Abstract
Budding yeast Sgt1 is required for kinetochore assembly, and its homologues have a role in cAMP signalling in fungi and pathogen resistance in plants. The function of mammalian Sgt1 is unknown. We report that RNA interference-mediated depletion of Sgt1 from HeLa cells causes dramatic alterations of the mitotic spindle and problems in chromosome alignment. Cells lacking Sgt1 undergo a mitotic delay due to activation of the spindle checkpoint. The checkpoint response, however, is significantly weakened in Sgt1-depleted cells, and this correlates with a dramatic reduction in kinetochore levels of Mad1, Mad2 and BubR1. These effects are explained by a problem in kinetochore assembly that prevents the localization of Hec1, CENP-E, CENP-F, CENP-I, but not CENP-C, to mitotic kinetochores. Our studies implicate Sgt1 as an essential protein and a critical assembly factor for the mammalian kinetochore, and lend credit to the hypothesis of a kinetochore assembly pathway that is conserved from yeast to man.
Keywords: Sgt1, Hec1, Mad2, CENP, kinetochore, spindle checkpoint
Introduction
Kinetochores assemble on centromeric DNA to mediate the interaction of chromosomes with the mitotic spindle (Cleveland et al, 2003). Mammalian kinetochores exist during interphase as pre-kinetochores, poorly characterized structures containing a subset of the mitotic kinetochore markers (Pluta et al, 1995). CENP-A, -B, -C, -G, -H and -I reside at kinetochores throughout the cell cycle, whereas CENP-E, CENP-F, Hec1 (homologue of budding yeast Ndc80p) and the components of the spindle checkpoint are only present on mitotic kinetochores (Cleveland et al, 2003). The mechanisms of pre-kinetochore and mitotic kinetochore assembly in mammals are largely uncharacterized. We report that assembly of human kinetochores critically requires Sgt1 (also known as Sugt1; Kitagawa et al, 1999). Budding yeast Sgt1p binds Skp1p to assemble the Cbf3p complex, the core component of the kinetochore in this organism (Kitagawa et al, 1999). Sgt1 is also required for pathogen resistance in plants (Gray, 2002). These functions probably derive from Sgt1 binding to Hsp90, of which Sgt1 may be a co-chaperone (Garcia-Ranea et al, 2002; Hubert et al, 2003; Liu et al, 2003b; Lu et al, 2003; Takahashi et al, 2003; Lee et al, 2004). Sgt1 has not been characterized in mammals. We report here that the phenotype of RNA interference (RNAi)-mediated Sgt1 depletion in HeLa cells defines an essential role in kinetochore assembly.
Results And Discussion
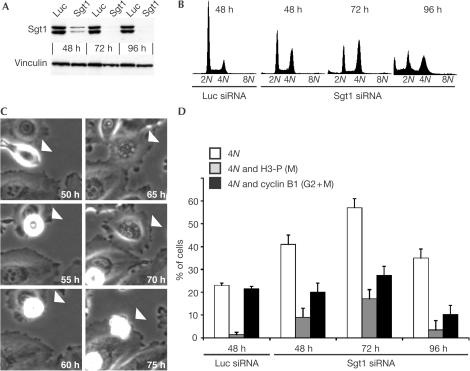
HsSgt1 exists in two isoforms due to alternative splicing (Niikura & Kitagawa, 2003). We designed short interfering RNA (siRNA) duplexes (Elbashir et al, 2001) that silenced both isoforms in HeLa cells. Sgt1 levels were greatly reduced between 48 and 72 h after siRNAs transfection (Fig 1A and supplementary Fig 1 online). Concomitantly, there was an accumulation of 4N cells becoming predominant at 72 h (Fig 1B). At 96 h, there was a large sub-G1 population, indicative of cell death. Time-lapse microscopy showed that HeLa cells lacking Sgt1 round up and are subsequently delayed in mitosis for several hours, finally exiting mitosis aberrantly without dividing and becoming multinucleated (Fig 1C). We applied a trivariate flow cytometry protocol to correlate DNA content with cyclin B1 (high during the entire G2–M) and H3-P (M-phase marker). Accumulation of H3-P-positive 4N cells between 48 and 72 h was observed, indicative of mitotic arrest (Fig 1D). In addition, a large fraction of 4N cells was negative for G2 or M markers, indicative of tetraploid G1 cells. Time-lapse microscopy suggests that these cells result from aberrant mitotic exit caused by loss of Sgt1. Thus, cells lacking Sgt1 are temporarily delayed in mitosis and eventually become tetraploid G1 cells after failing to divide.
Figure 1.
Silencing of Sgt1 affects progression through mitosis. (A) Western blot of HeLa cell lysates harvested at the indicated time points after transfection with siRNA duplexes against Sgt1. Control injections of anti-luciferase (Luc) siRNAs are also shown. Both splicing isoforms of Sgt1 are silenced. (B) DNA content was analysed by FC on PIstained cells. Cells lacking Sgt1 accumulate with 4N DNA, with an increase in a sub-G1 population 96-h after siRNA transfection. (C) A cell (white arrowhead) lacking Sgt1 enters mitosis and remains arrested for several hours, finally exiting without dividing. This cell eventually died around 75 h. (D) Trivariate FC analysis of cells lacking Sgt1. White bars: Cells with 4N DNA content; grey bars: cells with 4N DNA content that were H3-P positive (mitotic cells, M); black bars: cells with 4N DNA content that were positive for Cyclin B1 (total of G2- and M-phase cells). Note the increase of H3-P-positive cells following Sgt1 depletion, with a concomitant modest increase in G2–M cells at 72 h. More than 20% of the cell population had 4N DNA content but stained negative for mitotic markers, indicative of a tetraploid G1 population.
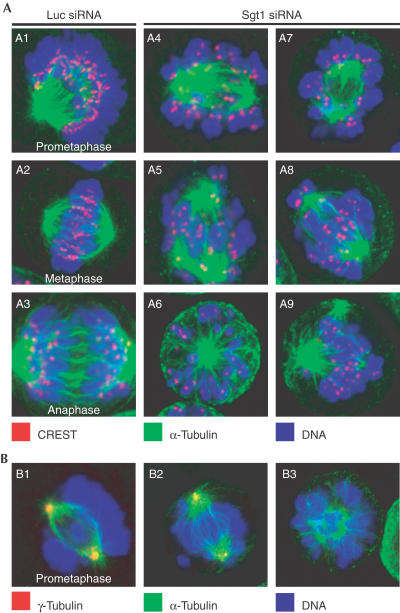
To investigate the cause of mitotic delay, we visualized mitotic spindle and kinetochores. This revealed severe chromosome congression and alignment problems (Fig 2, panels A4–A9). Cells presented a disordered mass of often hyper-condensed chromosomes, most of which had failed to align. Rare metaphase configurations were observed, but one or more unaligned chromosomes were always detected. Multipolar spindles and fragmented spindle poles were also frequent (see also Fig 2, panels B2 and B3), and many cells contained micronuclei, indicative of G1 cells that had aborted mitosis.
Figure 2.
Spindle defects in HeLa cells lacking Sgt1. (A) CREST (red), α-tubulin (green) and DNA (blue) were visualized to investigate spindle structure. Panels A1–A3: Normal control cells at different stages of mitosis; panels A4–A9: spindle defects in interfered cells. (B) Centrosome problems revealed with an anti-γ-tubulin antibody (red). Panel B1: A normal control cell; panels B2 and B3: two interfered cells with fragmented and faint γ-tubulin staining.
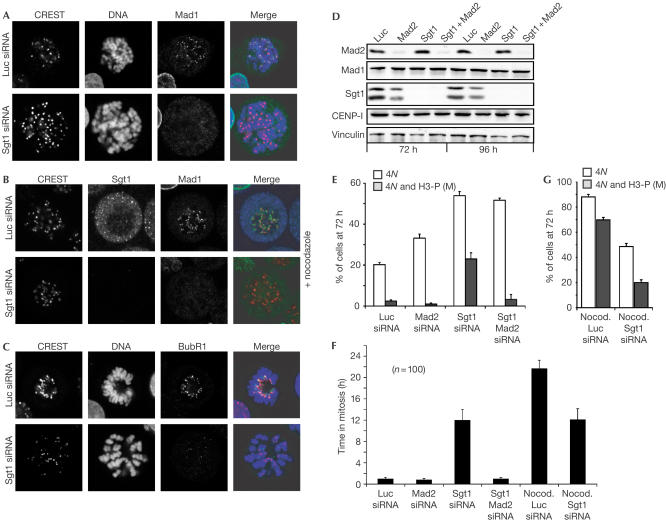
Problems in chromosome alignment activate the mitotic spindle checkpoint (MSC) (Musacchio & Hardwick, 2002). We asked whether the MSC was responsible for mitotic delay caused by Sgt1 depletion. MSC proteins Mad1 and Mad2 are recruited to kinetochores before microtubule–chromosome attachment or if microtubule–kinetochore interactions are disrupted with spindle poisons (Musacchio & Hardwick, 2002). Within the detection limits of our experiment, the kinetochores of Sgt1-depleted cells appeared to be devoid of Mad1 even after addition of nocodazole (Fig 3A,B). The same was true for Mad2 (not shown). At times, a faint signal was observed near a small number of kinetochores (1–3) in nocodazole-treated cells, but at markedly reduced levels relative to control cells (not shown). Of note, BubR1 was also unable to localize to the kinetochores of Sgt1-depleted cells (Fig 3C).
Figure 3.
Mitotic delay of cells lacking Sgt1 is caused by the spindle checkpoint. (A) Mad1 is absent from the kinetochores of Sgt1-depleted cells despite evident lack of microtubule attachment. (B) Addition of nocodazole did not cause a return of Mad1 to kinetochores. (C) Also BubR1 was missing from the kinetochores of Sgt1-depleted cells. (D) Parallel silencing of Sgt1 and Mad2 by RNAi as revealed by western blot on total cell lysates 72 and 96 h after siRNAs transfection. Mad1, CENP-I and vinculin are shown as controls. (E) Bivariate FC analysis on the indicated samples. White bars: 4N cells; grey bars: 4N cells that were H3-P positive. Dual ablation of Mad2 and Sgt1 relieves the mitotic block caused by Sgt1 ablation. (F) Cells lacking Sgt1 are unable to maintain a mitotic arrest beyond an average of 11 h, even if nocodazole is added. Black bars: Duration of mitosis in cells filmed by time-lapse microscopy from 48 to 96 h after siRNA transfection. (G) Sgt1-depleted cells cannot mount a full-fledge MSC response in nocodazole. White bars: 4N cells; grey bars: 4N cells that were positive for H3-P. The lower percentage of 4N cells in the total cell population after Sgt1 depletion is explained by a G1 arrest, as documented in SF 2. Note that the scale of this figure is different from that used in (E).
Thus, depletion of Sgt1 causes a severe reduction of the kinetochore levels of three MSC components. It has been shown, however, that the MSC is maintained under conditions that cause a substantial decrease of kinetochore Mad1 and Mad2 (Martin-Lluesma et al, 2002; DeLuca et al, 2003). To test if Mad2 was required to maintain the mitotic arrest caused by loss of Sgt1, we silenced Sgt1 and Mad2 by RNAi (Fig 3D,E). The accumulation of mitotic cells caused by Sgt1 loss was relieved by Mad2 depletion, indicating that the MSC is activated after loss of Sgt1 despite substantial kinetochore depletion of Mad1, Mad2 and BubR1 (Fig 3E). Sgt1-depleted cells, however, remain arrested in mitosis for 11 h on average, significantly shorter than the average 24-h arrest on addition of nocodazole (Fig 3F). In principle, this may indicate that sister chromatids slowly achieve bipolar attachment, a condition that satisfies the MSC allowing transit through mitosis. In this case, cells would be expected to arrest longer (24 h or more as in control cells) if the process of kinetochore–microtubule attachment was reverted. Addition of nocodazole to cells lacking Sgt1, however, did not restore a normal duration of the MSC (Fig 3F), which remained limited to 11 h. The proportion of mitotic cells was also the same with or without nocodazole (compare Fig 3E with Fig 3G) or taxol (not shown). This shows that cells do not exit mitosis because the MSC has been satisfied, and we conclude that depletion of Sgt1 reduces the potency of MSC response.
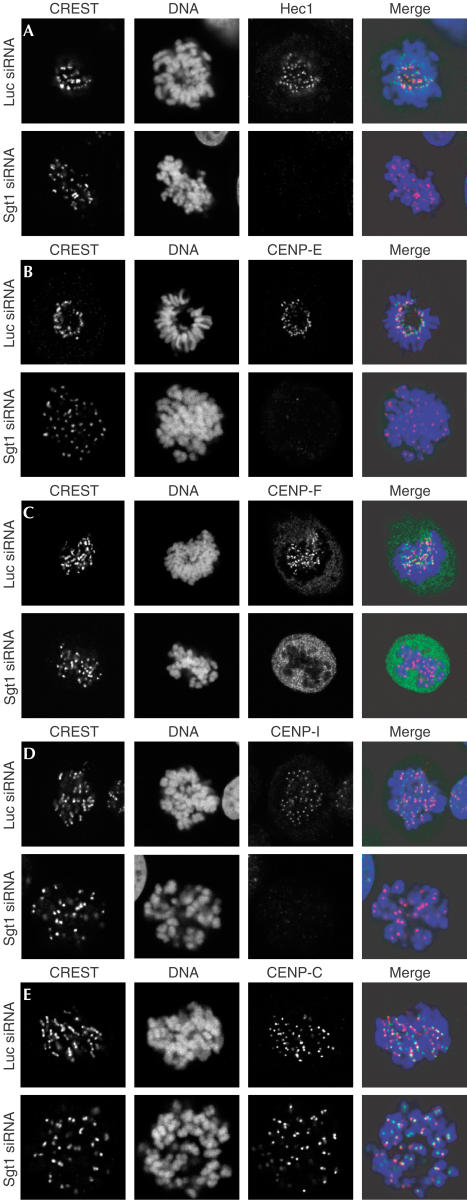
Alterations of the mitotic spindle and decreased levels of kinetochore Mad1 and Mad2 have been observed after depletion of kinetochore proteins such as Hec1, Nuf2, CENP-C, CENP-E or CENP-I (Wood et al, 1997; Fukagawa et al, 1999; McEwen et al, 2001; Martin-Lluesma et al, 2002; Nishihashi et al, 2002; DeLuca et al, 2003; Liu et al, 2003a), suggesting the possibility that Sgt1 depletion causes damage to kinetochores. We probed a subset of kinetochore markers by confocal immunofluorescence analysis of Sgt1-depleted HeLa cells. Hec1 did not localize to mitotic kinetochores (Fig 4A). The same was true for CENP-E and CENP-F (Fig 4B,C). Next, we addressed constitutive kinetochore residents. CENP-I, an inner kinetochore protein, was missing from kinetochores of Sgt1-depleted cells (Fig 4D). CENP-C, conversely, localized normally (Fig 4E). Because CENP-C requires CENP-A for kinetochore localization in HeLa and other cell types (Howman et al, 2000; Van Hooser et al, 2001; Goshima et al, 2003), we assume that CENP-A localization is not affected. Consistently, CENP-I is not required for CENP-A or CENP-C localization in HeLa cells (Goshima et al, 2003; Liu et al, 2003a).
Figure 4.
A kinetochore defect in Sgt1-depleted HeLa cells. (A) Localization of Hec1 to kinetochores was compared in control and Sgt1-depleted cells in the absence of spindle poisons. The CREST antiserum stains the inner kinetochore. In control cells, Hec1 is localized at the periphery of CREST staining. There was no kinetochore localization of Hec1 after Sgt1 depletion. (B) Localization of CENP-E to the outer kinetochore is affected by loss of Sgt1. (C) Localization of CENP-F is also affected, whereas there is substantial residual cytoplasmic staining. (D) CENP-I is absent from the inner kinetochore of cells lacking Sgt1. (E) Localization of CENP-C is unaffected.
Depletion of Sgt1 results in a marked alteration of kinetochores. All mitotic kinetochore markers and one pre-kinetochore marker tested (Mad1, Mad2, BubR1, Hec1, CENP-E, and CENP-F, CENP-I) were mislocalized. Loss of kinetochore integrity activates an MSC response of reduced potency, with cells exiting mitosis aberrantly (presumably because they cannot complete attachment), becoming tetraploid G1 cells and eventually dying (supplementary Fig 2 online). Reduced potency of the MSC correlates with extensive kinetochore damage caused by Sgt1 depletion, and the residual MSC response may be due to traces of Mad1 and Mad2 at kinetochores of Sgt1-depleted cells. A clear understanding of the mechanisms of MSC signal amplification is required to address this issue rigorously. Our phenotype is similar to the one caused by loss of CENP-I: chromosome misalignments, scrambled spindles, mislocalization of CENP-F, a failure to complete cytokinesis, a dramatic reduction of kinetochore Mad1 or Mad2, and a mitotic delay of reduced potency relative to control cells (Nishihashi et al, 2002; Goshima et al, 2003; Liu et al, 2003a). Cells lacking CENP-I, however, retain kinetochore BubR1, which is lost from kinetochores of cells lacking Sgt1 (Liu et al, 2003a).
What are the parallels between Sgt1 function in yeast and human cells? Budding yeast SGT1 is a dosage suppressor of SKP1. In this organism, Skp1 is a component of the Cbf3p kinetochore complex and of the SCF ubiquitin–ligase (Kitagawa & Hieter, 2001), and its binding to Bub1 is required for mitotic delay induced by kinetochore tension defects (Kitagawa et al, 2003). Sgt1p does not incorporate into Cbf3p and may be required to activate the Ctf13p subunit, a step also involving Skp1p and Hsp90 (Kaplan et al, 1997; Kitagawa et al, 1999; Stemmann et al, 2002). Our discovery that the function of Sgt1 in kinetochore assembly is conserved in humans suggests the intriguing possibility that the budding yeast and mammalian kinetochores share their assembly pathway. With the exception of SKP1, the vertebrate homologue of which has not been linked to kinetochore assembly and does not seem to localize to kinetochores of animal cells (Freed et al, 1999; Gstaiger et al, 1999), the homologues of genes encoding the Cbf3p complex have not been identified in other species. Human Sgt1 is a highly soluble protein with nuclear and cytoplasmic localization that is not visible at kinetochores (SF 1). Sgt1 is devoid of catalytic domains, but contains protein interaction motifs such as TPR and p23 repeats. The latter identify Sgt1 as Hsp90 co-chaperone (Garcia-Ranea et al, 2002; Hubert et al, 2003; Liu et al, 2003b; Lu et al, 2003; Takahashi et al, 2003; Lee et al, 2004). Co-chaperones are specificity factors that direct chaperones to specific classes of substrates in localized functional contexts (Young et al, 2003). In view of these findings, we speculate that Sgt1 may act as a kinetochore assembly factor controlling kinetochore incorporation of CENP-I and/or other unknown kinetochore components. Alternatively, cells lacking Sgt1 may be unable to retain CENP-I and other proteins to the centromere region. The fundamental role of Sgt1 in kinetochore assembly suggests that Sgt1 may be the vehicle leading us to the identification of the core constituents of the mammalian kinetochore.
Methods
Cell culture and RNA interference HeLa cells were cultured in high-glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Life Technologies) in a humidified 37°C incubator with 5% CO2. Sgt1 siRNA (5′-AAGGCUUUGGAACAGAAACCA-3′, top strand) was from Dharmacon, Inc. (USA). siRNAs targeting Mad2 (5′-AAGAGUCGGGACCACAGUUUA-3′, top strand) and GL2 luciferase have been described (Martin-Lluesma et al, 2002). We transfected annealed doublestranded siRNAs into HeLa cells using oligofectamine reagent (Invitrogen). 5-Bromodeoxyuridine (BrdU) and nocodazole (Sigma) were used at 33 μM and 200 ng/ml, respectively.
Antibodies Human Sgt1 (NM_006704) was subcloned into pRSET-B (Invitrogen) and expressed in a soluble form in Escherichia coli BL21(DE3) (Invitrogen) after induction with 0.2 mM isopropyl-β-D-thiogalactoside at 37°C for 3 h. The purified protein was concentrated to 10 mg/ml and used for immunization. Mad1 and Mad2 were expressed and purified as described (Sironi et al, 2001) and used for immunization. Mouse monoclonal antibodies (mAbs) against Sgt1 and Mad1 were generated by fusing the splenocytes to NS-2 mouse myeloma cells 3 days after the final boost. Single-cell cloning was used to generate cell lines producing mouse mAbs against Sgt1 (An32 and Ci47) and Mad1 (A. De Antoni and A. Musacchio, unpublished). A rabbit anti-Mad2 polyclonal antiserum was raised by immunization with pure 6His-Mad2. We used mouse mAbs against γ-tubulin (Sigma) and Sgt1 (BD Biosciences), and rabbit polyclonal antibodies against α-tubulin (Sigma) and CENP-F (Novus-Biologicals). Mouse mAb against BubR1 was purchased from Chemicon International. Anti-centromere autoimmune serum (CREST) was from Antibodies Inc. Rabbit polyclonal antibodies against CENP-I, Hec1 and CENP-C have been described (Saitoh et al, 1992; Martin-Lluesma et al, 2002; Liu et al, 2003a).
Immunodetection Cells grown on coverslips pretreated with 15 μg/ml poly(D)lysine (Sigma) were fixed in 4% paraformaldehyde in PIPES buffer (80 mM PIPES 5 mM EGTA, 2 mM MgCl2) for 5 min, treated with phosphate-buffered saline (PBS) plus 0.1% Triton X-100 for 10 min, and washed three times in PBS for 5 min. In the case of Hec1, the cells were incubated for 5 min in extraction buffer (0.1% Triton X-100, 100 mM PIPES, 300 mM sucrose, 1 mM EGTA, 1 mM MgCl2) before fixation in 4% paraformaldehyde. Primary antibodies diluted in PBS were applied to the coverslips and incubated for 60 min before PBS washing. Secondary antibodies against mouse, rabbit and human conjugated to Cy5, or AlexaFluor 488 were typically diluted 1:150. DNA was stained with 50 μg/ml propidium iodide (PI) for 30 s.
Microscopy Wide-field fluorescence microscopy images were acquired using a BX61 (Olympus) motorized fluorescence microscope equipped with a B/W cooled CCD camera (C5985 Hamamatsu). A Bio-Rad MRC 1024 confocal microscope equipped with a 20 mW Kr–Ar laser was used for confocal analysis. Time-lapse microscopy was performed with an IX70 inverted microscope (Olympus) equipped with an incubation chamber (Solent Scientific). The system includes a digital camera (Sensys, Roper Scientific) to acquire high-resolution images. Microscope stage motorization, filter wheels and image acquisition and processing were controlled by Metamorph (Universal Imaging).
Flow cytometry To detect cyclin B1, phosphoH3 and DNA, cells were washed with PBS, detached from tissue culture plates and fixed in suspension at 2 × 106 cells/ml in 1% formaldehyde in PBS for 15 min at different time points after siRNA transfection. After PBS washing, the pellet was resuspended in ice-cold 70% ethanol and stored at 4°C. For immunodetection, cells were washed twice in PBS and permeabilized in 0.1% Triton X-100 in PBS for 10 min. After blocking in 5% normal goat serum (NGS) in PBS for 20 min, cells were incubated with 2.5 μg/ml anti-cyclin B1 mouse mAb (Pharmingen) and anti-P-H3 rabbit polyclonal antibody (Upstate) 1:100 in PBS plus 1% NGS for 3 h at 37°C. Cells were rinsed in PBS and incubated for 1 h with FITC-conjugated goat anti-mouse (1:50, Sigma) and Cy5-conjugated goat anti-rabbit (1:50, Jackson Immunoresearch) antibodies. Cells were washed again and resuspended in 1 ml of a solution containing 1 μg/ml PI (Sigma) in PBS and 12.5 μl RNase 1 mg/ml in PBS and stained overnight at 4°C before acquisition. To detect BrdU and DNA, exponentially growing cells were incubated with 30 μM BrdU (Calbiochem Corp.) for 16 h at 37°C, fixed in 70% ethanol and kept at 4°C. After DNA denaturation, the cells were washed in 0.1 M sodium tetraborate (pH 8.5). Cells were permeabilized with 0.1% Triton X-100 in PBS (Sigma), and incubated with 200 μl of anti-BrdU mAb (Becton Dickinson) diluted 1:5 in PBS plus 1% NGS for 1 h at 20°C in the dark. After three PBS washings, the pellet was incubated with 200 μl of FITC-conjugated goat anti-mouse antibody (Sigma) diluted 1:50 in PBS plus 1% NGS. For DNA quantification, cells were finally resuspended in 2 ml of PI solution containing 2 μg/ml PI in PBS and 25 μl of RNase 1 mg/ml in water, and stained overnight at 4°C in the dark. Samples were acquired on a FACSCalibur (Becton Dickinson) flow cytometer. At least 10,000 events were acquired. Analysis was performed using CellQuest 3.3 (Becton Dickinson).
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Figures
Acknowledgments
We thank J. Kilmartin and T. Yen for reagents, and M. Melixetian, A. Ballabeni and the Musacchio group for discussions. A.M. thanks the Association for International Cancer Research, the Italian Association for Cancer Research and the Consiglio Nazionale delle Ricerche for support. P.S. is a fellow of the Danish Research Academy. W.C.E. is a Principal Research Fellow of The Wellcome Trust. K.K. was supported by the Cancer Center Support Grant CA21765 from the National Cancer Institute, by the National Institutes of Health grant GM68418, and by the American Lebanese Syrian Association Charities (ALSAC). A.M. is an EMBO Young Investigator and a Fellow of the Italian Foundation for Cancer Research.
References
- Cleveland DW, Mao Y, Sullivan KF (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112: 407–421 [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Howell BJ, Canman JC, Hickey JM, Fang G, Salmon ED (2003) Nuf2 and hec1 are required for retention of the checkpoint proteins mad1 and mad2 to kinetochores. Curr Biol 13: 2103–2109 [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498 [DOI] [PubMed] [Google Scholar]
- Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T, Jackson PK (1999) Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev 13: 2242–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa T, Pendon C, Morris J, Brown W (1999) CENP-C is necessary but not sufficient to induce formation of a functional centromere. EMBO J 18: 4196–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ranea J, Mirey G, Camonis J, Valencia A (2002) p23 and HSP20/α-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett 529: 162. [DOI] [PubMed] [Google Scholar]
- Goshima G, Kiyomitsu T, Yoda K, Yanagida M (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J Cell Biol 160: 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM (2002) Plant defence: a new weapon in the arsenal. Curr Biol 12: R352–R354 [DOI] [PubMed] [Google Scholar]
- Gstaiger M, Marti A, Krek W (1999) Association of human SCF(SKP2) subunit p19(SKP1) with interphase centrosomes and mitotic spindle poles. Exp Cell Res 247: 554–562 [DOI] [PubMed] [Google Scholar]
- Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KH (2000) Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci USA 97: 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, Dangl JL (2003) Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J 22: 5679–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KB, Hyman AA, Sorger PK (1997) Regulating the yeast kinetochore by ubiquitin-dependent degradation and Skp1p-mediated phosphorylation. Cell 91: 491–500 [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Hieter P (2001) Evolutionary conservation between budding yeast and human kinetochores. Nat Rev Mol Cell Biol 2: 678–687 [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P (1999) SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol Cell 4: 21–33 [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Abdulle R, Bansal PK, Cagney G, Fields S, Hieter P (2003) Requirement of Skp1–Bub1 interaction for kinetochore-mediated activation of the spindle checkpoint. Mol Cell 11: 1201–1213 [DOI] [PubMed] [Google Scholar]
- Lee YT, Jacob J, Michowski W, Nowotny M, Kuznicki J, Chazin WJ (2004) Human Sgt1 binds HSP90 through the CS domain and not the TPR domain. J Biol Chem 3: 3. [DOI] [PubMed] [Google Scholar]
- Liu ST, Hittle JC, Jablonski SA, Campbell MS, Yoda K, Yen TJ (2003a) Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat Cell Biol 17: 17. [DOI] [PubMed] [Google Scholar]
- Liu Y, Burchsmith T, Schiff M, Feng S, Dinesh-Kumar SP (2003b) Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J Biol Chem 28: 28. [DOI] [PubMed] [Google Scholar]
- Lu R, Malcuit I, Moffett P, Ruiz MT, Peart J, Wu AJ, Rathjen JP, Bendahmane A, Day L, Baulcombe DC (2003) High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J 22: 5690–5699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lluesma S, Stucke VM, Nigg EA (2002) Role of hec1 in spindle checkpoint signaling and kinetochore recruitment of mad1/mad2. Science 297: 2267–2270 [DOI] [PubMed] [Google Scholar]
- McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ (2001) CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell 12: 2776–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Hardwick KG (2002) The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol 3: 731–741 [DOI] [PubMed] [Google Scholar]
- Niikura Y, Kitagawa K (2003) Identification of a novel splice variant: human SGT1B (SUGT1B). DNA Seq 14: 436–441 [DOI] [PubMed] [Google Scholar]
- Nishihashi A, Haraguchi T, Hiraoka Y, Ikemura T, Regnier V, Dodson H, Earnshaw WC, Fukagawa T (2002) CENP-I is essential for centromere function in vertebrate cells. Dev Cell 2: 463–476 [DOI] [PubMed] [Google Scholar]
- Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC (1995) The centromere: hub of chromosomal activities. Science 270: 1591–1594 [DOI] [PubMed] [Google Scholar]
- Saitoh H, Tomkiel J, Cooke CA, Ratrie H III, Maurer M, Rothfield NF, Earnshaw WC (1992) CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70: 115–125 [DOI] [PubMed] [Google Scholar]
- Sironi L, Melixetian M, Faretta M, Prosperini E, Helin K, Musacchio A (2001) Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J 20: 6371–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmann O, Neidig A, Kocher T, Wilm M, Lechner J (2002) Hsp90 enables Ctf13p/Skp1p to nucleate the budding yeast kinetochore. Proc Natl Acad Sci USA 99: 8585–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Casais C, Ichimura K, Shirasu K (2003) HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc Natl Acad Sci USA 100: 11777–11782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser AA, Ouspenski II, Gregson HC, Starr DA, Yen TJ, Goldberg ML, Yokomori K, Earnshaw WC, Sullivan KF, Brinkley BR (2001) Specification of kinetochore-forming chromatin by the histone H3 variant CENP-A. J Cell Sci 114: 3529–3542 [DOI] [PubMed] [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LS, Cleveland DW (1997) CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91: 357–366 [DOI] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F (2003) More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci 28: 541–547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures