and identified Exo2 (Fig 1A). Treatment of monkey BSC1 cells with 50 μM Exo2 potently inhibits exit of VSVGts045-enhanced green fluorescent protein (GFP) from the ER to the Golgi apparatus (Fig 1B). Similarly to brefeldin A, Exo2 collapses the Golgi apparatus accompanied by transfer of its contents into the ER as indicated by the relocation of the KDEL receptor from the Golgi to the ER in a way that parallels the effect of brefeldin A (Fig 1C). In contrast to the effect of brefeldin A, however, which releases COPI coatomers from Golgi cisternae and AP1 clathrin adaptor complexes from the TGN to the cytosol (Lippincottschwartz et al, 1989), treatment with Exo2 promotes only the selective dissociation of COPI but leaves AP-1 intact (Fig 1D). Also unlike brefeldin A, Exo2 has no detectable effect on the integrity of the TGN, as evidenced by the lack of effect on the localization of the TGN marker TGN46 (Fig 1E). Thus, Exo2 selectively ablates the Golgi apparatus thereby blocking anterograde transport from the ER.
Figure 1.
Exo2 selectively disrupts Golgi apparatus but not TGN. (A) Chemical structure of Exo2. (B) Exo2 blocks VSVG exit from ER. Time lapse of VSVGts045-GFP after temperature shift from 40 to 32°C. (C–E) Exo2 selectively disrupts Golgi apparatus. Cells treated with 50 μM of Exo2 or 5 μM of BFA were stained for KDEL receptor (C), clathrin adapter AP1 (D) and TGN protein TGN46 (E).
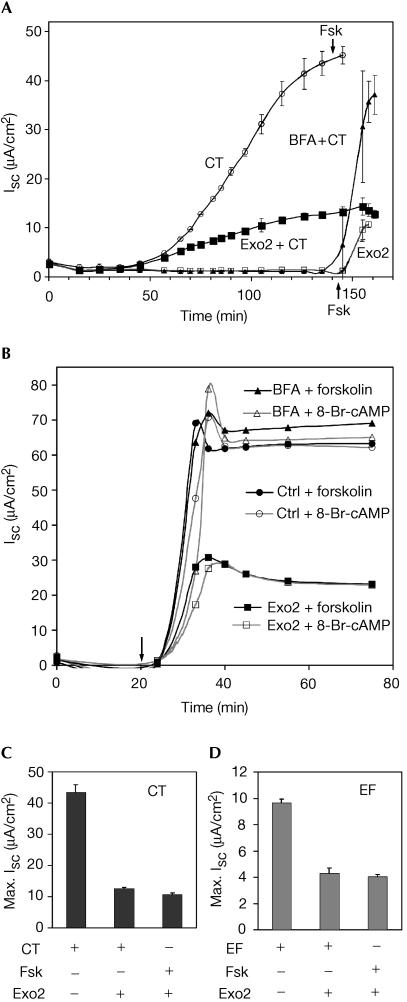
To test whether Exo2 impairs retrograde traffic, we examined its effects on the ability of CT to enter host cells and cause toxicity. When CT moves from the PM into the ER of the human intestinal cell line T84, the enzymatic A chain is retro-translocated to the cytosol where it rapidly activates adenylyl cyclase and induces a Cl− secretory response (Isc) that can be measured in real time by electrophysiology (Fig 2A). In T84 cells pretreated with Exo2, CT induces an Isc, but the maximal Isc induced is strongly attenuated. In contrast, pretreatment of T84 cells with brefeldin A causes a complete inhibition of CT-induced Isc, but not the maximal Isc, consistent with our previous results (Lencer et al, 1993; Fujinaga et al, 2003). These data show that CT can move from the cell surface to the ER in T84 cells treated with Exo2. Although Isc is strongly attenuated in Exo2-treated cells, the maximal Isc induced by CT is comparable to the maximal Isc induced by the cAMP agonist forskolin (Fig 2A). Thus, Exo2 has an effect on cAMP-induced Cl− secretion unrelated to retrograde toxin transport from PM to ER or on the retro-translocation of CT to the cytosol.
Figure 2.
Exo2 inhibits Cl− secretion in T84 cells. (A) Short-circuit current (Isc, mean±s.d.) in polarized T84 cells treated with CT alone (open circles), Exo2 alone (open squares), CT with Exo2 (solid squares), and CT with BFA (solid triangles). Forskolin was added at 140 min (Fsk, arrows). (B) Exo2 acts downstream of cAMP. T84 cells were pretreated with vehicle alone DMSO (circles), BFA (triangles) or Exo2 (squares), and Isc (arrow) was induced with forskolin (filled symbols) or 8-Br-cAMP (open symbols). Averages of duplicates are shown. (C,D) Maximal Cl− secretion responses induced by CT (C) or anthrax EF (D) in the presence or absence of forskolin.
This proposal was verified when we compared the effects of Exo2 on Cl− secretion induced by forskolin or by the membrane-permeant cAMP analogue 8-Br-cAMP. Exo2 inhibits Cl− secretion induced by both agonists equally (Fig 2B). Brefeldin A, in contrast, has no effect on the time course of maximal Isc induced by either agonist (Fig 2B). Furthermore, the maximal Isc induced by CT in cells treated with Exo2 was always equal to the maximal Isc induced by forskolin (Fig 2C). Thus, one effect of Exo2 is to inhibit a component(s) of the Isc downstream of the second messenger cAMP. Indeed, the Isc induced by anthrax toxin oedema factor (EF) (Fig 2D) or pertussis adenylate cyclase (AC) toxin (data not shown) applied to T84 cells was also attenuated by treatment with Exo2. EF and AC toxins are themselves potent adenylyl cyclases that are translocated to the cytosol directly across the endosomal membrane or PM, respectively.
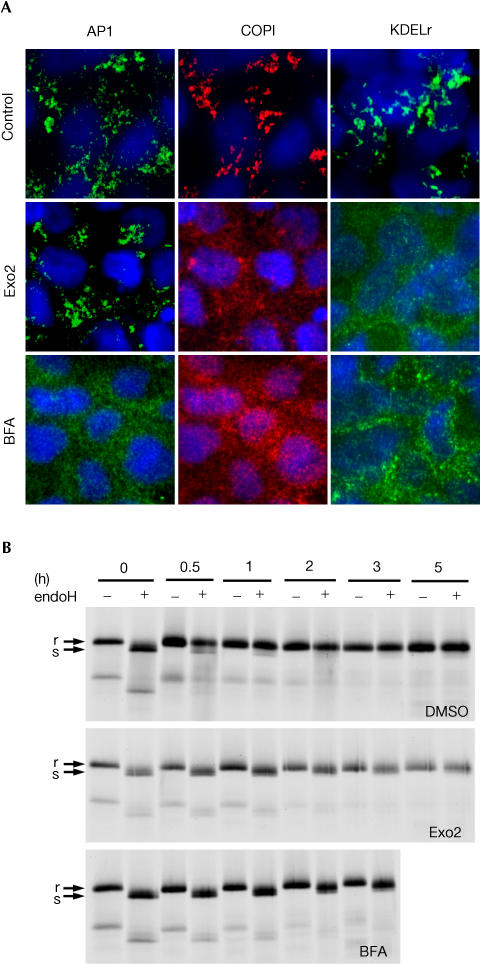
Although Exo2 has an inhibitory effect of on cAMP-induced Cl− secretion in T84 cells, there was no detectable effect on the time course of CT action, indicating that CT can still move from the cell surface to the ER and implying that Exo2 may not remove the Golgi apparatus in T84 cells. This, however, is not the case. As in BSC1 cells, treatment of T84 cells with Exo2 for up to 3 h disperses the Golgi proteins β-COP and the KDEL receptor to the cytosol and has no effect on the localization of AP1 to the TGN (Fig 3A). In contrast, brefeldin A applied under the same conditions elicits the expected collapse of both the TGN and Golgi markers. Collapse of the Golgi apparatus in T84 cells by Exo2 was confirmed by demonstrating a complete block in the Golgispecific oligosaccharide modification of newly synthesized VSVG (Fig 3B). Thus, Exo2 disrupts the COPI pathway and the Golgi apparatus in T84 cells, but does not release AP1 or overtly disrupt the TGN.
Figure 3.
Exo2 collapses the Golgi apparatus in polarized T84 cells. (A) Confocal Z-series of T84 monolayers treated with Exo2 (50 μM) or BFA (5 μM) labelled for AP1, COPI coatomer and KDEL receptor. (B) Phosphorimage of metabolically labelled VSVG-GFP pulse (0 h) and acquisition of endoH resistance (r, upper band) in T84 monolayers treated with DMSO alone (upper panel), Exo2 (middle panel) or brefeldin A (lower panel).
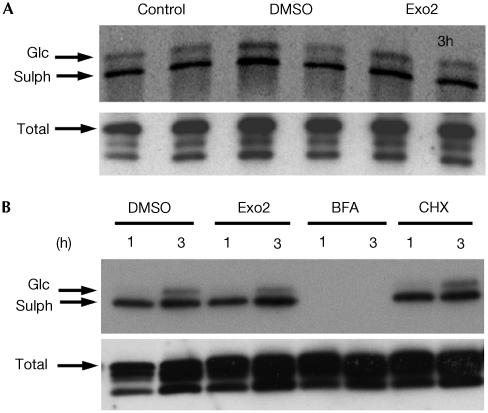
Finally, we directly measured the effect of Exo2 on transport of CT from the PM through the TGN and into the ER. For these experiments, we used the B-subunit of CT (CTB-GS) modified to contain sulphation and N-glycosylation motifs. This allows the detection of toxin entry into the TGN and ER respectively (Fujinaga et al, 2003) by virtue of the intracellular compartmentalization of the sulphotransferase and N-glycosylation activities to the TGN and ER (Gavel & von Heijne, 1990; Moore, 2003). The mutant toxin CTB-GS also lacks the KDEL motif recognized by the KDEL receptor for COPI-dependent retrograde traffic from Golgi to ER. As shown in Fig 4, the CTB-GS internalized in T84 (Fig 4A) or Vero (Fig 4B) cells is equally sulphated and glycosylated in the presence or absence of Exo2. As expected, treatment with brefeldin A completely prevents modification of the internalized CTB-GS because the toxin cannot reach the TGN as the drug collapses the TGN and Golgi apparatus back into the ER. We ruled out the possibility that a decrease in the amount of proteins reaching the TGN caused by Exo2 could increase the availability of sulphotransferase to modify internalized CT, since identical levels of sulphation and glycosylation were found in Vero cells treated with cycloheximide (Fig 4B). Furthermore, retrograde transport from the TGN to the ER is not affected since the relative amount of sulphated toxin that is glycosylated in the ER is not altered (Fig 4). From these results, we conclude that in cells treated with Exo2 CT can move directly from the TGN to ER independently of COPI and bypassing the Golgi apparatus entirely.
Figure 4.
Exo2 does not inhibit CT transport from TGN to ER. (A) Phosphorimage showing in duplicate the 35S-sulphation (lower band) and N-glycosylation (upper band) of CTB-GS internalized for 3 h in T84 cells in the absence of vehicle (control), or treated with DMSO or Exo2. An immunoblot of the total cell lysate shows equal loading of CTB-GS (total). (B) Phosphorimage showing the 35S-sulphation (lower band) and N-glycosylation (upper band) of CTB-GS internalized for 1 or 3 h in Vero cells treated with DMSO, Exo2, brefeldin A or cycloheximide (CHX).
Discussion
There are at least two pathways used by certain bacterial toxins and viruses to reach the ER from the outside of target cells. The pathway taken by CT, Shiga toxin (Stx), SV40 virus and polyoma virus is a lipid-dependent sorting pathway (Lencer & Tsai, 2003). These toxins and viruses bind glycolipids in lipid rafts at the cell surface and co-opt them to enter the cell. In the case of CT and probably Stx, the raft glycolipids carry the toxins all the way from the PM to the ER (Falguieres et al, 2001; Fujinaga et al, 2003). The lipid pathway is independent of sorting by the KDEL receptor and COPI-coated transport vesicles (Girod et al, 1999; Fujinaga et al, 2003). Here, we provide an explanation for this independence using the novel small molecule Exo2. Exo2 collapses the Golgi apparatus but leaves the TGN intact. Our results show that the lipid pathway used by CT bypasses the Golgi apparatus and moves the toxin directly from the TGN to the ER, at least in cells treated with Exo2.
A direct pathway for transport from PM to ER was previously suggested for the membrane protein caveolin and cholesterol (Smart et al, 1996) and for the GM1-binding virus SV40 (Pelkmans et al, 2001, 2002; Tsai et al, 2003). Thus, in some pathways, such as the one followed by CT, the Golgi apparatus is completely dispensable for retrograde transport to the ER. Indeed, the ER has the ability to interact directly with the PM as evidenced by the results of recent studies that demonstrate a direct role for the ER in restitution of the PM and in certain forms of phagocytosis (Gagnon et al, 2002; Kagan & Roy, 2002; Guermonprez et al, 2003).
The second pathway for the toxins to move from PM to ER is a protein-dependent sorting pathway. This pathway is typified by the proposed mechanism of action of Pseudomonas exotoxin A (Chaudry et al, 1990). Pseudomonas ExoA has a KDEL-like C-terminal sequence that may bind the KDEL receptor after removal of the C-terminal residue and is essential for function. Thus, ExoA appears to move into the ER by co-opting COPI-coated retrograde transport vesicles.
The COPI pathway, however, cannot be required for CT to move from the TGN to ER for two reasons. First, Exo2 induces the disassembly of the Golgi apparatus, releases COPI coats to the cytosol and disperses the KDEL receptor to the ER but has no effect on toxin action. Just as with brefeldin A, the function of the Golgi apparatus is completely disrupted by Exo2 because the virus protein VSVG protein cannot move from the ER to Golgi. However, Exo2 has no detectable effect on the TGN and no detectable effect on CT transport from the TGN to ER, or on retro-translocation of the toxin to the cytosol. The second reason is that a mutant CT that lacks functional KDEL motifs moves from the PM to the ER and induces toxicity with almost the same efficiency as the wild-type toxin (Lencer et al, 1995; Fujinaga et al, 2003). These results are consistent with recent studies on retrograde trafficking by Shiga toxin (Girod et al, 1999) and ricin toxin (Chen et al, 2003; Llorente et al, 2003), which also lack KDEL motifs. Both Shiga and ricin toxins move backwards from the PM to ER via COPI-independent pathways. Furthermore, genetic disruption of COPI activity has no influence on the toxicity of ricin or CT (Chen et al, 2002).
CT does, however, interact with the KDEL receptor to make toxin action more efficient (Lencer et al, 1995). Once in the ER, the toxin can move back to the Golgi in anterograde vesicles, and the KDEL receptor acts to return the toxin to the ER (Fujinaga et al, 2003), allowing more toxin to enter the cytosol. Such recycling by the KDEL receptor is dependent on COPI-coated vesicles, and this explains why in some studies CT trafficking into the Golgi and ER appears to depend on COPI-mediated vesicle transport (Majoul et al, 1998).
The mechanism(s) and site(s) of Exo2 action remain unknown. In these studies, we find that, in addition to dispersing the Golgi apparatus, Exo2 inhibits the Isc in T84 cells by interfering with a step downstream of the generation of intracellular cAMP. These steps involve phosphorylation of the apical membrane Cl− channel CFTR by protein kinase A and activation of basolateral K+ channels and Na–K–Cl co-transporters by unknown mechanisms (Barrett, 1997). Exo2 could act at any one or several of these sites. As Exo2 has structural features resembling nucleotides, it could be that Exo2 acts by inhibiting one or more kinases. Protein kinases are involved in ER and Golgi membrane dynamics (Davidson et al, 1992; Jamora et al, 1999; Aridor & Balch, 2000) and are required for COPII-dependent export from the ER (Aridor & Balch, 2000). Such a block in ER export leads to consumption of the Golgi apparatus. Thus, the dual effects of Exo2 on vesicular traffic and Cl− transport in T84 cells may be explained by inhibition of kinases with overlapping sensitivity for Exo2. Our attempts to demonstrate an effect of Exo2 on protein kinase A in vitro, however, were not successful, and other types and multiplicity of targets are also possible. Nonetheless, it remains probable that the mechanism of action of Exo2 on the Isc will overlap mechanistically with its effects on membrane dynamics in the Golgi apparatus.
Methods
Cells culture, antibodies, recombinant adenovirus, recombinant CTB-GS
T84 and Vero cells (ATCC, Rockville, MD) were used as described previously (Fujinaga et al, 2003). Antibodies were as follows: monoclonal anti-AP1 (Sigma, St Louis, MO), rabbit anti-β-COP (Affinity Bioreagents, Golden, CO), sheep anti-TGN46 (Serotec, Raleigh, NC), monoclonal anti-KDEL receptor (BD Biosciences Pharmingen, San Diego, CA) and monoclonal anti-VSVG (P5D4) from Dr Angela Wandinger-Ness (Univ. New Mexico, Albuquerque, NM). Recombinant adenovirus expressing VSVGts045-GFP (Feng et al, 2003) and rabbit anti-CTB and recombinant CTB-GS were as described (Lencer et al, 1995).
Confocal fluorescence microscopy
T84 monolayers were fixed in 3.7% paraformaldehyde, permeabilized in 0.2% Triton X-100 and 0.5% BSA, immunostained and imaged as described (Feng et al, 2003). Zseries were at 0.2 μm steps and deconvolved using AutoQuant (Troy, NY).
Electrophysiology, transport of VSVG-GFP and CTB-GS in T84 cells
T84 monolayers were infected with VSVGts045-GFP adenovirus in phosphate-buffered saline with 0.2 mM EDTA and EGTA for 2 h at 40°C, washed and cultured in media for 40 h at 40°C until studied as described (Feng et al, 2003). Sulphation and glycosylation of CTB and electrophysiology were performed as described (Fujinaga et al, 2003). Exo2 (50 μM) and brefeldin A (5 μM) were added both apically and basolaterally.
Acknowledgments
We thank Dr E. Hewlett and Dr R.J. Collier for supplying Pertussis AC and Anthrax EF toxins and Bill Ciesla for performing the AC toxin experiment. This work was supported by the Howard Hughes Medical Institute's Medical Student Research Fellowship (A.P.J.), and NIH grants P01 GM62566 (T.K.), DK48106 and DK57827 (W.I.L.) and DK34854 of the Harvard Digestive Diseases Center.
References
- Aridor M, Balch WE (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem 275: 35673–35676 [DOI] [PubMed] [Google Scholar]
- Barrett KE (1997) Integrated regulation of intestinal epithelial transport: intercellular and intracellular pathways. Am J Physiol 272: C1069–C1076 [DOI] [PubMed] [Google Scholar]
- Chaudry VK, Jinno Y, Fitzgerald D, Pastan I (1990) Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci USA 87: 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Hu T, Mikoryak C, Draper RK (2002) Retrograde transport of protein toxins under conditions of COPI dysfunction. Biochim Biophys Acta 1589: 124–139 [DOI] [PubMed] [Google Scholar]
- Chen A, AbuJarour RJ, Draper RK (2003) Evidence that the transport of ricin to the cytoplasm is independent of both Rab6A and COPI. J Cell Sci 15: 3503–3510 [DOI] [PubMed] [Google Scholar]
- Davidson HW, McGowan CH, Balch WE (1992) Evidence for the regulation of exocytic transport by protein phosphorylation. J Cell Biol 116: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD (1992) Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360: 350–352 [DOI] [PubMed] [Google Scholar]
- Falguieres T, Mallard F, Baron C, Hanau D, Lingwood C, Goud B, Salamero J, Johannes L (2001) Targeting of shiga toxin Bsubunit to retrograde transport route in association with detergent-resistant membranes. Mol Biol Cell 12: 2453–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Yu S, Lasell TK, Jadhav AP, Macia E, Chardin P, Melancon P, Roth M, Mitchison T, Kirchhausen T (2003) Exo1: a new chemical inhibitor of the exocytic pathway. Proc Natl Acad Sci USA 100: 6469–6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga Y, Wolf AA, Rodigherio C, Wheeler H, Tsai B, Allen L, Jobling MG, Rapoport T, Holmes RK, Lencer WI (2003) Gangliosides that associate with lipid rafts mediate transport of cholera toxin from the plasma membrane to the ER. Mol Biol Cell 14: 4783–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M (2002) Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110: 119–131 [DOI] [PubMed] [Google Scholar]
- Gavel Y, von Heijne G (1990) Sequence differences between glycosylated and non-glycosylated Asn–X–Thr/Ser acceptor sites: implications for protein engineering. Protein Eng 3: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R (1999) Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol 1: 423–430 [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S (2003) ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425: 397–402 [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V (1999) Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98: 59–68 [DOI] [PubMed] [Google Scholar]
- Johannes L, Tenza D, Antony C, Goud B (1997) Retrograde transport of KDEL-bearing B-fragment of shiga toxin. J Biol Chem 272: 19554–19561 [DOI] [PubMed] [Google Scholar]
- Kagan JC, Roy CR (2002) Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol 4: 945–954 [DOI] [PubMed] [Google Scholar]
- Lencer WI, Tsai B (2003) The intracellular voyage of cholera toxin: going retro. Trends Biol Chem 28: 639–645 [DOI] [PubMed] [Google Scholar]
- Lencer WI, de Almeida JB, Moe S, Stow JL, Ausiello DA, Madara JL (1993) Entry of cholera toxin into polarized human intestinal epithelial cells. Identification of an early brefeldin A sensitive event required for A 1-peptide generation. J Clin Invest 92: 2941–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer WI, Constable C, Moe S, Jobling MG, Webb HM, Ruston S, Madara JL, Hirst TR, Holmes RK (1995) Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol 131: 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincottschwartz J, Yuan LC, Bonifacino JS, Klausner RD (1989) Rapid distribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane recycling from Golgi to ER. Cell 56: 801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincottschwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD (1991) Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67: 601–616 [DOI] [PubMed] [Google Scholar]
- Llorente A, Lauvrak SU, van Deurs B, Sandvig K (2003) Induction of direct endosome to endoplasmic reticulum transport in Chinese hamster ovary (CHO) cells (LdlF) with a temperaturesensitive defect in epsilon-coatomer protein (epsilon-COP). J Biol Chem 278: 35850–35855 [DOI] [PubMed] [Google Scholar]
- Majoul I, Sohn K, Wieland FT, Pepperkok R, Pizza M, Hillemann J, Soling HD (1998) KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J Cell Biol 143: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KL (2003) The biology and enzymology of protein tyrosine Osulfation. J Biol Chem 278: 24243–24246 [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Kartenbeck J, Helenius A (2001) Caveolar endocytosis of simian virus 40 reveals a new twostep vesicular-transport pathway to the ER. Nat Cell Biol 3: 473–483 [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Puntener D, Helenius A (2002) Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 292: 535–539 [DOI] [PubMed] [Google Scholar]
- Sandvig K, Garred Ø, Prydz K, Kozlov JV, Hansen SH, van Deurs B (1992) Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature (Lond) 358: 510–511 [DOI] [PubMed] [Google Scholar]
- Sixma TK, Stein PE, Hol WG, Read RJ (1993) Comparison of the B-pentamers of heat-labile enterotoxin and verotoxin-1: two structures with remarkable similarity and dissimilarity. Biochemistry 32: 191–198 [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Donzell WC, Anderson RG (1996) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem 271: 29427–29435 [DOI] [PubMed] [Google Scholar]
- Tsai B, Rapoport T (2002) Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J Cell Biol 159: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B, Rodighiero C, Lencer WI, Rapoport T (2001) Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104: 937–948 [DOI] [PubMed] [Google Scholar]
- Tsai B, Gilbert JM, Stehle T, Lencer W, Benjamin TL, Rapoport TA (2003) Gangliosides are receptors for murine polyoma virus and SV40. EMBO J 22: 4346–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J et al. (1999) Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol 147: 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow JC, Feng Y, Perlman ZE, Kirchhausen T, Mitchison TJ (2003) Phenotypic screening of small molecule libraries by high throughput cell imaging. Comb Chem High Throughput Screen 6: 279–286 [DOI] [PubMed] [Google Scholar]