Not long ago, physiologists and biophysicists only half-jokingly referred to chloride channels as 'the dark side'. However, in scarcely more than 15 years, molecular and structural tools, in conjunction with increasingly sophisticated physiological techniques, have established chloride channels as interesting subjects in their own right. We now appreciate that chloride channels have key roles in cell-volume regulation, the stabilization of membrane potential, vesicular acidification and epithelial transport. Furthermore, when things go wrong with chloride channels, physiological chaos ensues. Such disease states offer opportunities to learn about the fundamental properties of these channels, which might lead to the development of better tools, to improved understanding and, ultimately, to possible therapies, as Picollo and colleagues show in this issue of EMBO reports (Picollo et al, 2004).
The CLC family of voltage-gated chloride channels provides several examples of the problems of chloride-channel dysfunction (Jentsch et al, 2002). Both dominant and recessive forms of congenital myotonia are linked to mutations in the gene encoding CLC-1, which is the skeletal muscle chloride channel that normally functions in repolarization. CLC-5 is abundant in the kidney and mutations in this channel underlie Dent's disease, which is characterized by low molecular weight proteinuria, renal calcifications and kidney stones. Mouse knockouts of Clcnk1, which encodes CLC-k1, have diabetes insipidus and dilute urine. Closely related to this channel are human CLC-Ka and -Kb, and mutations in the latter are linked to a form of Bartter's syndrome (Simon et al, 1997). The classical form of this disease is characterized by salt wasting and hypotension owing to the inability of the thick ascending limb (TALH) and the distal convoluted tubule (DCT) to reabsorb sodium chloride actively. Therefore, compounds that selectively block CLC-Kb have attracted interest as potential therapeutic agents for the management of hypertension. Furthermore, although a human form of nephrogenic diabetes insipidus has not, so far, been linked to mutations in the gene encoding CLC-Ka, specific blockers of this channel could be useful for inducing water diuresis to reduce cardiac load, for example, after heart failure.
One severe limitation of studying chloride channels has been the lack of specific pharmacological tools with which to distinguish between two closely related chloride channels, such as CLC-Ka and -Kb. Human CLC-Ka and -Kb have high sequence similarity, as do mouse CLC-K1 and -K2 (∼90 and ∼80% respectively; Kieferle et al, 1994). Interspecies similarities are also high. Clarification of the distinct roles of these channels came from a series of elegant studies from the group of Fumiaki Marumo. Matsumura et al (1999) suggested that CLC-K1 must localize to the thin ascending limb (tAL) of Henle's loop, because Clcnk1−/− mice have nephrogenic diabetes insipidus. An antibody that recognizes both CLC-K1 and -K2 stained only the TALH and DCT of kidney sections from Clcnk1−/− mice, which led Kobayashi et al (2001) to deduce that CLC-K2 normally resides in the TALH and represents the rodent orthologue of CLC-Kb. Therefore, human CLC-Ka and -Kb correspond to rat CLC-K1 and -K2, respectively. Moreover, the biophysical properties of CLC-Ka and -Kb remained elusive until recently, owing to inconsistent functional expression. The identification of an accessory subunit, barttin, which enhances the residency of both ion channels in the plasma membrane (Estévez et al, 2001) finally allowed the resolution of functional CLC-K channel currents with distinct biophysical signatures.
With this finding came another twist. Mutations in barttin are linked to a form of Bartter's syndrome that presents with deafness (Birkenhäger et al, 2001). Both CLC-Ka and -Kb colocalize with barttin in the cochlear cells that secrete a potassium-rich endolymph, and so these proteins determine the excitability of the sensory hair cells. Therefore, in addition to the effect on renal salt absorption, mutations in barttin also disrupt both pathways for chloride movement, limit potassium secretion and interfere with hearing. Defective CLC-Kb causes Bartter's syndrome without deafness, because the CLC-Ka/barttin channels in the ear can compensate. It is clear that the ability of drugs to distinguish between these two similar channels, as well as other CLC channels, is crucial to avoid renal, auditory and other side effects.
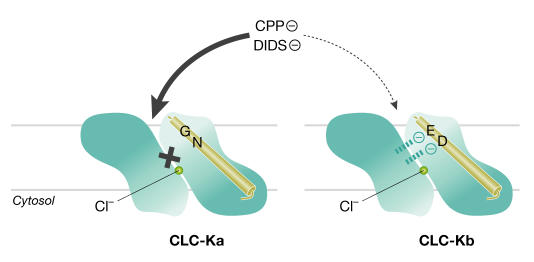
The 2-(p -chlorophenoxy)propionic acid (CPP) derivatives potently inhibit CLC-1 from the intracellular side of the channel (Estévez et al, 2003). Surprisingly, they also block CLC-K1 channels from the extracellular side, with negligible effects on CLC-2 and -5 (Liantonio et al, 2002, 2004). Picollo et al (2004) have now shown that 3-phenyl-CPP, as well as the unrelated stilbene blocker DIDS, inhibits the human CLC-Ka and -Kb channels with differential sensitivities. Like Estévez and colleagues, they used the crystal structure of a bacterial CLC channel (Dutzler et al, 2002) as a map to identify residues that were likely to confer the fivefold higher extracellular sensitivity of CLC-Ka to these drugs (Fig 1). Mutagenesis and subsequent functional expression pinpointed the neutral amino acids N68 and G72 as crucial for the higher inhibition of CLC-Ka by 3-phenyl-CPP and DIDS, respectively. Furthermore, this block was diminished when replaced by the negatively charged D68 and E72, which are found in CLC-Kb. The side groups of these amino acids project into the external mouth of the channel pore, so electrostatic interactions at these points might either permit (CLC-Ka) or impede (CLC-Kb) the interaction of the negatively charged groups of either drug with other binding sites deeper within the pore. If this is indeed the mechanism for extracellular blockage, then it might provide a basis for designing specific inhibitors of other CLC channels.
Figure 1.
Model for CPP and DIDS block of CLC-Ka. The preferential extracellular block of CLC-Ka (left) by 3-phenyl-CPP and the unrelated stilbene blocker DIDS is facilitated by neutral residues within helix B (N68 and G72). Negatively charged residues at the same positions in CLC-Kb (D68 and E72; right) might electrostatically interfere with drug interaction at a deeper site. The yellow circle indicates a chloride ion at the selectivity site. These findings were obtained with the aid of the bacterial CLC-channel crystal structure, which also confirmed earlier work indicating that CLC channels function as dimers of two monomeric pores. For simplicity, this figure shows only one pore unit for each channel.
Clearly, many hurdles must be overcome before these findings can be put to therapeutic use. Circulating levels of drugs presumably would target CLC-Ka at the tAL basolateral membrane. Moreover, there is some evidence for the presence of this channel at the apical membrane. In this case, it is important to note that CPP compounds are secreted by the kidney and so would reach the target. Although the urine levels of 3-phenyl-CPP have yet to be measured in an animal model, such studies would address the questions of whether therapeutic levels are achievable and what unanticipated side effects might result. Also, DIDS blocks non-CLC chloride channels, such as volume-sensitive and calcium-activated chloride channels. The molecular identity of these channels remains obscure, but the possibility that CPP derivatives might also inhibit other channels clearly must be ruled out before therapeutic applications are considered further.
On one level, Picollo and colleagues have given us a tool to study channel-blocking mechanisms; that is, how a perfect fit is achieved in the pore of a channel. In a broader sense, their findings provide a much-needed structural basis for rational drug design, the fruits of which are eagerly awaited.

References
- Birkenhäger R et al. (2001) Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Med 29: 310–314 [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a CLC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415: 287–294 [DOI] [PubMed] [Google Scholar]
- Estévez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl− channel βsubunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 414: 558–561 [DOI] [PubMed] [Google Scholar]
- Estévez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M (2003) Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron 38: 47–59 [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503–568 [DOI] [PubMed] [Google Scholar]
- Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ (1994) Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci USA 91: 6943–6947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F (2001) Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12: 1327–1334 [DOI] [PubMed] [Google Scholar]
- Liantonio A et al. (2002) Molecular requisites for drug binding to muscle CLC-1 and renal CLC-K channel revealed by the use of phenoxy-alkyl derivatives of 2-(p-chlorophenoxy)propionic acid. Mol Pharmacol 62: 265–271 [DOI] [PubMed] [Google Scholar]
- Liantonio A et al. (2004) Investigations of pharmacologic properties of the renal CLC-K1 chloride channel co-expressed with barttin by the use of 2-(p-chlorophenoxy)propionic acid derivatives and other structurally unrelated chloride channels blockers. J Am Soc Nephrol 15: 13–20 [DOI] [PubMed] [Google Scholar]
- Matsumura Y et al. (1999) Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet 21: 95–98 [DOI] [PubMed] [Google Scholar]
- Picollo A, Liantonio A, Didonna MP, Elia L, Conte Camerino D, Pusch M (2004) Molecular determinants of differential pore blocking of kidney CLC-K chloride channels. EMBO Rep 5: 584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D et al. (1997) Mutations in the chloride channel gene CLCNKB, cause Bartter's syndrome type III. Nat Genet 17: 171–178 [DOI] [PubMed] [Google Scholar]