Abstract
Insertion of β-barrel proteins into the outer membrane of mitochondria is mediated by the TOB complex. Known constituents of this complex are Tob55 and Mas37. We identified a novel component, Tob38. It is essential for viability of yeast and the function of the TOB complex. Tob38 is exposed on the surface of the mitochondrial outer membrane. It interacts with Mas37 and Tob55 and is associated with Tob55 even in the absence of Mas37. The Tob38–Tob55 core complex binds precursors of β-barrel proteins and facilitates their insertion into the outer membrane. Depletion of Tob38 results in strongly reduced levels of Tob55 and Mas37 and the residual proteins no longer form a complex. Tob38-depleted mitochondria are deficient in the import of β-barrel precursor proteins, but not of other outer membrane proteins or proteins of other mitochondrial subcompartments. We conclude that Tob38 has a crucial function in the biogenesis of β-barrel proteins of mitochondria.
Keywords: β-barrel proteins, mitochondria, protein insertion, Tob38, TOB complex
Introduction
A distinct group of mitochondrial outer membrane proteins consists of membrane-embedded β-barrel proteins (Gabriel et al, 2001; Rapaport, 2003). The other biological membranes that contain β-barrel proteins are the outer membranes of Gram-negative bacteria and of chloroplasts (Schleiff et al, 2003; Wimley, 2003). Most probably, this reflects the evolutionary origin of mitochondria from an endosymbiont that belonged to the class of Gram-negative bacteria.
The signals that target β-barrel precursor proteins to their subcellular location and the mechanism of their insertion into the membrane are only partly understood (Rapaport, 2003; Johnson & Jensen, 2004). In the case of mitochondria, the precursors are initially recognized by the receptor components of the TOM complex of the mitochondrial outer membrane. They are then translocated through the import pore of the TOM complex (Rapaport & Neupert, 1999; Model et al, 2001; Rapaport, 2002). From the TOM complex, β-barrel precursors are transferred to the TOB/SAM complex (Kozjak et al, 2003; Paschen et al, 2003; Wiedemann et al, 2003). The major component of this complex is Tob55 (also named Sam50/Omp85). Tob55 was found to be essential for viability of yeast cells and to promote the insertion of β-barrel proteins into the mitochondrial outer membrane (Kozjak et al, 2003; Paschen et al, 2003; Gentle et al, 2004). Tob55 has sequence similarity to the highly conserved bacterial protein Omp85, which was proposed to mediate the insertion of β-barrel proteins into the bacterial outer membrane (Voulhoux et al, 2003). Sequence analysis suggested that Tob55 homologues are found in the outer membrane of mitochondria of all eukaryotes (Paschen et al, 2003; Gentle et al, 2004). The other known component of the TOB complex is the outer membrane protein Mas37 (Kozjak et al, 2003; Paschen et al, 2003; Wiedemann et al, 2003; Johnson & Jensen, 2004).
Many questions as to the structure and function of the TOB complex remain to be answered. Here we report on the identification and characterization of a new component of the TOB complex, Tob38, which is crucial for the biogenesis of mitochondrial β-barrel proteins. Together with Tob55, Tob38 forms a functional TOB core complex and is essential for the integrity and function of the TOB complex and thus for viability of yeast cells.
Results
Identification of Tob38
Mitochondria from both a wild-type yeast and a strain carrying a His8 tag at the amino terminus of Tob55 (Tob55His; Paschen et al, 2003) were lysed with digitonin and Ni-NTA beads were added. Proteins retained on the beads selectively with the Tob55His mitochondria were identified by mass spectrometry. In addition to Mas37 and Tob55His, a protein of 38 kDa was found that was termed Tob38. Tob38 is encoded by an essential gene (ORF Yhr083w; Niedenthal et al, 1999) and is predicted to consist of 330 amino-acid residues. Our searches in various databases failed to identify prokaryotic or eukaryotic proteins that have significant sequence similarity to Tob38. It shows a weak sequence resemblance to an uncharacterized protein in the yeast Schizosaccharomyces pombe, which in turn was suggested to be a homologue of metaxin 2, an outer membrane protein of mammalian mitochondria.
A strain in which the chromosomal copy of the TOB38 gene was replaced by a version encoding Tob38 with a Myc tag at the carboxy terminus (Tob38Myc) grew like wild type (Fig 1A). After subcellular fractionation, the Myc-tagged protein was found in the mitochondrial fraction (Fig 1B), as previously suggested by proteomic approaches (Huh et al, 2003; Sickmann et al, 2003).
Figure 1.
Tob38 is present on the surface of mitochondria. (A) Myc-tagged Tob38 is functional. Wild-type cells and cells containing Myc-tagged Tob38 (Tob38Myc) were analysed by drop dilution test for their growth at 30°C on the indicated media. (B) Mitochondrial and post-mitochondrial fractions (M and P, respectively) of wild-type and Tob38Myc cells were subjected to SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting using antibodies directed against the Myc tag (anti-Myc) and marker proteins for the cytosol (anti-hexokinase) and the mitochondrial outer membrane (anti-Tom20). (C,D) Mitochondria isolated from cells containing either C-terminally Myc-tagged Tob38 (C) or N-terminally His-tagged Tob38 (D) were incubated in the absence or presence of proteinase K (+PK). Samples were analysed as above with the indicated antibodies. Anti-His, antibodies against the His tag; AAC (ADP/ATP carrier), integral inner membrane protein. (E) Wild-type mitochondria were subjected to PK treatment and to alkaline extraction (Alk. Ex.) and analysed as above. Aco (aconitase), soluble matrix protein; P and S, pellet and supernatant fraction, respectively; Tom70, outer membrane protein exposed to the cytosol.
Tob38, a peripheral outer membrane protein
To determine the submitochondrial location of Tob38, mitochondria were treated with proteinase K (PK). Both C-terminally Myc-tagged Tob38 and N-terminally His-tagged Tob38 were degraded and could no longer be immunodecorated with antibodies against the respective tag (Fig 1C,D). In addition, when polyclonal antibodies against recombinant Tob38 were used, immunoreactivity was lost after protease treatment of mitochondria (Fig 1E). Thus, the bulk of the protein and both its termini appear to be exposed to the cytosol.
After alkaline treatment of mitochondria, Tob38 fractionated in the supernatant, in contrast to membrane-integrated marker proteins (Fig 1E). When radiolabelled Tob38 precursor was incubated with isolated mitochondria, it became associated with the organelles. Treatment with PK resulted in the formation of several proteolytic fragments. These fragments were not observed when soluble Tob38 precursor was incubated with PK.
In conclusion, Tob38 appears to be a peripheral rather than an integral protein of the outer membrane. Various computer programs failed to predict membrane-anchoring β-sheets and/or helical transmembrane segments.
Tob38 is part of the TOB complex
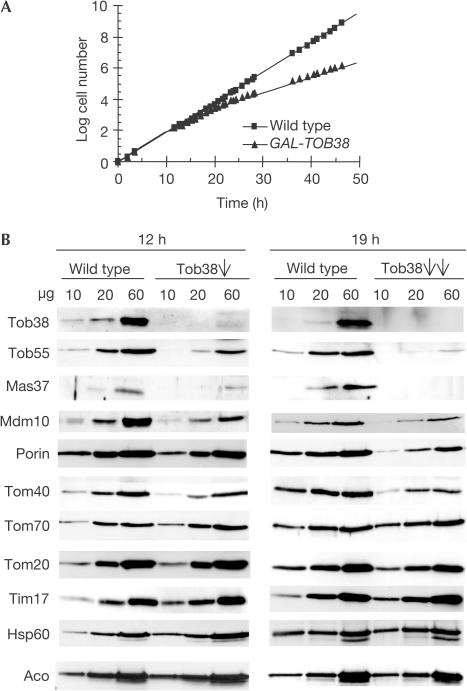
A yeast strain was constructed in which the TOB38 gene was under the control of the GAL10 promoter. In the presence of galactose, these cells grew at a similar rate as wild type. In contrast, in the presence of glucose, growth of the GAL10-TOB38 strain was slowed down after 12 h and was strongly reduced after 19 h (Fig 2A). This incomplete halt of growth agrees well with previous results of a systematic genomic analysis (Niedenthal et al, 1999).
Figure 2.
Tob38 is essential for the biogenesis of β-barrel proteins. (A) Downregulation of Tob38 affects cell growth. Wild-type cells and cells expressing Tob38 under control of the GAL10 promoter (GAL-TOB38) were shifted from galactose-containing medium to glucose-containing medium at time zero. (B) Levels of Tob38, Mas37, Tob55 and of β-barrel proteins are reduced in mitochondria from cells depleted of Tob38 for 12 h (Tob38, single arrow) or 19 h (Tob38, double arrows), as determined by immunodecoration with antibodies against the indicated proteins.
GAL-TOB38 cells as well as isogenic wild-type cells were grown for either 12 or 19 h in the presence of glucose. Tob38 was depleted in mitochondria from the GAL-TOB38 cells grown on glucose (Fig 2B). The levels of a number of other mitochondrial proteins were determined by immunodecoration (Fig 2B). Mas37 and Tob55 were present at strongly reduced levels as early as 12 h after the shift to glucose. Depletion of Tob38 for 19 h resulted in even lower amounts of Mas37 and Tob55 (5–15% of wild-type levels) and in reduced amounts of the β-barrel proteins Tom40, Mdm10 and porin (35–60% of wild-type levels; Fig 2B). In contrast, other proteins of the various mitochondrial subcompartments were present at roughly control levels (82–104% of wild-type levels; Fig 2B).
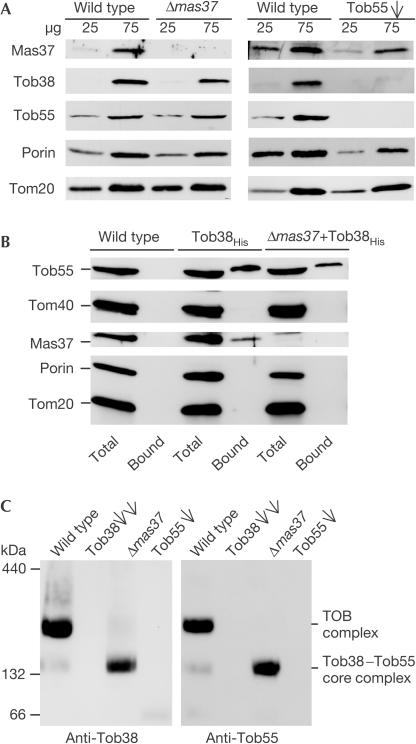
The levels of Tob38 in mitochondria from Δmas37 cells were slightly reduced. Downregulation of Tob55, in contrast, caused a much stronger decrease (Fig 3A). Hence, the levels of Tob38 and Tob55 appear to be co-regulated. Deletion of Mas37, conversely, did not affect the levels of Tob55, and depletion of Tob55 resulted in a moderate reduction in the amount of Mas37.
Figure 3.
Tob38 is a constituent of the TOB complex. (A) Mitochondria isolated from Δmas37 strain, from cells depleted of Tob55 (Tob55, single arrow) and from corresponding wild-type cells were analysed by SDS–PAGE and immunodecoration with antibodies against the indicated proteins. (B) Mitochondria containing either the authentic (wild type) or the His-tagged form of Tob38 (Tob38His) and mitochondria from cells deleted of MAS37 containing Tob38His (Δmas37+Tob38His) were lysed and subjected to Ni-NTA affinity purification. Equal amounts of total and bound fractions were analysed as in (A). (C) Mitochondria isolated from wild-type, Tob38-depleted (Tob38, double arrows), Δmas37 and Tob55-depleted (Tob55, single arrow) cells were lysed with Triton X-100 and analysed by BNGE and immunodecoration with antibodies against Tob38 and Tob55.
Pull-down assays were performed using mitochondria from a strain that lacked wild-type Tob38 and, instead, expressed Tob38 with a His tag at the C terminus (Tob38His). These cells grew like wild-type cells (data not shown), and therefore the protein appears to be fully functional. The two known components of the TOB complex, Tob55 and Mas37, were co-purified with Tob38His. Such interaction was not observed for other outer membrane proteins (Fig 3B and data not shown). About 80% of Tob55 present in the mitochondria was co-isolated with Tob38, but only 15% of Mas37 (average of four experiments). We conclude that Tob38 is part of the TOB complex and tightly interacts with Tob55. It appears that the interaction with Mas37 is more labile.
Does the interaction of Tob38 with Tob55 depend on the presence of Mas37? Pull-down assays were performed with mitochondria isolated from a Δmas37 strain containing Tob38His. Tob38His was co-isolated with Tob55 even in the absence of Mas37 although with reduced efficiency (Fig 3B). Thus, the association of Tob38 with Tob55 is not strictly dependent on the presence of Mas37.
The TOB complex was further analysed by blue native gel electrophoresis (BNGE) and immunodecoration with antibodies against Tob55 and Tob38. After solubilization of wild-type mitochondria with Triton X-100, the complex migrated with an apparent molecular mass of about 200–210 kDa (Fig 3C; Kozjak et al, 2003; Paschen et al, 2003; Wiedemann et al, 2003). A weak band with an apparent molecular mass of approximately 160 kDa containing both Tob55 and Tob38 was also present. A TOB core complex of such smaller molecular mass has been observed previously with Mas37-deficient mitochondria (Paschen et al, 2003; Wiedemann et al, 2003). Thus, the 160 kDa species appears to represent minor amounts of TOB complex in which Mas37 is not present or has dissociated from the TOB holo complex. In fact, the deletion of MAS37 resulted in the absence of the TOB complex and the presence of Tob55 and Tob38 only in the TOB core complex of about 160 kDa (Fig 3C).
In mitochondria depleted of Tob38 or Tob55, neither the TOB complex nor the TOB core complex could be detected (Fig 3C). Thus, both the TOB complex and the TOB core complex contain Tob38 and Tob55. In conclusion, Tob38 is an integral and crucial component of the TOB complex.
Tob38 and membrane insertion of β-barrel proteins
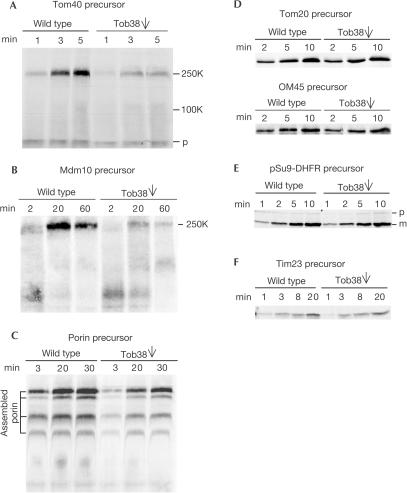
Is Tob38 involved in the import of β-barrel membrane proteins and if so at which stage? Precursors of Tom40 and Mdm10 bind to the TOB complex, and thereby an import intermediate of about 250 kDa is formed (Model et al, 2001; Paschen et al, 2003; Wiedemann et al, 2003). As shown here, in Tob38-depleted mitochondria, the 250 kDa intermediates were present at strongly reduced levels (Fig 4A,B). The import of porin precursor, a β-barrel protein that does not show defined 250K intermediates (Kozjak et al, 2003; Wiedemann et al, 2003; Gentle et al, 2004), was analysed using the formation of assembled complexes as a criterion. This reaction was inhibited in Tob38-depleted cells (Fig 4C). Import of porin, however, was less dependent on Tob38 than import of Tom40 and Mdm10. In contrast to the β-barrel precursors, other proteins of the various mitochondrial subcompartments (Fig 4D–F) were imported into mitochondria from Tob38-depleted cells with similar efficiencies as into mitochondria from wild-type cells.
Figure 4.
Tob38 is required for the insertion and assembly of β-barrel precursor proteins. (A–C) Mitochondria isolated from wild-type and Tob38-depleted cells (Tob38, single arrow) cells were incubated with radiolabelled precursors of Tom40 (A), Mdm10 (B) and porin (C) for various time periods. Mitochondria were re-isolated and analysed by BNGE followed by autoradiography. The 250 and 100 kDa assembly intermediates (250K, 100K) and the precursor form (p) of Tom40, the 250 kDa assembly intermediate of Mdm10 (250K) and the assembled complexes of porin are indicated. (D) Import of radiolabelled Tom20 or OM45 precursors was performed as above. Samples were subjected to alkaline extraction and the pellet fractions were analysed by SDS–PAGE and autoradiography. (E,F) Import of radiolabelled pSu9-DHFR precursor (E) and Tim23 (F) into the indicated mitochondria. Samples were re-isolated, treated with PK and analysed as in (D). p and m, precursor and mature forms of pSu9-DHFR, respectively.
Is Tob38 part of the 250 kDa import intermediate? Mitochondria from a Tob38His strain were incubated with Tom40 precursor under conditions that favour accumulation of the 250 kDa import intermediate. After lysis with digitonin and incubation with Ni-NTA beads, the 250 kDa intermediate was completely removed from the lysate (Fig 5A). We conclude that Tob38 is an integral component of the 250 kDa intermediate complex.
Figure 5.
Tob38 interacts with precursors of β-barrel proteins. (A) Wild-type, Tob38His and Δmas37+Tob38His mitochondria were incubated with radiolabelled Tom40 precursor. Mitochondria were re-isolated, resuspended in a buffer containing 1% digitonin and halved. One-half was analysed directly by BNGE, whereas the other was incubated with Ni-NTA beads. The unbound material was analysed. The precursor form of Tom40 (p) and the Tom40 import intermediates (250K and 250K core) are indicated. (B) The indicated radiolabelled precursors were imported into mitochondria as in (A). After lysis, a portion (total 20%) was removed. The rest was incubated with Ni-NTA beads. These were washed and eluted with SDS-containing buffer (bound). Fractions were analysed by SDS–PAGE and autoradiography.
In mitochondria lacking Mas37, a smaller Tob55-containing import intermediate of Tom40 is formed (Fig 5A, 250K core; Paschen et al, 2003; Wiedemann et al, 2003). This smaller complex was suggested to reflect a TOB core complex that lacks Mas37 (Wiedemann et al, 2003). When a similar assay was performed with Δmas37 mitochondria expressing Tob38His, this 250K core intermediate was completely retained on the Ni-NTA beads (Fig 5A). Thus, Tob38 is a constituent of a TOB core complex built of Tob55 and Tob38.
To demonstrate that Tob38 interacts directly or indirectly with precursors of β-barrel proteins during their import, radiolabelled precursors of Tom40 and Mdm10 were incubated with Tob38His mitochondria. After lysis and incubation with Ni-NTA beads, the precursors were indeed co-isolated with Tob38His in contrast to Tom20 (Fig 5B). Thus, Tob38 interacts with precursors of β-barrel proteins on their import pathway. This was true even in the absence of Mas37 (Fig 5B). Thus, Tob38 is a component of the TOB complex and is required for binding of β-barrel precursors.
Discussion
We have identified Tob38, a new component with an essential role in the biogenesis of mitochondrial β-barrel proteins. Tob38, together with Mas37 and the membrane embedded Tob55, forms the TOB complex in the mitochondrial outer membrane. Tob38 appears to be present at the cytoplasmic surface of the mitochondria as a peripheral membrane protein. It is tightly bound to Tob55 and the levels of Tob38 are strongly reduced in Tob55-depleted cells. Thus, Tob38 is most probably anchored to mitochondria via Tob55.
A recent analysis of global protein expression in yeast provides some hints about the stoichiometry of the various components in the TOB complex. In this study, Tob38 and Mas37 were found to be present at a roughly 1:1 molar ratio (1,470 and 1,580 molecules per cell, respectively), whereas the number of Tob55 molecules per cell could not be determined (Ghaemmaghami et al, 2003). The TOB complex was proposed to contain one molecule of Mas37 (Wiedemann et al, 2003), therefore also one copy of Tob38 may be present per complex.
What are the functions of the three components of the TOB complex? Tob55 is highly conserved in evolution and is able to form a pore in lipid membranes. Therefore, it may mediate the insertion process of β-barrel precursors. The function of Mas37 is not yet resolved. The deletion of the protein is lethal in yeast only at elevated temperatures (Gratzer et al, 1995). Thus, it may have a stabilizing role in the complex rather than a catalytic one.
Tob38, in contrast to Mas37, is essential for the function of the TOB complex. Together with the membrane-embedded Tob55, Tob38 forms an active TOB core complex in the absence of Mas37. Depletion of Tob38 results in strongly reduced levels of Tob55 and Mas37. We did not detect a subcomplex of Mas37 with Tob55 on depletion of Tob38. Hence, Tob38 is required for the stability and assembly of the TOB complex.
The location of Tob38 on the surface of mitochondria raises the possibility of additional functions of the protein. It may promote interactions of the TOB complex with cytosolic chaperones, helping to keep the β-barrel precursors in an import-competent conformation before their interaction with the TOM complex. After their translocation through the TOM complex, β-barrel precursor proteins are transferred to the TOB complex. Tob38 could be involved in mediating a transient association between these two complexes, thereby facilitating the transfer of precursor proteins. Furthermore, as the pore structure formed by Tob55 is probably involved in the membrane insertion of precursor proteins, Tob38 could also function as a regulator of this pore.
A deeper understanding of the function of Tob38 will require a careful dissection of the multistep import pathways of β-barrel membrane proteins.
Methods
Yeast strains and growth conditions
Standard genetic techniques were used for growth and manipulation of yeast strains (Sherman et al, 1986). The wild-type strain YPH499 was used. The GAL10-TOB38 strain was constructed by replacing 105 base pairs upstream of the TOB38 reading frame with a GAL10 promoter-containing cassette in YPH499. For depletion of Tob38, GAL-TOB38 cultures were shifted from lactate medium containing 0.1% galactose to lactate medium containing 2% glucose. Wild-type cultures were grown as controls in the same way. PCR-mediated gene manipulation was used to replace the chromosomal copy of Yhr083w by a gene expressing a Myc or His tag after the coding sequence of Tob38 (Lafontaine & Tollervey, 1996), or to introduce a His tag at the N terminus of the protein.
Biochemical methods
Subcellular and submitochondrial fractionations and in vitro import experiments were performed as described previously (Waizenegger et al, 2003). For pull-down assays, mitochondria were dissolved in lysis buffer (20 mM Tris–HCl, 50 mM NaCl, 10% glycerol, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 mM imidazole, 0.25% digitonin, pH 7.4). After a clarifying spin (20 min, 125,000g), the supernatants were incubated with Ni-NTA beads.
Blue native gel electrophoresis
Mitochondria (50–150 μg) were lysed in 45 μl of detergent-containing buffer (1% digitonin or 0.5% Triton X-100 in 20 mM Tris–HCl, 0.1 mM EDTA, 50 mM NaCl, 10% glycerol, 1 mM PMSF, pH 7.4). After incubation at 4°C for 15 min and a clarifying spin (15 min, 20,600g), 5 μl of sample buffer (5% (w/v) Coomassie brilliant blue G-250, 100 mM Bis-Tris, 500 mM 6-aminocaproic acid, pH 7.0) was added, and the mixture was analysed on a 6–13% gradient blue native gel (Rapaport & Neupert, 1999).
Antibodies against Tob38
The DNA sequence encoding Tob38 was cloned into the pQE-30 vector (Qiagen) for expression of the recombinant protein in Escherichia coli BL-21 cells. The protein was purified on Ni-NTA agarose column. Antibodies against Tob38 were raised in rabbits.
Acknowledgments
We thank P. Heckmeyer and H. Germeroth for technical assistance, Dr J. Regula for the mass spectroscopic analysis of proteins, and Dr M. Yaffe and Dr S. Kohlwein for the antibodies against Mdm10 and Mas37. This work was supported by the Deutsche Forschungsgemeinschaft (D.R.), SFB 594, the Fonds der Chemischen Industrie (W.N.), and predoctoral fellowships from the Boehringer Ingelheim Fonds (T.W. and S.A.P.) and the Minerva Stiftung (S.J.H.).
References
- Gabriel K, Buchanan SK, Lithgow T (2001) The α and β: protein translocation across mitochondrial and plastid outer membranes. Trends Biochem Sci 26: 36–40 [DOI] [PubMed] [Google Scholar]
- Gentle I, Kipros G, Beech P, Waller R, Lithgow T (2004) The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol 164: 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S et al. (2003) Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- Gratzer S et al. (1995) Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol 129: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK et al. (2003) Global analysis of protein localization in budding yeast. Nature 425: 686–691 [DOI] [PubMed] [Google Scholar]
- Johnson AE, Jensen RE (2004) Barreling through the membrane. Nat Struct Mol Biol 11: 113–114 [DOI] [PubMed] [Google Scholar]
- Kozjak V et al. (2003) An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem 278: 48520–48523 [DOI] [PubMed] [Google Scholar]
- Lafontaine D, Tollervey D (1996) Onestep PCR mediated strategy for the construction of conditionally expressed and epitope tagged yeast proteins. Nucleic Acids Res 24: 3469–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model K et al. (2001) Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat Struct Biol 8: 361–370 [DOI] [PubMed] [Google Scholar]
- Niedenthal R, Riles L, Güldener U, Klein S, Johnston M, Hegemann JH (1999) Systematic analysis of S. cerevisiae chromosome VIII genes. Yeast 15: 1775–1796 [DOI] [PubMed] [Google Scholar]
- Paschen SA et al. (2003) Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 426: 862–866 [DOI] [PubMed] [Google Scholar]
- Rapaport D (2002) Biogenesis of the mitochondrial TOM complex. Trends Biochem Sci 26: 191–197 [DOI] [PubMed] [Google Scholar]
- Rapaport D (2003) How to find the right organelle-targeting signals in mitochondrial outer membrane proteins. EMBO Rep 4: 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D, Neupert W (1999) Biogenesis of Tom40, core component of the TOM complex of mitochondria. J Cell Biol 146: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff E et al. (2003) Prediction of the plant β-barrel proteome: a case study of the chloroplast outer envelope. Protein Sci 12: 748–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks J (1986) Methods in Yeast Genetics: A Laboratory Course. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Sickmann A et al. (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc Natl Acad Sci USA 100: 13207–13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J (2003) Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299: 262–265 [DOI] [PubMed] [Google Scholar]
- Waizenegger T, Stan T, Neupert W, Rapaport D (2003) Signal-anchor domains of proteins of the outer membrane of mitochondria: structural and functional characteristics. J Biol Chem 278: 42064–42071 [DOI] [PubMed] [Google Scholar]
- Wiedemann N et al. (2003) Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424: 565–571 [DOI] [PubMed] [Google Scholar]
- Wimley WC (2003) The versatile β-barrel membrane protein. Curr Opin Struct Biol 13: 404–411 [DOI] [PubMed] [Google Scholar]