Abstract
The binding and pore formation abilities of Cry1A and Cry1Fa Bacillus thuringiensis toxins were analyzed by using brush border membrane vesicles (BBMV) prepared from sensitive (YDK) and resistant (YHD2) strains of Heliothis virescens. 125I-labeled Cry1Aa, Cry1Ab, and Cry1Ac toxins did not bind to BBMV from the resistant YHD2 strain, while specific binding to sensitive YDK vesicles was observed. Binding assays revealed a reduction in Cry1Fa binding to BBMV from resistant larvae compared to Cry1Fa binding to BBMV from sensitive larvae. In agreement with this reduction in binding, neither Cry1A nor Cry1Fa toxin altered the permeability of membrane vesicles from resistant larvae, as measured by a light-scattering assay. Ligand blotting experiments performed with BBMV and 125I-Cry1Ac did not differentiate sensitive larvae from resistant larvae. Iodination of BBMV surface proteins suggested that putative toxin-binding proteins were exposed on the surface of the BBMV from resistant insects. BBMV protein blots probed with the N-acetylgalactosamine-specific lectin soybean agglutinin (SBA) revealed altered glycosylation of 63- and 68-kDa glycoproteins but not altered glycosylation of known Cry1 toxin-binding proteins in YHD2 BBMV. The F1 progeny of crosses between sensitive and resistant insects were similar to the sensitive strain when they were tested by toxin-binding assays, light-scattering assays, and lectin blotting with SBA. These results are evidence that a dramatic reduction in toxin binding is responsible for the increased resistance and cross-resistance to Cry1 toxins observed in the YHD2 strain of H. virescens and that this trait correlates with altered glycosylation of specific brush border membrane glycoproteins.
Development of resistance by target insect pests is one of the major concerns associated with long-term use of insecticides based on Bacillus thuringiensis δ-endotoxins. These so-called Cry toxins are synthesized during sporulation as proteinaceous crystals (36). Upon ingestion by a sensitive insect, the Cry1 protoxin form (130 to 140 kDa) is solubilized and activated by midgut proteinases to a toxic form (55 to 65 kDa) that binds to specific receptors on the brush border membrane of midgut cells. After binding, toxins oligomerize and insert into the membrane, forming pores that lead to cell lysis and insect death (1).
To date, only Plutella xylostella (diamondback moth) has attained high levels of resistance and cross-resistance to B. thuringiensis insecticides in the field (10), while other insect species have developed resistance after laboratory selection. Although different mechanisms of resistance have been proposed, the best-characterized mechanism is alteration of binding to specific receptors in the midgut (10).
Heliothis virescens (tobacco budworm) has the ability to develop resistance and cross-resistance to B. thuringiensis toxins after selection in the laboratory (31, 15, 16). In this insect, three populations of receptors (receptors A, B, and C) for Cry1 toxins have been proposed (40, 22). According to this model, receptor A binds the Cry1A, Cry1Fa, and Cry1Ja toxins; receptor B binds Cry1Ab and Cry1Ac; and receptor C binds only Cry1Ac.
Notably, the Cry1Ac-selected YHD2 strain developed the highest level of resistance to this toxin reported and cross-resistance to Cry1Aa, Cry1Ab, and Cry1Fa (16). When the properties of binding of Cry1A toxins to brush border membrane vesicles (BBMV) prepared from midguts of resistant YHD2 larvae were analyzed, only Cry1Aa showed reduced binding. Both Cry1Ab and Cry1Ac bound specifically to BBMV from YHD2 with the same parameters with which they bound to BBMV from sensitive YDK larvae (25). No differences in toxin stability or irreversible binding of Cry1Ac were detected between sensitive and resistant larvae in the same study. These results suggested that alteration of binding to the shared Cry1A receptor was the mechanism of resistance against these toxins.
Disruption of a cadherin superfamily gene (HevCadLP) is a mechanism of Cry1A resistance in YHD2 larvae (11). Since cadherin-like proteins bind Cry1A toxins in Manduca sexta and Bombyx mori (23, 33), it is probable that elimination of HevCadLP reduces Cry1A toxin binding. Although hypothetical, the absence of this protein in YHD2 insects would explain the loss of Cry1Aa binding observed by Lee et al. (25). This hypothesis identifies HevCadLP as the shared Cry1A receptor (receptor A) in H. virescens.
The YHD2 strain has been under continuous Cry1Ac selection since the experiments conducted by Lee et al. (25), and the resistance ratio is believed to have increased (Gould, unpublished data). The increased resistance suggests that YHD2 has changed and that an additional mechanism(s) of resistance to Cry1Ac may have developed in this strain, in addition to the lack of full-length HevCadLP expression. In fact, the mechanism reported by Gahan et al. (11) explains only 40 to 80% of the Cry1Ac resistance in YHD2, and other linkage groups contribute to Cry1Ac resistance in this strain (18).
In the present study, bioassays with YDK and YHD2 neonates indicated that resistance to Cry1Ac had increased compared to the resistance described by Lee et al. (25). The goal of this study was to identify additional resistance mechanisms that are currently present in the YHD2 strain. These mechanisms lead to reduced Cry1Ab and Cry1Ac toxin binding and pore formation compared to the results of Lee et al. (25). Reduced toxin binding correlates with altered glycosylation of specific BBMV proteins.
MATERIALS AND METHODS
Insect strains and bioassays.
The origins of H. virescens strains YDK and YHD2, as well as bioassay protocols, have been described previously (16). F1 larvae were obtained after mating YDK and YHD2 adults, as described by Gould et al. (16). For bioassays, five concentrations of each toxin were tested in diet incorporation bioassays performed with neonate larvae for 7 days. Mortality data were analyzed by using the probit procedure in SAS (SAS Institute) with correction for control mortality.
Due to the high levels of resistance of YHD2 larvae to Cry1Ac, 10-day tests of growth of neonates on a toxin-containing diet were also carried out to obtain more accurate resistance ratios for this toxin. Larval weight was log transformed, and a regression of log weight on Cry1Ac toxin concentration was run. The toxin concentration that was necessary to decrease the 10-day larval weight to 10% of the expected weight (when no toxin was present in the diet) was estimated. Resistance ratios were calculated by dividing the concentration of toxin that decreased the growth rate by 10-fold for YHD2 larvae or larvae from the F1 generation of YDK × YHD2 crosses by the estimated concentration that decreased the growth rate by 10-fold for YDK larvae.
Bacterial strains and toxin purification.
Bacterial strains that produce individual toxins and toxin activation and purification procedures have been described by Jurat-Fuentes and Adang (22). Fractions containing pure toxin (as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) were pooled and stored at −80°C until they were used. Protein concentrations were determined by the method of Bradford (4).
Midgut isolation and BBMV preparation.
Midguts were isolated from fifth-instar H. virescens larvae, washed in ice-cold MET buffer (250 mM mannitol, 17 mM Tris-HCl, 5 mM EGTA; pH 7.5), frozen on dry ice, and stored at −80°C.
BBMV were prepared by the MgCl2 precipitation method described by Wolfersberger et al. (43). The final BBMV pellet was suspended in ice-cold TBS buffer (25 mM Tris-HCl [pH 7.5], 3 mM KCl, 100 mM NaCl), and the protein concentration was determined by the method of Bradford (4).
The activity of aminopeptidase, a marker enzyme for lepidopteran brush border membrane preparations (39), was assayed by using leucine p-nitroanilide as the substrate (13). The aminopeptidase activity was five to eight times higher in BBMV preparations than in the initial homogenate (data not shown).
Labeling of Cry1 toxins.
Cry1A toxins were labeled with Na125I by using the chloramine-T method (12). Toxins (1 μg) were labeled with 0.5 mCi of Na125I. The specific activities of 125I-labeled toxins were 20 to 40 mCi/mg (based on input toxin). The purity of 125I-labeled toxins was verified by SDS-PAGE and radiography (data not shown).
Cry1Ac and Cry1Fa were biotinylated with EZ-Link Sulfo-NHS-LC-Biotin (Pierce) as previously described (22).
Radiolabeling (Cry1Aa, Cry1Ab, and Cry1Ac) and biotinylation (Cry1Ac and Cry1Fa) do not alter Cry1 toxin activity (22, 40).
Binding experiments with intact BBMV.
Specific binding assays were performed three times in duplicate as described by Jurat-Fuentes and Adang (22). Radioactivity was measured with a Beckman Gamma 4000 detector. The amount of nonspecific binding was determined by adding the corresponding unlabeled toxin at a concentration of 1 μM to the initial reaction mixtures. Specific binding was determined by calculating the difference between total binding and nonspecific binding.
Qualitative binding assays were performed with 200 μg of BBMV proteins per ml and 0.1 nM 125I-labeled Cry1Ac in 0.1 ml of binding buffer (TBS buffer plus 0.1% bovine serum albumin [BSA]) at room temperature for 1 h. For biotinylated toxins, BBMV were incubated with 12 nM biotinylated Cry1Ac or Cry1Fa. After centrifugation, the pellets were washed twice with 0.5 ml of binding buffer, and the final BBMV pellets were solubilized and electrophoresed in SDS-10% PAGE gels. For 125I-labeled toxins, gels were dried and exposed to film (Kodak XAR-5) for 1 h to detect the presence of bound toxin.
Bound biotinylated toxins were detected by Western blotting. BBMV and bound toxin were electrophoresed and transferred to polyvinylidene difluoride (PVDF) Q membrane filters (Millipore) in transfer buffer (48 mM Tris, 390 mM glycine, 0.1% [wt/vol] SDS, 20% methanol; pH 8.3). After blocking in TBST buffer (25 mM Tris-HCl [pH 7.5], 3 mM KCl, 100 mM NaCl, 0.1% Tween 20) containing 3% BSA, the membranes were incubated with streptavidin-peroxidase conjugate in TBST buffer plus 0.1% BSA for 1 h. Biotinylated toxins were visualized by using ECL reagents (Amersham Pharmacia) as recommended by the manufacturer.
BBMV permeability assay.
BBMV solute permeability was measured by using the data for 450-nm light scattered at a 90o angle from incidence by a BBMV suspension in a stop flow spectrofluorimeter (model RSM 1000; On-line Instrument Systems, Bogart, Ga.).
Freshly prepared BBMV were suspended in 10 mM Tris-HCl (pH 7.5), diluted to a concentration of 0.4 mg/ml in the same buffer, and incubated for 1 h on ice and then for 10 min at room temperature before the light-scattering experiments were started. Baseline measurements were obtained by injecting identical volumes of BBMV and isosmotic buffer (10 mM Tris-HCl, pH 7.5) into the cuvette in the spectrofluorimeter sample compartment. Incident 450-nm light scattered at a 90o angle from incidence was monitored for 60 s by obtaining five measurements per second. To determine the amount of light scattered after osmotically induced shrinkage, measurements were obtained after simultaneous injection of identical volumes of BBMV and a hyperosmotic solution (500 mM KCl, 10 mM Tris-HCl; pH 7.5). In preliminary trials, the response of BBMV from YDK insects to Cry1Ac was dose dependent, while the nontoxic molecule Cry1Ea had no effect on overall BBMV permeability (data not shown). Based on these results, a 100 nM toxin dose (final concentration in cuvette) in hyperosmotic buffer was selected.
Because YHD2 insects are resistant to all toxins used in this study, nystatin, a pore-forming macrolide, served as a positive control. A total of 835 U of nystatin (Sigma) in dimethyl sulfoxide was added to the hyperosmotic buffer. Dimethyl sulfoxide in hyperosmotic buffer had no effect on BBMV permeability (data not shown).
Toxin-induced pore formation in BBMV was monitored by determining the decrease in the amount of light scattered due to reswelling of the vesicles. To quantify the data, rates of swelling of BBMV during a 1-min period after toxin addition were calculated by using the RSM 1000 data-fitting software. Kinetic swelling traces were fitted to a first-order process (Y = A0·e[−k1t]) as a best fit suggested by the robust global fitting program (I. B. C. Matheson; On-line Instrument Systems). The results are expressed below as the calculated rate for each toxin treatment minus the rate observed for the hyperosmotic solution alone. The rates presented below are the means of at least three measurements obtained with independent BBMV preparations and light-scattering experiments.
Ligand blotting.
For ligand blotting, BBMV proteins (15 μg) were separated by SDS-PAGE and electrotransferred onto PVDF filters. Protein-blotted filters were blocked with TBST buffer containing 3% BSA and then incubated with 0.05 nM 125I-Cry1Ac at room temperature for 90 min. After washing, the filters were allowed to dry and then exposed to Kodak XAR-5 film with an intensifying screen at −80°C for 24 to 48 h.
Immunoblotting to detect aminopeptidase N (APN) was performed by using a 1:3,000 dilution of serum developed against the 120-kDa APN protein from M. sexta. Bound antibodies were detected by using anti-rabbit horseradish peroxidase conjugate (Sigma) and were visualized by enhanced chemiluminescence (Santa-Cruz Biotechnology, Santa Cruz, Calif.).
Lectin blotting was conducted by probing BBMV proteins on PVDF filters with 1 μg of peroxidase-labeled soybean agglutinin (SBA) (Sigma) per ml for 1 h. After washing, SBA blots were developed by using an enhanced chemiluminescence substrate (Santa-Cruz Biotechnology).
The intensity of SBA recognition of selected BBMV proteins was quantified by spot densitometry by using AlphaImager software (Alpha Innotech Corporation, San Leandro, Calif.). This program assigns an integrated density value to each protein, and the sum of the signal intensities of all the proteins is defined an integrated density value of 100%. The density values obtained in at least four independent experiments were used in one-way analysis of variance statistical tests with α < 0.050 (SigmaStat statistical software; SPSS Science, Chicago, Ill.) to test for statistically significant differences in SBA recognition between the strains.
Radioiodination of BBMV surface proteins.
BBMV surface proteins were radioiodinated with Na125I by using a lactoperoxidase-catalyzed reaction (20). All reactions were carried out on ice with ice-cold materials. Briefly, 100 μg of BBMV proteins was suspended in 100 μl of phosphate-buffered saline (135 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1.7 mM KH2PO4; pH 7.5) containing protease inhibitors (complete; Pierce). Lactoperoxidase (0.4 mg/ml; from bovine milk; Sigma) was included in each reaction mixture. Na125I was treated with 1 mM sodium sulfite to reduce any I2 (which passes through the cell membrane) to I− (which does not pass through the cell membrane), and then 0.15 mCi was added to each reaction mixture. Reactions were carried out by adding six 10-μl aliquots of a 0.03% hydrogen peroxide solution at 2-min intervals and were stopped by centrifugation. 125I-labeled BBMV pellets were washed four times with ice-cold phosphate-buffered saline and resuspended in 150 μl of the same buffer. Ten microliters of each sample was loaded on an SDS-8% PAGE gel. The gels were dried and exposed to Kodak XAR-5 photographic film with an intensifying screen at −80°C for less than 1 h to detect radiolabeled surface proteins.
RESULTS
Bioassays.
The Cry1A and Cry1Fa toxins were very active against YDK larvae but not against YHD2 larvae (Table 1). Cry1Ea was inactive against both YDK and YHD2 larvae. YHD2 larvae were resistant to Cry1Ac and cross resistant to Cry1Aa, Cry1Ab, and Cry1Fa, as previously reported (16, 25).
TABLE 1.
Toxicities and resistance ratios of Cry1 toxins for sensitive (YDK) and resistant (YHD2) H. virescens neonates
| Toxin | YDK LC50 (μg/ml)a | YHD2 LC50 (μg/ml) | Resistance ratiob |
|---|---|---|---|
| Cry1 Aac | 2.07 (1.06-4.87) | >55d | >25 |
| Cry1 Ab | 7.56 (4.49-19.55) | >900d | >119 |
| Cry1 Ac | 0.93 (0.33-1.39) | >2,000d | >2,000 |
| Cry1 Fa | 3.86 (2.67-5.54) | >130d | >33 |
| Cry1 Ea | >560 | >560 | NAe |
LC50, 50% lethal concentration.
The resistance ratio was determined as follows: 50% lethal concentration for YHD2/50% lethal concentration for YDK.
The units for the Cry1 Aa 50% lethal concentration are micrograms per square centimeter of diet.
No mortality was observed at this concentration of toxin.
NA, not applicable.
No mortality of YHD2 larvae was observed when 2 mg of Cry1Ac toxin per ml was used. Since Lee et al. (25) reported that 0.5 mg/ml is the 50% lethal concentration of this toxin for YHD2, this is evidence that resistance to Cry1Ac increased after further laboratory selection. In our bioassays, we did not observe any mortality at the highest Cry1Aa, Cry1Ab, and Cry1Fa concentrations used, and when the results were compared with the results for YDK larvae, cross-resistance to these toxins was evident.
Although YHD2 mortality was not observed at any toxin concentration tested, the 1,000-μg/ml concentration of Cry1Ac caused decreased growth of the YHD2 larvae. Therefore, to obtain a resistance ratio for this toxin, we performed 10-day growth tests with neonates fed a diet containing Cry1Ac (Table 2). From these growth bioassays, a 73,700-fold ratio for resistance against Cry1Ac was obtained for YHD2 larvae. In these experiments, larvae from the F1 generation of the cross between YDK and YHD2 adults were sixfold resistant to Cry1Ac relative to YDK larvae. Since susceptibility of the F1 larvae was independent of the sex of the resistant progenitor, maternal inheritance and sex linkage of Cry1Ac resistance in YHD2 were considered unimportant.
TABLE 2.
Resistance ratios for Cry1Ac for different strains
| Strain | Intercept | Slope | Cry1Ac concn for 1/10 growtha (μg/ml of diet) | Resistance ratio |
|---|---|---|---|---|
| YDK | 2.570 | −23.55 | 0.042 | 1.0 |
| YDK × YHD2 | 2.797 | −3.436 | 0.291 | 6.8 |
| YHD2 × YDK | 2.776 | −4.707 | 0.212 | 5.0 |
| YHD2 | 2.887 | −0.0003 | 3,125.0 | 73,703 |
Concentration of toxin that decreased the growth rate by 10-fold.
Our bioassays showed that resistance to Cry1Ac had increased in the YHD2 strain when the results were compared to the results of previous studies (25) and that resistance is inherited as an incompletely recessive autosomal trait, as previously demonstrated (16).
Binding assays performed with the sensitive (YDK) and resistant (YHD2) strains.
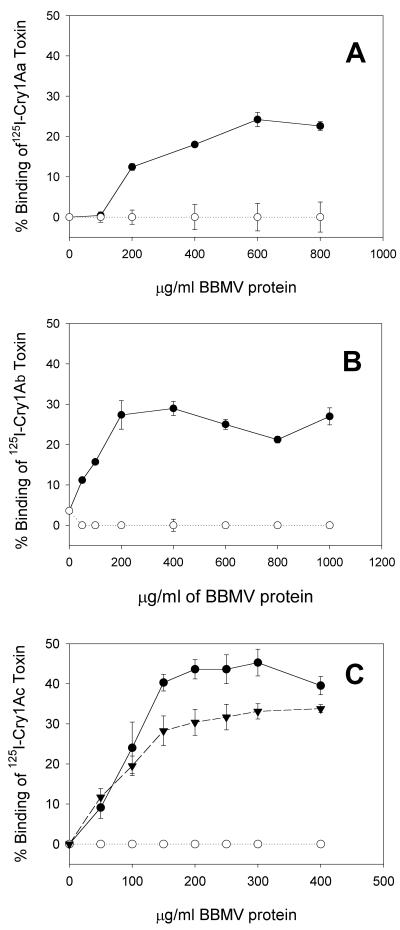
We tested BBMV prepared from YDK and YHD2 larvae for the ability to bind three 125I-labeled Cry1A toxins. Figure 1 shows that all 125I-Cry1A toxins bound specifically to BBMV from sensitive YDK larvae. The maximum specific binding of each 125I-Cry1A toxin to YDK BBMV was in agreement with the data of Lee et al. (25). For BBMV from the resistant YHD2 strain, the lack of 125I-Cry1Aa binding was anticipated based on the results of Lee et al. (25). Unexpectedly, 125I-Cry1Ab and 125I-Cry1Ac did not bind to YHD2 BBMV (Fig. 1). Even at the highest BBMV concentration tested no specific binding to BBMV from YHD2 was observed for any of the 125I-Cry1A toxins tested. This indicated that the YHD2 strain had undergone genetic changes in toxin binding since it was tested by Lee et al. (25).
FIG. 1.
Specific binding of 125I-labeled Cry1Aa (A), Cry1Ab (B), and Cry1Ac (C) toxins to BBMV from YDK (•), YHD2 (○), or F1 (▾) insects. Vesicles at different concentrations were incubated with 125I-Cry1A toxin at a concentration of 0.3 nM (Cry1Aa) or 0.1 nM (Cry1Ab and Cry1Ac) for 1 h. Binding reactions were stopped by centrifugation. The nonspecific binding in the presence of 1,000 nM unlabeled toxin was subtracted from the total binding. The bars indicate the standard errors of the means.
We also measured 125I-Cry1Ac binding to BBMV prepared from larvae of the F1 progeny resulting from mating of sensitive and resistant moths. 125I-Cry1Ac bound specifically to the F1 BBMV, although the extent of specific binding was slightly reduced compared to the extent of specific binding to YDK BBMV (Fig. 1C). 125I-Cry1Ac binding was independent of the sex of the resistant parent, showing that the binding trait is not sex linked (data not shown). Binding data for F1 BBMV suggested that there was recessive inheritance of reduced binding.
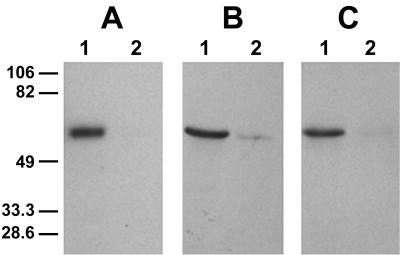
Because Cry1Fa was highly toxic to YDK larvae and because it shares binding sites with Cry1A toxins in H. virescens BBMV (22), we tested the hypothesis that YHD2 larvae that were cross resistant to Cry1Fa had reduced Cry1Fa binding. Since iodination inactivates Cry1Fa (30), we biotinylated Cry1Fa, and binding of this molecule to BBMV was detected by enhanced chemiluminescence. The intensity of the toxin signal (Fig. 2) provided a qualitative measure of binding. As a control, we compared Cry1Ac binding detected by two methods: radiography of bound 125I-Cry1Ac (Fig. 2A) and Western blotting with biotinylated Cry1Ac (Fig. 2B). Decreased toxin binding to YHD2 BBMV was observed by both techniques. Figure 2C shows the results of the biotinylated Cry1Fa binding experiment performed with sensitive and resistant BBMV. Cry1Fa binding to YDK like Cry1Ac binding was observed, and Cry1Fa binding to YHD2 BBMV was undetectable.
FIG. 2.
Binding of 125I-Cry1Ac (A), biotinylated Cry1Ac (B), and biotinylated Cry1Fa (C) toxins to BBMV from YDK (lanes 1) or YHD2 (lanes 2) insects. Toxins were incubated with BBMV proteins (20 μg) for 1 h. Binding reactions were stopped by centrifugation, and washed pellets were separated by SDS-PAGE. Gels were dried and autoradiographed (A) or transferred to PVDF filters. Biotinylated Cry1Ac (B) and Cry1Fa (C) were detected by using streptavidin-peroxidase conjugate and enhanced chemiluminescence.
The results of the toxin-binding experiments demonstrated that increased resistance and cross-resistance in the YHD2 strain correlated with a reduction in toxin binding.
Permeability assays.
The rationale for the permeability assays was as follows. If YHD2 resistance to Cry1 toxins were due to reduced toxin binding, toxin-induced pore formation should be reduced in BBMV from this strain. The light-scattering technique indirectly measures a Cry toxin's capacity to permeate membranes (i.e., form pores) (6). In a hyperosmotic environment, Cry toxin pores allow entry of KCl and water into BBMV, which results in BBMV swelling and decreased scattered light. The rate of BBMV reswelling provides a measure of the rate of membrane permeation by the toxins.
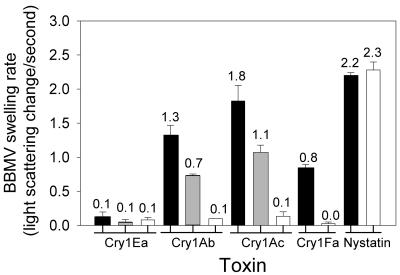
The results of light-scattering experiments performed with the Cry1Ab, Cry1Ac, and Cry1Fa toxins are shown in Fig. 3. Each toxin increased the membrane permeability of YDK BBMV but not the membrane permeability of YHD2 BBMV. Nystatin, a pore-forming antibiotic, permeated both YDK and YHD2 vesicles, confirming that both types of BBMV could respond in the light-scattering assay. The light-scattering experiments supported the conclusion that Cry1Ab, Cry1Ac, and Cry1Fa toxin binding was reduced in BBMV of YHD2 larvae compared with YDK BBMV.
FIG. 3.
Rates (decreases in scattered light per second) of BBMV swelling after challenges with KCl hyperosmotic solutions containing specific Cry1 toxins. YDK (solid bars), F1 (gray bars), and YHD2 (open bars) vesicles were mixed with a hyperosmotic solution containing toxin or nystatin. The rates of swelling after shrinkage as calculated from 1-min measurements are shown along with the standard errors of the means.
Using the light-scattering technique, we measured Cry1-induced membrane permeation in BBMV from the F1 larvae used in the binding experiments (Fig. 3). The rationale for this experiment was that since toxin binding was observed in F1 BBMV, toxin-induced pore formation should also be restored. Cry1Ab- and Cry1Ac-induced rates of swelling in F1 BBMV were intermediate between those in YHD2 and YDK BBMV. These results agreed with the binding data and provided further evidence of the recessive nature of the resistance trait.
Toxin-binding molecules in sensitive and resistant BBMV.
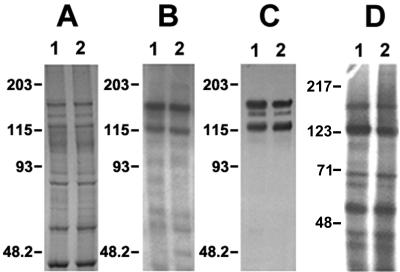
We performed ligand blotting to ascertain if reduced toxin binding was correlated with absence of toxin-binding molecules in the YHD2 vesicles. The patterns of 125I-Cry1Ac toxin-binding molecules in BBMV from YDK and YHD2 appeared to be identical (Fig. 4B). Identical results were obtained whether 125I-Cry1Aa, 125I-Cry1Ab, or biotinylated Cry1Fa was used as the probe (data not shown).
FIG. 4.
Ligand blotting, immunoblotting, and BBMV surface protein radioiodination. For ligand blotting, 20 μg of BBMV proteins from YDK (lane 1) or YHD2 (lane 2) insects was separated by SDS-PAGE and either stained with Coomassie blue (A) or electrotransferred to PVDF filters (B and C). The filters were probed with 0.1 nM 125I-Cry1Ac toxin for 1 h (B) and then washed, dried, and exposed to photographic film for 12 h. Immunoblotting was completed by probing BBMV proteins with serum against the 120-kDa APN from M. sexta (C). For BBMV surface protein iodination (D), 100 μg of BBMV proteins from YDK (lane 1) or YHD2 (lane 2) insects was radiolabeled as described in Materials and Methods.
A specific 170-kDa APN protein from H. virescens BBMV, which has been suggested to represent a shared Cry1A-binding protein, has been shown to catalyze both toxin binding and pore formation (29). Because of this, we studied the possibility that the absence of this toxin-binding molecule would lead to the reduced toxin binding observed in the BBMV binding assays. Immunoblots prepared with BBMV proteins from YDK and YHD2 were probed with serum developed against the 120-kDa APN from M. sexta, which cross-reacts with APNs from H. virescens (2). As shown in Fig. 4C, no detectable changes in either quantity or size of the 170-kDa APN were observed when BBMV from YDK and YHD2 insects were compared.
To test the possibility that the content of BBMV surface proteins was changed in resistant larvae, BBMV surface proteins were radiolabeled by using a lactoperoxidase-catalyzed reaction. As shown in Fig. 4D, no obvious differences in surface-exposed proteins were observed between BBMV from YDK insects and BBMV from YHD2 insects.
Therefore, the absence of toxin-binding molecules did not appear to be the reason for the reduction in toxin binding to BBMV from YHD2.
Protein blots of YHD2 and YDK BBMV probed with SBA.
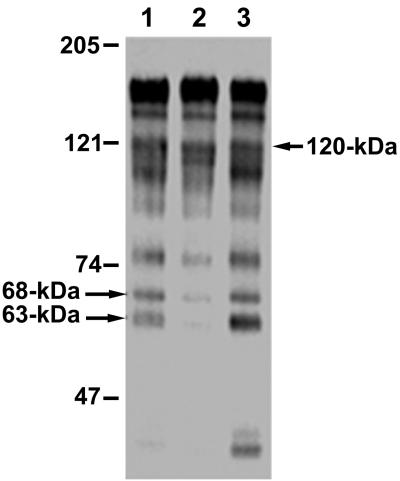
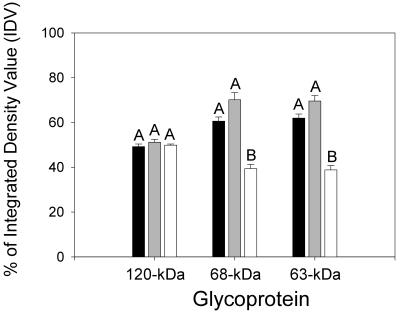
The first step in Cry1Ac recognition of its midgut receptors is dependent on N-acetylgalactosamine (GalNAc) moieties (21). Therefore, we tested for GalNAc residues on BBMV proteins by probing blots with the GalNAc-specific lectin from soybean (SBA). SBA bound to 170- and 120-kDa BBMV proteins, as expected from previous analyses of H. virescens BBMV (24, 26). Several proteins that were smaller than 100 kDa were also detected in our SBA blots (Fig. 5). Interestingly, some of these proteins, especially 68- and 63-kDa proteins, exhibited reduced SBA recognition in BBMV from YHD2. These proteins regained SBA recognition levels observed for YDK vesicles in BBMV from F1 crosses (Fig. 5, lane 3). To quantify the differences, binding of SBA to the 68- and 63-kDa BBMV proteins was quantified by spot densitometry (Fig. 6). One-way analysis of variance statistical tests revealed that SBA recognition of the 68- and 63-kDa proteins was decreased in BBMV from the YHD2 insects compared with the recognition in YDK or F1 vesicles. Densitometry measurements of SBA binding to the 120-kDa APN in the different strains were used as a control. The results suggest that there is a correlation among decreased toxin binding, resistance, and altered glycosylation of the proteins.
FIG. 5.
SBA lectin blots of BBMV from YDK, YHD2, and F1 larvae. BBMV proteins (15 μg) from YDK (lane 1), YHD2 (lane 2), or F1 (lane 3) insects were separated by SDS-PAGE, electrotransferred to PVDF filters, and probed with peroxidase-conjugated SBA to detect GalNAc moieties. Detection was by enhanced chemiluminescence. The arrows indicate proteins selected for spot densitometry analysis.
FIG. 6.
Spot densitometry of SBA binding to 120-, 68- and 63-kDa BBMV proteins from YDK (solid bars), F1 (gray bars), and YHD2 (open bars) larvae. Binding of SBA to protein bands was analyzed by using the AlphaImager software as described in Materials and Methods. Different letters indicate significant differences at the 95% confidence level for the intensity of SBA binding for each protein.
DISCUSSION
We found that the increased resistance in YHD2 larvae is due to reduced Cry1Aa, Cry1Ab, Cry1Ac, and Cry1Fa binding. Using the light-scattering technique, we correlated for the first time decreased binding with the absence of toxin-induced pore formation in BBMV from resistant larvae. Crosses between YDK and YHD2 moths resulted in F1 larvae that had sixfold resistance to Cry1Ac, slightly reduced binding, and a level of pore formation intermediate between the levels of YDK and YHD2 larvae. This resistance inheritance pattern is consistent with previous descriptions of YHD2 resistance. Most of the resistance in the YHD2 strain is autosomal and incompletely recessive, yet it is closer to codominance than to complete recessivity (10, 16).
YHD2 larvae selected for Cry1Ac resistance were highly cross resistant to Cry1Fa (16; this study). The YHD2 larvae had reduced Cry1Fa binding (Fig. 2) and Cry1Fa-induced pore formation (Fig. 3). These results support the model proposed by Tabashnik et al. (38) to explain cross-resistance in diamondback moth larvae. Briefly, this model predicts cross-resistance for Cry1 toxins that share binding sites and have amino acid similarity in the loops of toxin domain II.
Reduced toxin binding could be due to an absence of binding proteins in YHD2 insects. Analysis of Cry1Ac-binding proteins in H. virescens is complicated by the number of binding proteins (8, 14, 22, 25). Nevertheless, in ligand blot experiments we did not detect any changes in 125I-Cry1Ac-binding (Fig. 4), 125I-Cry1Aa-binding, 125I-Cry1Ab-binding, or biotinylated Cry1Fa-binding proteins in BBMV from resistant YHD2 larvae. Only 125I-Cry1Ac ligand blots are described in this paper. 125I-Cry1Ac bound 170-, 120-, and 110-kDa APN molecules plus smaller proteins in BBMV from both YDK and YHD2 insects. Previously published ligand blots of other B. thuringiensis-resistant insect species also show unchanged patterns of Cry toxin-binding proteins (25, 32). Furthermore, radioiodination of BBMV surface proteins did not reveal differences between YDK and YHD2 vesicles.
The failure to detect a 200-kDa binding protein (the predicted size of HevCadLP) on ligand blots deserves mention. Although YHD2 larvae have a disrupted cadherin gene, HevCadLP, YDK larvae should express HevCadLP (11). This protein probably binds Cry1A toxins in susceptible insects, although this has not been experimentally determined. Previously, Jurat-Fuentes and Adang (22) commented that detection of a 200-kDa protein on Cry1A ligand blots was inconsistent. We do not know whether HevCadLP was degraded or was not present in BBMV preparations used in the previous study (22) or in the present study. The ligand blot properties of a cadherin-like protein (BtR175) in B. mori suggest another explanation. While native BtR175 binds Cry1Aa with high affinity, denatured BtR175 exhibits greatly reduced Cry1Aa binding that is barely detected on ligand blots (33).
Carbohydrate moieties are involved in Cry1Ac (24), Cry1Ab5 (9), and Cry1B (19) toxin binding to insect BBMV. For example, Cry1Ac recognizes GalNAc moieties on APN as a first binding step (21), and mutations in Cry1Ac eliminate binding via GalNAc to APN (5, 27). In Caenorhabditis elegans, altered glycosylation mediated by a glycosyltransferase causes resistance and cross-resistance to B. thuringiensis Cry toxins (17). To study if altered glycosylation was related to Cry1Ac resistance in YHD2, lectin blotting with SBA was performed. Although SBA recognizes galactose with low affinity, it is the lectin with the highest affinity for GalNAc moieties (34). SBA lectin blots showed decreased recognition of 68- and 63-kDa brush border proteins that were readily detected in BBMV from sensitive YDK and F1 larvae. Thus, a change in glycosylation of the 68- and 63-kDa proteins is a recessive trait that correlates with reduced toxin binding and resistance. Preliminary experiments performed in our laboratory confirmed that Cry1Ac binds to the carbohydrate moieties recognized by SBA (GalNAc) in these proteins (J. L. Jurat-Fuentes, unpublished data). Also, 68- and 63-kDa proteins containing GalNAc have been found in mosquito midgut microvillae (42).
Domain II of Cry1Aa exhibits structural similarity to the plant lectins jacalin (35) and Maclura pomifera agglutinin (28), among other carbohydrate-binding proteins (5). Both jacalin and M. pomifera lectins bind with high affinity to Galβ(1,3)GalNAc residues, although they also recognize other galactose and galactosamine derivatives, which complicates glycoprotein analyses with these lectins. From our results, we hypothesized that there is a resistance mechanism in YHD2 insects that results in reduced binding of different Cry1 toxins by altering a shared carbohydrate-binding epitope recognized by domain II of these toxins.
Altered glycosylation is known to have important consequences for pathogenesis (3, 7), as well as insect resistance (37). In some cases, modifications of specific carbohydrates mask recognition epitopes used by pathogens (41). In our case, reduced toxin binding and pore formation due to altered glycosylation of toxin-binding moieties might be one of the molecular mechanisms involved in Cry1 resistance in YHD2. Further work aimed at identifying these toxin-binding glycoproteins and the specific alterations that lead to reduced toxin binding is currently being performed in our laboratory.
Acknowledgments
This research was supported in part by the NRI Competitive Grants Program of the U.S. Department of Agriculture.
We thank Juan Ferré for critically reading a draft of the manuscript.
REFERENCES
- 1.Aronson, A. I., C. Geng, and L. Wu. 1999. Aggregation of Bacillus thuringiensis Cry1A toxins upon binding to target insect larval midgut vesicles. Appl. Environ. Microbiol. 65:2503-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, D. J., J. L. Jurat-Fuentes, D. Dean, and M. J. Adang. 2001. Bacillus thuringiensis Cry1Ac and Cry1Fa δ-endotoxin binding to a novel 110-kDa aminopeptidase in Heliothis virescens is not N-acetylgalactosamine mediated. Insect Biochem. Mol. Biol. 31:909-918. [DOI] [PubMed] [Google Scholar]
- 3.Bond, A., A. Alavi, J. S. Axford, B. E. Bourke, F. E. Bruckner, M. A. Kerr, J. D. Maxwell, K. J. Tweed, M. J. Weldon, P. Youinou, and F. C. Hay. 1997. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J. Autoimmun. 10:77-85. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Burton, S. L., D. J. Ellar, J. Li, and D. J. Derbyshire. 1999. N-acetylgalactosamine on the putative insect receptor aminopeptidase N is recognised by a site on the domain III lectin-like fold of a Bacillus thuringiensis insecticidal toxin. J. Mol. Biol. 287:1011-1022. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, J., and D. J. Ellar. 1993. An analysis of Bacillus thuringiensis δ-endotoxin action on insect-midgut-membrane permeability using a light-scattering assay. Eur. J. Biochem. 214:771-778. [DOI] [PubMed] [Google Scholar]
- 7.Chabot, D. J., H. Chen, D. S. Dimitrov, and C. C. Broder. 2000. N-linked glycosylation of CXCR4 masks coreceptor function for CCR5-dependent human immunodeficiency virus type 1 isolates. J. Virol. 74:4404-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowles, E. A., H. Yunovitz, J. F. Charles, and S. S. Gill. 1995. Comparison of toxin overlay and solid-phase binding assays to identify diverse Cry1A(c) toxin-binding proteins in Heliothis virescens midgut. Appl. Environ. Microbiol. 61:2738-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denolf, P., K. Hendrickx, J. Van Damme, S. Jansens, M. Peferoen, D. Degheele, and J. Van Rie. 1997. Cloning and characterization of Manduca sexta and Plutella xylostella midgut aminopeptidase N enzymes related to Bacillus thuringiensis toxin-binding proteins. Eur. J. Biochem. 248:748-761. [DOI] [PubMed] [Google Scholar]
- 10.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 11.Gahan, L. J., F. Gould, and D. G. Heckel. 2001. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293:857-860. [DOI] [PubMed] [Google Scholar]
- 12.Garczynski, S. F., J. W. Crim, and M. J. Adang. 1991. Identification of putative insect brush border membrane-binding molecules specific to Bacillus thuringiensis δ-endotoxin by protein blot analysis. Appl. Environ. Microbiol. 57:2816-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garczynski, S. F., and M. J. Adang. 1995. Bacillus thuringiensis Cry1A(c) delta-endotoxin binding aminopeptidase in the Manduca sexta midgut has a glycosyl-phosphatidylinositol anchor. Insect Biochem. Mol. Biol. 25:409-415. [Google Scholar]
- 14.Gill, S. S., E. A. Cowles, and V. Francis. 1995. Identification, isolation and cloning of a Bacillus thuringiensis Cry1Ac toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J. Biol. Chem. 270:27277-27282. [DOI] [PubMed] [Google Scholar]
- 15.Gould, F., A. Martínez-Ramírez, A. Anderson, J. Ferré, F. J. Silva, and W. J. Moar. 1992. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl. Acad. Sci. USA 89:7986-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould, F., A. Anderson, A. Reynolds, L. Bumgarner, and W. Moar. 1995. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 88:1545-1559. [Google Scholar]
- 17.Griffitts, J. S., J. L. Whitacre, D. F. Stevens, and R. V. Aroian. 2001. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293:860-864. [DOI] [PubMed] [Google Scholar]
- 18.Heckel, D., L. Gahan, F. Gould, and A. Anderson. 1997. Identification of a linkage group with a major effect on resistance to Bacillus thuringiensis Cry1Ac endotoxin in the tobacco budworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 90:75-86. [Google Scholar]
- 19.Hoffmann, C., P. Lüthy, R. Hütter, and V. Pliska. 1988. Binding of the delta endotoxin from Bacillus thuringiensis to brush-border membrane vesicles of the cabbage butterfly (Pieris brassicae). Eur. J. Biochem. 173:85-91. [DOI] [PubMed] [Google Scholar]
- 20.Huber, C. T., and M. Morrison. 1973. Heterogeneity of the outer membrane of mitochondria. Biochemistry 12:4274-4282. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins, J. L., M. K. Lee, A. Valaitis, A. Curtiss, and D. Dean. 2000. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J. Biol. Chem. 275:14423-14431. [DOI] [PubMed] [Google Scholar]
- 22.Jurat-Fuentes, J. L., and M. J. Adang. 2001. Importance of Cry1 δ-endotoxin domain II loops for binding specificity in Heliothis virescens (L.). Appl. Environ. Microbiol. 67:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeton, T. P., and L. A. Bulla, Jr. 1997. Ligand specificity and affinity of BT-R1, the Bacillus thuringiensis Cry1A toxin receptor from Manduca sexta, expressed in mammalian and insect cell cultures. Appl. Environ. Microbiol. 63:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowles, B. H., P. J. K. Knight, and D. J. Ellar. 1991. N-acetylgalactosamine is part of the receptor in insect gut epithelia that recognizes an insecticidal protein from Bacillus thuringiensis. Proc. R. Soc. London 245:31-35. [DOI] [PubMed] [Google Scholar]
- 25.Lee, M. K., F. Rajamohan, F. Gould, and D. H. Dean. 1995. Resistance to Bacillus thuringiensis CryIA δ-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl. Environ. Microbiol. 61:3836-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, M. K., T. H. You, B. A. Young, J. A. Cotrill, A. P. Valaitis, and D. H. Dean. 1996. Aminopeptidase N purified from gypsy moth brush border membrane vesicles is a specific receptor for Bacillus thuringiensis Cry1Ac toxin. Appl. Environ. Microbiol. 62:2845-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, M. K., T. H. You, F. L. Gould, and D. H. Dean. 1999. Identification of residues in domain III of Bacillus thuringiensis Cry1Ac toxin that affect binding and toxicity. Appl. Environ. Microbiol. 65:4513-4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, X., A. Thompson, Z. Zhang, H. Ton-that, J. Biesterfeldt, C. Ogata, L. Xu, R. A. Johnston, and N. M. Young. 1998. Structure of the complex of Maclura pomifera agglutinin and the T-antigen disaccharide, Galβ1,3GalNAc. J. Biol. Chem. 273:6312-6318. [DOI] [PubMed] [Google Scholar]
- 29.Luo, K., S. Sangadala, L. Masson, A. Mazza, R. Brousseau, and M. J. Adang. 1997. The Heliothis virescens 170 kDa aminopeptidase functions as “receptor A” by mediating specific Bacillus thuringiensis δ-endotoxin binding and pore formation. Insect Biochem. Mol. Biol. 27:735-743. [DOI] [PubMed] [Google Scholar]
- 30.Luo, K., D. Banks, and M. J. Adang. 1999. Toxicity, binding, and permeability analyses of four Bacillus thuringiensis Cry1 δ-endotoxins by use of brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl. Environ. Microbiol. 65:457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntosh, S. C., T. B. Stone, R. S. Jokerst, and L. Fuchs. 1991. Binding of Bacillus thuringiensis proteins to a laboratory-selected line of Heliothis virescens. Proc. Natl. Acad. Sci. USA 88:8930-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammed, S. I., D. E. Johnson, and A. I. Aronson. 1996. Altered binding of the Cry1Ac toxin to larval membranes but not to the toxin-binding protein in Plodia interpunctella selected for resistance to different Bacillus thuringiensis isolates. Appl. Environ. Microbiol. 62:4168-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagamatsu, Y., S. Toda, F. Yamaguchi, M. Ogo, M. Kogure, M. Nakamura, Y. Shibata, and T. Katsumoto. 1998. Identification of Bombyx mori midgut receptor for Bacillus thuringiensis insecticidal CryIA(a) toxin. Biosci. Biotechnol. Biochem. 62:718-726. [DOI] [PubMed] [Google Scholar]
- 34.Pereira, M. E. A., E. A. Kabat, and N. Sharon. 1974. Immunochemical studies on the specificity of soybean agglutinin. Carbohydr. Res. 37:89-102. [DOI] [PubMed] [Google Scholar]
- 35.Sankaranarayanan, R., K. Sekar, R. Banerjee, V. Sharma, A. Surolia, and M. Vijayon. 1996. A novel mode of carbohydrate recognition in jacalin, a Moraceae plant lectin with a β-prism fold. Nat. Struct. Biol. 3:596-603. [DOI] [PubMed] [Google Scholar]
- 36.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Small, G. J., and J. Hemingway. 2000. Differential glycosylation produces heterogeneity in elevated esterases associated with insecticide resistance in the brown planthopper Nilaparvata lugens Stål. Insect Biochem. Mol. Biol. 30:443-453. [DOI] [PubMed] [Google Scholar]
- 38.Tabashnik, B. E., T. Malvar, Y. B. Liu, N. Finson, D. Borthakur, B. S. Shin, S. H. Park, L. Masson, R. A. de Maagd, and D. Bosch. 1996. Cross-resistance of the diamondback moth indicates altered interactions with domain II of Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 62:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terra, W. R., and C. Ferreira. 1994. Insect digestive enzymes: properties, compartmentalization and function. Comp. Biochem. Physiol. B Comp. Biochem. 109:1-62. [Google Scholar]
- 40.Van Rie, J., S. Jansens, H. Hofte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis δ-endotoxins: importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 41.Varki, A. 1993. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3:97-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkins, S., and P. F. Billingsley. 2001. Partial characterization of oligosaccharides expressed on midgut microvillar glycoproteins of the mosquito Anopheles stephensi Liston. Insect Biochem. Mol. Biol. 31:937-948. [DOI] [PubMed] [Google Scholar]
- 43.Wolfersberger, M., P. Luethy, A. Maurer, P. Parenti, F. V. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. A Comp. Physiol. 86:301-308. [Google Scholar]