Abstract
The mechanism by which caspase-2 executes apoptosis remains obscure. Recent findings indicate that caspase-2 is activated early in response to DNA-damaging antineoplastic agents and may be important for the engagement of the mitochondrial apoptotic pathway. We demonstrate here that fully processed caspase-2 stimulates mitochondrial release of cytochrome c and Smac/DIABLO, but not apoptosis-inducing factor (AIF). This event occurs independently of several Bcl-2 family proteins, including Bax, Bak and Bcl-2, and inactivation experiments reveal that the proteolytic activity of caspase-2 is not required for the effect. Further, functional studies of mitochondria indicate that processed caspase-2 stimulates state 4 respiration and decreases the respiratory control ratio as a result of, in large part, an uncoupling effect. Combined, our data suggest that caspase-2 retains a unique ability to engage directly the mitochondrial apoptotic pathway, an effect that requires processing of the zymogen but not the associated catalytic activity.
Keywords: apoptosis, Bcl-2, caspase, cytochrome c, mitochondria
Introduction
Until recently, the prevailing view has been that caspase-9 activation represents the apex of the caspase cascade within the mitochondrial apoptotic pathway. However, this paradigm is currently being challenged by different lines of evidence indicating that caspase-2 may be important upstream of mitochondria during DNA-damage-induced apoptosis (Robertson et al, 2000, 2002; Lassus et al, 2002). Our previous findings demonstrated that caspase-2 activation occurs before cytochrome c release and caspase-9 activation in response to DNA damage induced by a clinically relevant concentration (10 μM) of the antineoplastic agent etoposide (Robertson et al, 2002). Further, undermining caspase-2 activation with z-VDVAD-fmk, a partial downregulation of the procasp-2 gene or short interfering RNA has been shown to confer a survival advantage on cells exposed to DNA-damaging agents by attenuating cytochrome c release, downstream caspase activation and other manifestations of apoptosis (Lassus et al, 2002; Robertson et al, 2002).
Caspase-2 is a caspase recruitment domain (CARD)-containing caspase (similar to caspase-9) that shares the highest degree of sequence similarity of any mammalian caspase with the Caenorhabditis elegans cell death protease CED-3 (Lamkanfi et al, 2002). The mechanism by which caspase-2 is activated remains unclear, although a recent study reported that it occurs by oligomerization without the help of the CED-4 homologue Apaf-1, and initial activation may not require processing of the proenzyme (Read et al, 2002). Further, of the initiator caspases identified to date, caspase-2 is the only caspase that apparently cannot cleave effector caspases (Van de Craen et al, 1999). These findings, combined with the fact that caspase-2−/− mice have a subtle developmental phenotype (Bergeron et al, 1998), have made it difficult to assign an emergent function to this protease during apoptosis.
The aim of the current study was to determine how caspase-2 is involved in the activation of the mitochondrial apoptotic pathway. The results indicate that processed, but not unprocessed, recombinant human caspase-2 induces a concentration-dependent release of cytochrome c and Smac/DIABLO, but not apoptosis-inducing factor (AIF), from mitochondria. This effect involves neither an induction of mitochondrial permeability transition (MPT) nor the activation of Bax or Bak. Further, inhibition of the catalytic activity of processed caspase-2 failed to block its effect, indicating that, instead of cleaving a protein substrate, caspase-2 may interact directly with the outer mitochondrial membrane (OMM) to trigger the release of intermembrane space proteins and disrupt organelle function.
Results And Discussion
We demonstrated previously that caspase-2 activation is important for mitochondrial cytochrome c release and other manifestations of apoptosis during etoposide-induced cell death (Robertson et al, 2002). To build on these findings and to evaluate more precisely the mechanism by which caspase-2 engages the mitochondrial apoptotic pathway, recombinant wild-type and mutant caspase-2 proteins (Guo et al, 2002) were generated and used to treat permeabilized cells or isolated liver mitochondria.
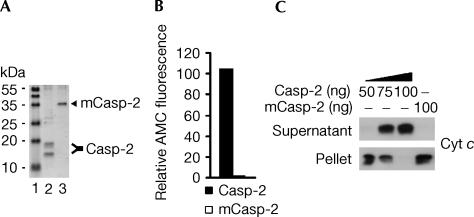
Recombinant proteins were expressed in Escherichia coli and affinity purified. Wild-type caspase-2 underwent autoprocessing to its active long and short subunits when expressed in bacteria (Fig 1A, lane 2), whereas inactive caspase-2, which contains a Cys303Ala activesite mutation, was not processed (Fig 1A, lane 3). As shown in Fig 1B, fluorometric analysis of VDVADase activity indicated that recombinant wild-type caspase-2 was active, whereas mutant caspase-2 was catalytically inactive.
Figure 1.
Processed caspase-2 induces a concentration-dependent release of cytochrome c in permeabilized Jurkat cells. (A) His6-tagged wild-type and mutant caspase-2 proteins were expressed in E. coli, Ni2+-affinity purified and analysed by SDS–polyacrylamide gel electrophoresis (PAGE) and Coomassie blue staining. Lane 1, molecular mass marker; lane 2, affinity-purified wild-type caspase-2; lane 3, affinity-purified mutant (m) caspase-2. (B) Enzyme activity of recombinant wild-type and mutant caspase-2 (200 ng) evaluated as the release of AMC from VDVAD-AMC. (C) Wild-type Jurkat cells (106) were permeabilized with digitonin and incubated with 50, 75 or 100 ng of wild-type caspase-2 or 100 ng of mutant caspase-2 for 10 min at 22°C. Subsequently, supernatant and pellet fractions were separated by SDS–PAGE and western blotted.
When wild-type Jurkat T cells (106) were permeabilized with 5 μg of digitonin and incubated with 50, 75 or 100 ng of recombinant caspase-2 for 10 min at 22°C, cytochrome c was released in a concentration-dependent manner that was complete at the highest concentration (Fig 1C). In contrast, mutant caspase-2 failed to trigger cytochrome c release, indicating that processing of caspase-2 is required for this effect (Fig 1C).
As caspase-2-induced cytochrome c release was rapid and complete in the permeabilized cell model, it was of interest to determine whether caspase-2 would induce a similar release of cytochrome c from isolated mitochondria. The reason was that, if caspase-2 needed to cleave Bid to engage mitochondria (Paroni et al, 2001; Guo et al, 2002), then incubating isolated mitochondria alone with the protease should render it ineffective. As seen in Fig 2A, when mitochondria (0.5 mg of protein) were incubated with 100 ng (lane 2) or 200 ng (lane 3) of active caspase-2 for 10 min at 22°C, cytochrome c was released, which is consistent with data reported previously (Guo et al, 2002). Next, to test whether this effect could be attributed to an induction of MPT, all available Ca2+ was chelated by the inclusion of 1 mM ethyleneglycoltetraacetic acid (EGTA), and mitochondria were incubated with 100 ng of caspase-2 for 10 min (Fig 2A, lane 4). The absence of any effect of EGTA on cytochrome c release suggested that MPT was not involved. Finally, as expected, mutant caspase-2 failed to induce any cytochrome c release, further illustrating the need for processing of caspase-2 to cause OMM permeabilization (Fig 2A, lane 1).
Figure 2.
Caspase-2 stimulates cytochrome c release and impairs mitochondrial function. (A) Isolated liver mitochondria (0.5 mg of protein) were incubated with 100 or 200 ng of wild-type caspase-2 or 200 ng of mutant caspase-2 in the presence or absence of 1 mM EGTA for 10 min at 22°C. Supernatants and pellets were separated by SDS–PAGE and western blotted. (B) Representative curves of mitochondrial oxygen consumption in the absence or presence of 200 ng of wild-type caspase-2. The concentrations of succinate, ADP and CCCP were 5 mM, 150 μM and 1 μM, respectively. (C) Quantification of changes in oxygen consumption induced by 100, 200 and 400 ng of wild-type caspase-2 or 400 ng of mutant caspase-2.
In view of the fact that cytochrome c release caused by caspase-2 was so prominent, experiments were performed to determine the effect this had on mitochondrial function. When mitochondria were energized with 5 mM succinate and then incubated with 100, 200 or 400 ng of active caspase-2 and oxygen consumption was measured (Fig 2B,C), a slight stepwise decrease in state 3 respiration (V3) was observed, suggesting that these organelles retained an ability to phosphorylate ADP (Fig 2C). Interestingly, a more prominent concentration-dependent increase in state 4 respiration (V4) was observed (Fig 2C), indicating that caspase-2 was able to induce an uncoupling of mitochondria. Further, when caspase-2-treated mitochondria were incubated with the uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) (1 μM) and respiration (VCCCP) was measured, a progressive decline in the rate of uncoupled respiration was observed (Fig 2C), which was most probably due to the concentration-dependent manner in which caspase-2 stimulated cytochrome c release. Combined, these results demonstrate that caspase-2 stimulates state 4 respiration (V4) (Fig 2C) and decreases the respiratory control ratio (RCR) as a result of, in large part, an uncoupling effect (Fig 2C).
A recent paper suggested that caspase-3 can cause permeabilization of the OMM with the help of proapoptotic Bcl-2 proteins (Ricci et al, 2003). In addition, the authors indicated that upon OMM permeabilization and cytochrome c release, caspase-3 can act back on mitochondria to dissipate membrane potential by disrupting the function of complexes I and II of the electron transport chain (Ricci et al, 2003). However, the concentrations of recombinant caspase-3 used in that study were ∼100-fold higher than the caspase concentrations used here. Nevertheless, it was of interest to compare the ability of caspase-2 to induce cytochrome c release with that of other caspases, including caspase-3, -6, -7 and -8. Whether an overexpression of the anti-apoptotic protein Bcl-2 would protect against caspase-induced OMM permeabilization was also investigated.
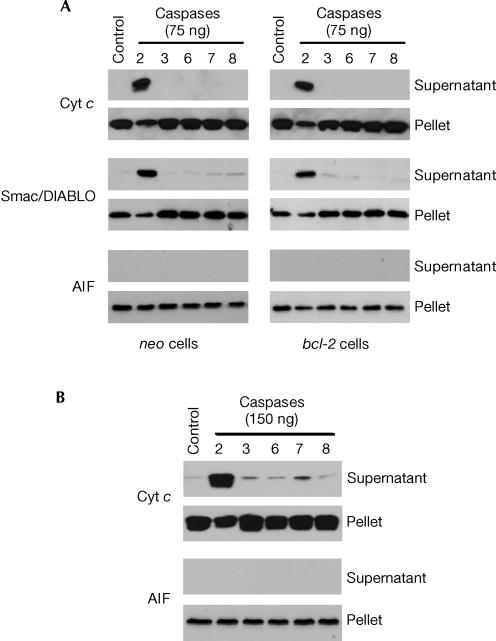
For these experiments, digitonin-permeabilized neo- and bcl-2-transfected Jurkat T cells (106) were used (Armstrong et al, 1996) and incubated with 75 ng of the different recombinant caspases for 10 min at 22°C. Consistent with the data presented in Fig 1C, treatment of permeabilized neo-transfected cells with 75 ng of active caspase-2 induced extensive cytochrome c release, whereas incubation with 75 ng of caspase-3, -6, -7 or -8 (BD Pharmingen, San Jose, CA) had no effect (Fig 3A). Importantly, the catalytic activities of caspase-3, -6, -7 and -8 were confirmed by fluorimetric analysis of DEVD-7-amino-4-methylcoumarin (AMC), VEID-AMC, DEVD-AMC and IETD-AMC cleavage, respectively (data not shown). It should be mentioned that caspase-9 was also tested. Although it failed to stimulate cytochrome c release, this could be because of the observation that we and others (Garcia-Calvo et al, 1998; Ryan et al, 2002) have made indicating that recombinant forms of caspase-9 possess extremely low activity as determined by LEHD-AMC cleavage (data not shown).
Figure 3.
Caspase-2-induced cytochrome c and Smac/DIALBO release is not inhibited by bcl-2 overexpression. (A) Neo- and bcl-2-transfected Jurkat cells (106) were permeabilized with digitonin and incubated with 75 ng of caspase-2, -3, -6, -7 or -8 for 10 min at 22°C. (B) Isolated liver mitochondria (0.5 mg of protein) were incubated with 150 ng of caspase-2, -3, -6, -7 or -8 for 10 min at 22°C. Supernatant and pellet fractions were separated by SDS–PAGE and western blotted.
Whether Smac/DIABLO was released together with cytochrome c was also investigated as Smac/DIABLO is a soluble proapoptotic protein residing in the mitochondrial intermembrane space. The results obtained were consistent with the cytochrome c release data in that only recombinant caspase-2 induced significant Smac/DIABLO release (Fig 3A). Further, bcl-2 overexpression failed to block caspase-2-induced cytochrome c or Smac/DIABLO release, suggesting that the putative mechanism accounting for OMM permeabilization does not involve Bcl-2 proteins. Finally, when isolated liver mitochondria (0.5 mg of protein) were incubated with 150 ng of caspase-2, -3, -6, -7 or -8 for 10 min, only caspase-2 induced significant cytochrome c release (Fig 3B).
AIF was originally described as a soluble protein that is present in the intermembrane space of mitochondria and is released in response to various apoptotic triggers (Susin et al, 1999). However, a recent study challenged this notion (Arnoult et al, 2003). Specifically, the authors' findings indicated that AIF is associated with the inner mitochondrial membrane, and its release can be dissociated from that of cytochrome c. Experiments to determine whether caspase-2 or the other recombinant caspases could stimulate AIF release in our experimental model revealed that, despite being a potent inducer of cytochrome c and Smac/DIABLO release, caspase-2 failed altogether to induce the release of AIF from either digitonin-permeabilized cells (Fig 3A) or isolated mitochondria (Fig 3B).
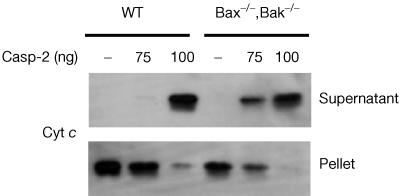
As a previous report indicated that caspase-2 was required for Bax translocation to mitochondria when intact cells were treated with etoposide (Lassus et al, 2002), we were interested in determining whether Bax or Bak was required for caspase-2-induced cytochrome c release. To test this, we compared the ability of caspase-2 to stimulate mitochondrial cytochrome c release from wild-type or Bax−/−,Bak−/− mouse embryonic fibroblasts (MEFs) (Wei et al, 2001). Specifically, cells were harvested, digitonin-permeabilized and incubated with 75 or 100 ng of active caspase-2 for 10 min at 22°C. As shown in Fig 4, caspase-2 was able to induce cytochrome c release in both cell types, although it appeared that the Bax−/−,Bak−/− cells were sensitive to 75 ng of caspase-2, whereas wild-type MEFs were not. In general, however, these data show that neither Bax nor Bak is required for the ability of caspase-2 to permeabilize the OMM and stimulate the release of intermembrane space proteins.
Figure 4.
Caspase-2 stimulates cytochrome c release from Bax−/−,Bak−/− MEFs. Wild-type (WT) or Bax,Bak-deficient cells were harvested, digitonin-permeabilized and incubated with 75 or 100 ng of wild-type caspase-2 for 10 min at 22°C. Supernatant and pellet fractions were separated by SDS–PAGE and western blotted.
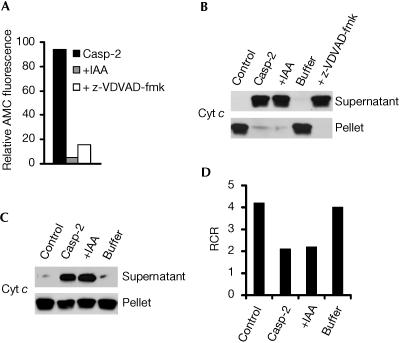
Although processing of the caspase-2 zymogen was needed for permeabilization of the OMM to occur, the next step was to determine whether the associated catalytic activity of processed caspase-2 was also required. For this purpose, catalytically inactive processed caspase-2 was generated by alkylating the active-site cysteine with 10 mM iodoacetamide (IAA) for 60 min at 37°C (Gerwin, 1967). The results showed that inactivated processed caspase-2, which retained only ∼5% of the proteolytic activity of the active enzyme (Fig 5A), elicited cytochrome c release to the same extent as non-inactivated processed caspase-2 when added to permeabilized Jurkat cells (Fig 5B) or isolated mitochondria (Fig 5C). Similar results were obtained when inactivation of processed caspase-2 was performed by incubating the caspase with its irreversible inhibitor z-VDVAD-fmk (Fig 5A,B). Further, when the IAA-inactivated processed caspase-2 protein and its buffer were separated by ultrafiltration and the buffer was used subsequently to treat permeabilized cells or isolated mitochondria, cytochrome c was not released (Fig 5B,C), indicating that IAA was not having a direct effect (Fig 5B,C). Finally, in addition to stimulating cytochrome c release, the IAA-inactivated processed enzyme induced a lowering of RCR that was comparable to that induced by catalytically active processed caspase-2 (Fig 5D). Combined, these data suggest that the proteolytic activity of processed caspase-2 is dispensable for the ability of this enzyme to engage and activate the mitochondrial apoptotic pathway.
Figure 5.
Proteolytic activity of caspase-2 is dispensable for its ability to engage mitochondria. (A) Enzyme activity of wild-type caspase-2 (200 ng), either untreated (Casp-2), treated with 10 mM IAA (+IAA) for 60 min at 37°C or treated with z-VDVAD-fmk (250 μM), as monitored by the release of AMC from VDVAD-AMC. (B) Wild-type Jurkat cells (106) were permeabilized with digitonin and incubated in the absence or presence of 100 ng of wild-type caspase-2 (Casp-2), inactivated processed caspase-2 or IAA-based inactivation buffer alone for 10 min at 22°C. (C) Isolated liver mitochondria (0.5 mg of protein) were incubated under the same conditions as described for (B) except that digitonin was omitted and the amount of recombinant caspase-2 was 150 ng. For (B,C), supernatant and pellet fractions were separated by SDS–PAGE and western blotted. (D) RCR measurement of mitochondrial suspensions incubated under the same conditions as described for (C).
Conclusions
Despite considerable evidence indicating that caspase-2 is activated early in response to a variety of apoptotic stimuli (Kumar et al, 1994; Wang et al, 1994; Harvey et al, 1997; Robertson et al, 2002), assigning an emergent function to this protease has been difficult. Recent findings have suggested that caspase-2 participates in the activation of the mitochondrial apoptotic pathway. In one study, overexpressed green-fluorescent-protein-labelled procaspase-2 was shown to locate to the nucleus, where it may be able to induce apoptosis by stimulating mitochondrial cytochrome c release by an unknown mechanism (Paroni et al, 2002). A separate study presented findings indicating that purified caspase-2 is able to trigger cytochrome c release in vitro, possibly by cleaving Bid (Guo et al, 2002). Finally, two reports, including one from this laboratory, presented evidence indicating that caspase-2 is important for the engagement of mitochondria in the presence of DNA-damaging agents (Lassus et al, 2002; Robertson et al, 2002).
In the current study, we demonstrate that recombinant processed caspase-2, but not caspase-3, -6, -7 or -8, permeabilizes the OMM directly and stimulates the release of cytochrome c and Smac/DIABLO, but not AIF. Whereas the ability of caspase-2 to permeabilize mitochondria depends on processing of the zymogen, the underlying mechanism appears not to involve an induction of MPT or to be modulated by pro- or anti-apoptotic Bcl-2 proteins. Instead, we propose that processed caspase-2 interacts directly with putative proteins and/or phospholipids located on the OMM, or at contact sites between the outer and inner membranes, to cause the release of proapoptotic proteins from the intermembrane space. Studies geared towards identifying the precise molecular requirements for caspase-2-induced engagement of the mitochondrial apoptotic pathway are ongoing in the laboratory.
Note added in proof: While this manuscript was in press, it was reported that caspase-2 activation occurs in a complex called the PIDDosome, which consists of the death-domain-containing protein PIDD and the adaptor protein RAIDD (Tinel & Tschopp, 2004).
Methods
Cell culture. Jurkat T lymphocytes and MEFs were cultured in RPMI 1640 complete medium supplemented with 10% (v/v) heat-inactivated fetal calf serum, 2% (w/v) glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified air/CO2 (19:1) atmosphere at 37°C. Cells were maintained in an exponential growth phase for all experiments.
Isolation of rat liver mitochondria. The liver of a male Sprague–Dawley rat was minced on ice, resuspended in 50 ml of MSH buffer (210 mM mannitol, 70 mM sucrose, 5 mM Hepes, pH 7.5) supplemented with 1 mM ethylenediaminetetraacetic acid (EDTA), and homogenized with a tight-fitting glass–Teflon motorized homogenizer. Homogenates were centrifuged at 600g for 8 min at 4°C. The supernatant was decanted and recentrifuged at 5,500g for 15 min to form a mitochondrial pellet that was resuspended in MSH buffer without EDTA and centrifuged again at 5,500g for 15 min. The final mitochondrial pellet was resuspended in MSH buffer at a protein concentration of 80–100 mg/ml.
Digitonin-permeabilized cells. Jurkat T cells or MEFs (106) were washed in PBS, resuspended in 100 μl of buffer (140 mM mannitol, 46 mM sucrose, 50 mM KCl, 1 mM KH2PO4, 5 mM MgSO4, 5 mM succinate, 1 mM EGTA, 5 mM Tris, pH 7.4) and added to the incubation chamber. Following a 2 min stabilization period, cells were permeabilized with 5 μg of digitonin, and 5 μM rotenone was added to maintain pyridine nucleotides in a reduced form.
Western blot analysis. Western blot analysis was carried out as described previously (Robertson et al, 2002). The antibodies used were anti-AIF (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-cytochrome c (1:2,500) (BD Pharmingen, San Jose, CA) or antismac/DIABLO (1:1,000) (Alexis Corporation, San Diego, CA).
Measurement of caspase activity. A 50–200 ng portion of recombinant caspase was combined with substrate dissolved in a standard reaction buffer, and cleavage of the fluorogenic peptide substrate (VDVAD-AMC, DEVD-AMC, VEID-AMC, IETD-AMC, LEHD-AMC) was monitored by AMC liberation in a Fluoroscan II plate reader (Labsystems, Stockholm, Sweden) using 355 nm excitation and 460 nm emission wavelengths.
Measurement of mitochondrial oxygen consumption. Mitochondria (1 mg/ml) were incubated in a buffer containing 140 mM mannitol, 46 mM sucrose, 50 mM KCl, 1 mM KH2PO4, 5 mM succinate, 5 mM Tris, pH 7.4, at 25°C. Rotenone (2 μM) was added to maintain pyridine nucleotides in a reduced form. Oxygen consumption by isolated rat liver mitochondria was measured using a Clark-type oxygen electrode (Yellow Spring Instrument Co., OH) at 25°C. Mitochondria with an RCR (defined as the rate of respiration in the presence of ADP divided by the rate obtained following the expenditure of ADP) above 4 were used for all experiments. Fresh mitochondria were prepared for each experiment and used within 4 h.
Expression and purification of caspase-2 proteins. Bacterial expression plasmids containing active or inactive recombinant caspase-2 (Guo et al, 2002) were expressed in E. coli strain BL21(DE3) as C- or N-terminal His6-tagged proteins using pET-21a or pET-28a vector (Novagen) and purified by standard Ni2+ affinity chromatography.
Acknowledgments
We thank Emad Alnemri for the caspase-2 and caspase-2 C-A bacterial expression constructs and Stanley Korsmeyer for the wild-type and Bax−/−,Bak−/− MEFs, as well as the neo- and bcl-2-transfected Jurkat T lymphocytes. This work was supported by grants from the Swedish Science Foundation (31X-02471-37A), the Swedish (3829-B02-07XBC) and Stockholm (03:173) Cancer Societies, and the European Commission (QLK3-CT-2002-01956). J.D.R. was supported by grant 5 F32 CA83273 from the National Cancer Institute, National Institutes of Health, and A.K. was supported by a grant from the Wenner-Gren Foundation.
References
- Armstrong RC et al. (1996) Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem 271: 16850–16855 [DOI] [PubMed] [Google Scholar]
- Arnoult D, Parone P, Martinou J-C, Antonsson B, Estaquier J, Ameisen JC (2003) Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol 159: 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron L et al. (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12: 1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA (1998) Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 273: 32608–32613 [DOI] [PubMed] [Google Scholar]
- Gerwin BI (1967) Properties of the single sulfhydryl group of streptococcal proteinase. A comparison of the rates of alkylation by chloroacetic acid and chloroacetamide. J Biol Chem 242: 451–456 [PubMed] [Google Scholar]
- Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 277: 13430–13437 [DOI] [PubMed] [Google Scholar]
- Harvey NL, Butt AJ, Kumar S (1997) Functional activation of Nedd2/ICH-1 (caspase-2) is an early process in apoptosis. J Biol Chem 272: 13134–13139 [DOI] [PubMed] [Google Scholar]
- Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA (1994) Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 β-converting enzyme. Genes Dev 8: 1613–1626 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P (2002) Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ 9: 358–361 [DOI] [PubMed] [Google Scholar]
- Lassus P, Opitz-Araya X, Lazebnik Y (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297: 1352–1354 [DOI] [PubMed] [Google Scholar]
- Paroni G, Henderson C, Schneider C, Brancolini C (2001) Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3. J Biol Chem 276: 21907–21915 [DOI] [PubMed] [Google Scholar]
- Paroni G, Henderson C, Schneider C, Brancolini C (2002) Caspase-2 can trigger cytochrome c release and apoptosis from the nucleus. J Biol Chem 277: 15147–15161 [DOI] [PubMed] [Google Scholar]
- Read SH, Baliga BC, Ekert PG, Vaux DL, Kumar S (2002) A novel Apaf-1-independent putative caspase-2 activation complex. J Cell Biol 159: 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci J-E, Gottlieb RA, Green DR (2003) Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol 160: 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S (2000) Distinct pathways for stimulation of cytochrome c release by etoposide. J Biol Chem 275: 32438–32443 [DOI] [PubMed] [Google Scholar]
- Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem 277: 29803–29809 [DOI] [PubMed] [Google Scholar]
- Ryan CA, Stennicke HR, Nava VE, Burch JB, Hardwick JM, Salvesen GS (2002) Inhibitor specificity of recombinant and endogenous caspase-9. Biochem J 366: 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA et al. (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446 [DOI] [PubMed] [Google Scholar]
- Tinel A, Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science, doi:10.1126/science.1095432 [DOI] [PubMed] [Google Scholar]
- Van de Craen M, Declercq W, Van den brande I, Fiers W, Vandenabeele P (1999) The proteolytic procaspase activation network: an in vitro analysis. Cell Death Differ 6: 1117–11124 [DOI] [PubMed] [Google Scholar]
- Wang L, Miura M, Bergeron L, Zhu H, Yuan J (1994) Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell 78: 739–750 [DOI] [PubMed] [Google Scholar]
- Wei MC et al. (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]