Abstract
Bone morphogenetic protein (BMP) signals pattern the dorsal neural tube, defining distinct neuronal progenitor cell domains along the dorsoventral axis of the developing spinal cord. These dorsally expressed BMPs appear to have a limited range of action. The mechanisms that regulate this range of action are unclear. We created a GAL4/UAS bigenic mouse system to overexpress BMP4 or a mutant form of BMP4 (mutBMP4), which lacks a subset of amino-terminal basic amino acids that limits its range of action, in the dorsal neural tube. UAS-Bmp4 and UAS-mutBmp4 responder genes were activated in the dorsal neural tube by a Wnt1-GAL4 transgene. Analysis of the spinal cords of bigenic embryos that expressed comparable levels of transgenic transcripts revealed that mutBMP4 acted more ventrally in the neural tube than BMP4. This suggests that the amino-terminal basic amino-acid motif of mature BMP4 controls long-range activity for dorsoventral patterning of the vertebrate neural tube.
Keywords: BMP, neural tube, dorsoventral pattern, transgenic mouse
Introduction
During the development of the vertebrate nervous system, a gradient of sonic hedgehog (SHH) patterns the ventral neural tube and defines ventral neuronal differentiation (Chiang et al, 1996; Ericson et al, 1997; Briscoe et al, 1999). In addition, bone morphogenetic protein (BMP) activity has been shown to regulate dorsal–ventral patterning of the vertebrate neural tube (Barth et al, 1999; Liem et al, 2000; Nguyen et al, 2000). The transforming growth factor-β (TGF-β) family members, BMP2, -4, -6, -7 and growth differentiation factor 7 (GDF7) are expressed in the surface ectoderm and the roof plate of the developing spinal cord, specifying dorsal neuronal fates (Dudley & Robertson, 1997; Lee et al, 1998). These dorsally expressed BMPs appear to have a relatively short range of action, raising questions as to how BMP activity is localized.
Heparin sulphate proteoglycans (HSPGs) present in the extracellular matrix (ECM) can regulate the activities of extracellular ligands either by directly binding to these ligands to enhance the formation of receptor signalling complexes or by sequestering these ligands in the ECM to limit physically their localization (Bernfield et al, 1999; Hartmann & Maurer, 2001). Previous studies have shown that a basic amino-acid-rich amino-terminal sequence preceding the first cysteine of the mature human BMP2 protein is essential for heparin binding in vitro (Ruppert et al, 1996). BMP4 also contains this basic amino-acid-rich N-terminal sequence (Koenig et al, 1994). Animal cap assays also demonstrated that mutation of the basic amino-acid core of BMP4 resulted in a protein that could act farther in distance than wild-type BMP4 (Ohkawara et al, 2002). The in vitro binding affinities of this mutant BMP4 (mutBMP4) for its type I receptor, BMPR-IA, and noggin were unchanged.
We designed a GAL4/UAS bigenic mouse system to compare the in vivo activities of BMP4 and mutBMP4 in the developing spinal cord. The responder loci, UAS-Bmp4 and UAS-mutBmp4, were targeted individually into the Hprt locus. These transgenes were ectopically expressed in the roof plate of mouse embryos using a Wnt1-GAL4 transgene (Rowitch et al, 1999). Our findings suggest that the N-terminal basic amino-acid core of the mature BMP4 is required to restrict its activity for dorsoventral patterning and differentiation of the neural tube.
Results
Introduction of UAS-Bmp4/mutBmp4 into the Hprt locus
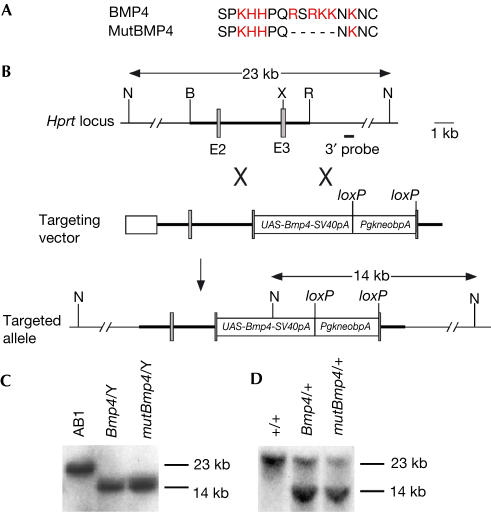
UAS-Bmp4 and UAS-mutBmp4 were introduced into the third exon of the Hprt locus by gene targeting in XY embryonic stem (ES) cells, respectively (Fig 1B). ES cell lines with correct targeting were identified by Southern blot analysis (Fig 1C). Three targeted ES cell clones for each transgene were used to generate male chimaeras for germline transmission, which was confirmed by Southern analysis (Fig 1D). Mice carrying the UAS-Bmp4 or UAS-mutBmp4 alleles were phenotypically normal. All the three lines for each of the two transgenes gave similar results when crossed to Wnt1-GAL4 transactivator mice.
Figure 1.
Generation of UAS-Bmp4 and UAS-mutBmp4 knockin mice. (A) The N-terminal amino-acid sequence preceding the first cysteine of the mature BMP4 protein is rich in basic amino acids (red). The mutant form of BMP4 (mutBMP4) used in this study has an in-frame five-amino-acid deletion. (B) Gene targeting strategy. UAS-Bmp4 (or UAS-mutBmp4) was inserted into exon 3 (E3) of the Hprt locus. The shaded boxes denote exons, and the thick line represents the homology used for gene targeting. B, BamHI; N, NcoI; R, EcoRI; TK, thymidine kinase expression cassette; X, XhoI. (C) Southern blot analysis of NcoI-digested DNA from control (AB1) and UAS-Bmp4 (Bmp4/Y) and UAS-mutBmp4 (mutBmp4/Y) targeted XY ES cell clones. Only one mutant band is detected because the Hprt gene is located on the X chromosome. (D) Southern hybridization of NcoI-digested tail DNA isolated from control (+/+) and agouti-pigmented daughters of male chimaeras (Bmp4/+, UAS-Bmp4 heterozygote; mutBmp4/+, UAS-mutBmp4 heterozygote).
Conditional expression of Bmp4 and mutBmp4
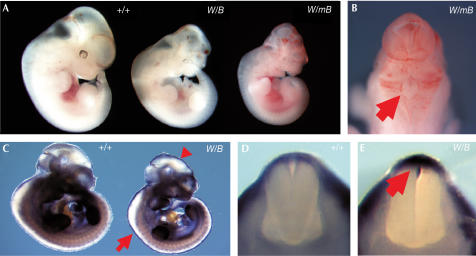
To generate bigenic mouse embryos, male Wnt1-GAL4 transgenic mice were crossed with female UAS-Bmp4 and UAS-mutBmp4 mice, respectively. At 10.5 days post-coitum (dpc), bigenic embryos for both types of transgenes were obtained at the expected mendelian frequencies (data not shown). At 10.5 dpc, approximately 10% of the bigenic embryos could be distinguished morphologically from controls (Table 1). By 11.5 dpc, both types of affected bigenic embryos were growth retarded and displayed a spectrum of phenotypic abnormalities (Fig 2A). Wnt1-GAL4tg/+; UAS-Bmp4tg/+ (abbreviated to Wnt1/Bmp4) bigenic embryos had midbrain exencephaly with blood pools and/or haemorrhage. In addition to these defects, Wnt1-GAL4tg/+; UAS-mutBmp4tg/+ (abbreviated to Wnt1/mutBmp4) bigenic embryos also showed what appeared to be ectopic blood vessel formation and eye abnormalities (Fig 2B). Death of the bigenic embryos was observed at a higher frequency at 11.5 and 12.5 dpc in comparison to 10.5 dpc (Table 1). Therefore, we focused our analysis on 11.0 dpc embryos.
Table 1.
Summary of bigenic embryo dissections
| Stage analysed (dpc) | Genotypea | Total number of bigenic embryos analysed | Number of morphologically abnormal embryos (%) | Number of resorption sites (%) |
|---|---|---|---|---|
| 10.5 | Wnt1/Bmp4 | 102 | 13 (13) | 3 (3) |
| Wnt1/mutBmp4 | 80 | 9 (11) | 4 (5) | |
| 11.5 | Wnt1/Bmp4 | 164 | 13 (8) | 10 (6) |
| Wnt1/mutBmp4 | 141 | 10 (7) | 10 (11) | |
| 12.5 | Wnt1/Bmp4 | 74 | 9 (12) | 7 (9) |
| Wnt1/mutBmp4 | 45 | 3 (7) | 6 (13) |
aWnt1/Bmp4, Wnt1-GAL4tg/+; UAS-Bmp4tg/+. Wnt1/mutBmp4, Wnt1-GAL4tg/+; UAS-mutBmp4tg/+.
Figure 2.
Analysis of Wnt1/Bmp4 and Wnt1/mutBmp4 bigenic embryos. (A) Morphology of 11.5 dpc wild-type (+/+) and bigenic (W/B, Wnt1/Bmp4; W/mB, Wnt1/mutBmp4) embryos. The bigenic embryos develop cranial defects. (B) Dorsal view of the mid- and hindbrain region of a Wnt1/mutBmp4 bigenic embryo. Note the excessive blood vessel formation and incomplete closure of the hindbrain (arrow). (C) Whole-mount in situ hybridization of 10.5 dpc wild-type and Wnt1/Bmp4 bigenic embryos using a Bmp4 riboprobe that recognizes both endogenous and transgenic transcripts. Note the ectopic transgene expression in the dorsal mid- and hindbrain (arrowhead) and the spinal cord (arrow). (D) Transverse section of the spinal cord region of a wild-type embryo showing endogenous Bmp4 expression in the surface ectoderm. (E) Transverse section of the spinal cord region of a Wnt1/Bmp4 embryo showing ectopic Bmp4 expression in the roof plate region (arrow).
The phenotypic diversity observed in the bigenic embryos suggested variations in transgene expression. To determine the spatial expression pattern of the activated UAS transgenes, we performed whole-mount in situ hybridization using a Bmp4 cDNA probe to visualize both transgene expression and endogenous Bmp4 expression. At 10.5 dpc, endogenous Bmp4 is expressed in the surface ectoderm along the spinal cord (Fig 2C,D). In Wnt1/Bmp4 embryos, Bmp4 transcripts were detected in a Wnt1specific pattern superimposed on the endogenous Bmp4 expression pattern. Notably, Bmp4 expression was detected in the surface ectoderm and also the roof plate region (Fig 2E), indicating that Wnt1-GAL4 expression had activated transgene expression in the dorsal neural tube. Among 28 bigenic embryos examined by whole-mount in situ hybridization, only two showed strong ectopic Bmp4 expression in the roof plate region and one showed relatively weaker expression. Thus, the penetrance of transgene activation in this system was only ∼10%. We obtained similar results with Wnt1-Gal4/UAS-lacZ bigenic mice (Rowitch et al, 1999). Only about 10% of these bigenic embryos showed strong lacZ expression along the entire length of the developing spinal cord.
As there was both phenotypic and transgene expression variation, we performed semiquantitative reverse transcription (RT)–PCR to identify bigenic embryos with comparable transgene expression levels. The RT–PCR was optimized for semiquantitative analysis for both Bmp4 and phosphoglycerate kinase 1 (Pgk1). We then selected four bigenic embryos for both Bmp4 and mutBmp4 that expressed the two responder transgenes at approximately the same levels for further analysis (supplementary Fig 1 online).
Dorsoventral patterning of the spinal cord
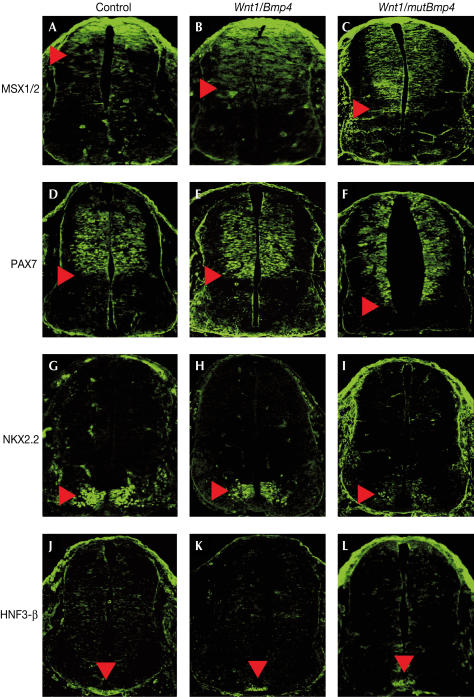
Msh-like 1 homeobox gene (Msx1) and Msx2 are expressed in the roof plate and the mesenchyme lateral to the neural tube (Bendall & Abateshen, 2000). A number of studies have shown that BMP4 signalling can induce Msx gene expression in many tissues and can be considered direct readouts of BMP4 signalling (Lee et al, 1998). In 11.0 dpc wild-type embryos, MSX1/2 was detected in a gradient in the dorsal third of the neural tube (Fig 3A). In Wnt1/Bmp4 bigenic embryos, MSX1/2 expression was detected in the dorsal half of the spinal cord (Fig 3B), indicating an upregulation in BMP4 signalling more ventrally. In Wnt1/mBmp4 bigenic embryos, MSX1/2 expression was detected even more ventrally (Fig 3C). In addition, the signals appeared to be evenly distributed in the dorsal neural tube, unlike the gradient of MSX1/2 expression that was present in both wild-type and Wnt1/Bmp4 embryos. These results suggest that mutBMP4 has a broader activity range relative to wild-type BMP4.
Figure 3.
Dorsoventral patterning of the spinal cords of bigenic embryos. Confocal fluorescent images of immunostained transverse sections of the spinal cords at the level of the forelimbs of 11.0 dpc control (A,D,G,J), Wnt1/Bmp4 (B,E,H,K) and Wnt1/mutBmp4 (C,F,I,L) embryos. (A–C) MSX1/2 immunofluorescence. MSX1/2 is expressed in the dorsal third of the spinal cord (A), with a ventral limit indicated (arrowhead). Relative to controls, MSX1/2 expression in Wnt1/Bmp4 embryos was expanded ventrally (B). In Wnt1/mutBmp4 embryos (C), expression was expanded even more ventrally in comparison with Wnt1/Bmp4 embryos. (D–F) PAX7 immunofluorescence. PAX7 is expressed in neural progenitor cells of the dorsal spinal cord (D). The ventral limit of PAX7 expression (arrowheads) was similar between controls and Wnt1/Bmp4 embryos (D,E). However, PAX7 expression was significantly expanded more ventrally in Wnt1/mutBmp4 embryos (F). (G–I) NKX2.2 immunofluorescence. NKX2.2 is expressed in V3 ventral interneurons of the ventral spinal cord (arrowheads). NKX2.2 immunostaining was similar between controls and Wnt1/Bmp4 bigenic embryos (G,H), but was reduced in Wnt1/mutBmp4 embryos (I). (J–L) HNF3-β immunofluorescence. HNF3-β expression in floor plate cells (arrowheads) was similar between controls and both types of bigenic embryos.
To investigate how dorsoventral patterning was affected by expanded BMP4 signalling, we examined the expression pattern of the dorsoventral neural markers PAX7 and NKX2.2. Pax7 expression is detected in dorsal neuronal progenitor cells after neural tube closure (Hynes et al, 1995). In 11.0 dpc wild-type embryos, PAX7 was detected in the dorsal half of the neural tube (Fig 3D). This pattern was also observed in Wnt1/Bmp4 bigenic embryos (Fig 3E). However, in Wnt1/mutBmp4 bigenic embryos, the PAX7 expression domain was significantly expanded more ventrally (Fig 3F). The ventral limit of the ectopic PAX7 expression domain was greater than the ventral limit of the ectopic MSX1/2 expression domain. In 11.0 dpc wild-type embryos, NKX2.2 expression is detected in V3 ventral interneurons (Fig 3G; Briscoe et al, 1999). In Wnt1/Bmp4 embryos, NKX2.2 expression in the ventral neural tube was similar to wild-type controls (Fig 3H). However, in Wnt1/mutBmp4 embryos, NKX2.2 expression levels were reduced in V3 interneurons (Fig 3I).
We next tested whether floor plate cells were affected in the bigenic embryos. The forkhead transcription factor HNF3-β is a marker of the floor plate (Sasaki & Hogan, 1993). In 11.0 dpc wild-type embryos, HNF3-β is expressed in floor plate cells of the neural tube (Fig 3J), in which the ventralizing signal SHH is generated. HNF3-β expression in both Wnt1/Bmp4 and Wnt1/mutBmp4 bigenic embryos was similar to wild-type controls (Fig 3K,L).
Discussion
The basic amino-acid-rich motif that is present in the mature BMP4 mediates interactions with the ECM and has been implicated in ligand diffusion (Koenig et al, 1994; Ruppert et al, 1996; Ohkawara et al, 2002). To test the hypothesis that this motif restricts the range of BMP action to pattern the dorsoventral axis of the vertebrate neural tube, we have used a GAL4/UAS binary transgenic mouse strategy. Bigenic mice were generated to express comparable levels of wild-type or mutant BMP4 in the developing dorsal neural tube to compare their activities during neural patterning and differentiation. Our analyses confirm that BMP4 can dorsally pattern the spinal cord (Liem et al, 1995). In addition, our data suggest that mutBMP4 can act at a farther distance than wild-type BMP4 in the developing neural tube, dorsally patterning the ventral spinal cord. Our results are consistent with the idea that the conserved basic amino-acid motif mediates the long-range activity (i.e. diffusion) of BMP4 in the developing central nervous system and presumably in other tissues by interactions with the ECM (Lee et al, 1998).
The cross-repressive interactions of class I and class II proteins are essential for defining and refining ventral progenitor cell domains (Briscoe & Ericson, 2001). SHH signalling has been shown to repress PAX7 expression in the ventral neural tube and induce NKX2.2 expression in V3 interneurons (Briscoe et al, 1999). We found that NKX2.2 expression in the ventral neural tube was reduced by the expanded BMP signalling. One of the direct consequences of expanded mutBMP4 activity was the ectopic expression of PAX7 (class I) in the ventral half of the neural tube. As a result, NKX2.2 expression in V3 interneurons was affected. HNF3-β expression in the floor plate was not affected by ectopic BMP4 signalling, indicating that mutBMP4 acts on target cells to modify their response to SHH signalling instead of directly regulating SHH signalling itself. Additional evidence to support this idea comes from the observation that there is ectopic Bmp4 expression in the floor plate cells of noggin-deficient mice, whereas Shh floor plate expression is unaffected (McMahon et al, 1998).
The basic amino-acid motif of BMP2 was initially identified by its ability to bind heparin (Koenig et al, 1994; Ruppert et al, 1996). Subsequent studies have reinforced this idea (Ohkawara et al, 2002). Many growth and differentiation factors that are involved in various developmental processes have heparin-binding sites. The interactions between growth factors and HSPGs have been postulated to be important for growth factor storage and stabilization, and ligand–receptor binding (Hartmann & Maurer, 2001). Our data, together with previous studies (Koenig et al, 1994; Ruppert et al, 1996; Ohkawara et al, 2002), are consistent with the idea that HSPG–BMP interactions may be important for correct dorsoventral patterning of the neural tube by limiting the free diffusion of BMPs. Thus, in tissues rich in HSPG, the range of action for BMPs would be limited, whereas in HSPG-poor regions, BMP would be able to act at a longer distance. If this is true, then Hspg mutations might result in dorsoventral patterning defects in mouse neural development. However, none of the current HSPG-deficient mouse mutants have been reported to have neural phenotypes, perhaps because there are many Hspg genes that could act redundantly (Forsberg & Kjellen, 2001). Alternatively, proteins that could block the heparin-binding activity of the basic amino-acid motif could also potentially regulate BMP diffusion.
Previous studies have shown that Drosophila DPP, the BMP2/4 orthologue, contains a similar basic amino-acid motif, has long-range activity and functions as a morphogen to pattern cell fields in several developmental contexts (Vincent & Briscoe, 2001). Thus, the role of this basic amino-acid core in restricting ligand diffusion may vary in different developmental contexts among different species. In vertebrates, the basic amino-acid motif found in BMP4 is also present in BMP2 and nodal, suggesting that the range of actions of these TGF-β family members is also regulated by HSPG in the ECM. However, this motif is not present in all BMPs. Perhaps during evolution, the N-terminal basic amino-acid core for each distinct BMP family ligand may have been modified in coordination with the distribution of HSPGs in specific tissues at specific times to mediate distinct BMP-dependent developmental processes.
Methods
Generation of UAS-Bmp4 and UAS-mutBmp4 mice. A mouse Bmp4 cDNA was modified to delete a 15 nucleotide (nt) sequence (5′-AGCGGTCCAGGAAGA-3′) from position 901 to 915, encoding the amino acids RSRKK that precede the first cysteine of the mature BMP4 protein (Fig 1A; Ohkawara et al, 2002). This BMP4 variant was termed mutant BMP4 (mutBMP4). UAS-Bmp4sV40pA and UAS-mutBmp4-SV40pA were generated by inserting the Bmp4 and mutBmp4 cDNAs into the plasmid pUAST, respectively (Brand & Perrimon, 1993). To generate the gene targeting vectors, UAS-Bmp4sV40pA (or UAS-mutBmp4-SV40pA) was ligated into the plasmid pPgkneobpA-loxB (Liu et al, 1999). The entire 4.2 kb UAS-Bmp4sV40pA (or UAS-mutBmp4-SV40pA)-loxP-PgkneobpA-loxP cassette was then inserted into the XhoI site of exon 3 of the mouse Hprt targeting vector R1V6.0I OLIGO (Zhang et al, 1994). An MC1tkpA cassette was introduced into the BamHI site of the Hprt targeting vector. The targeting vector was linearized at a unique BamHI site, electroporated into AB1 ES cells, and subsequently cultured in selective medium. Correctly targeted ES cell clones were identified by Southern hybridization using a 3′ external probe (Zhang et al, 1994). Targeted ES cell clones were injected into C57BL/6J (B6) blastocysts to generate male chimaeras. Germline transmission was obtained by crosses with B6 females. Mouse and embryo genotypes were determined by Southern blot.
Semiquantitative RT–PCR. Embryos (11 dpc) were dissected and the trunk below the hindlimb level was isolated for total RNA extraction. cDNA was prepared using reverse transcriptase according to the manufacturer's instructions (Roche). The concentration of all cDNA from different samples was adjusted to 1 ng/μl. PCR was performed using a RoboCycler temperature cycler (Stratagene) to semiquantitate Bmp4 (or mutBmp4) transgene and Pgk1 mRNA expression. Each 50 μl PCR reaction contained 1.5 mM MgCl2, 20 pmol of each primer, 0.25 U (for Bmp4-SV40pA) and 0.15 U (for Pgk1) Taq polymerase, 3 mM (for Bmp4-SV40 pA) and 5 mM (for Pgk1) dNTPs, and 2 ng cDNA. PCR for transgene expression consisted of a 3 min initial denaturation step at 94°C. Thermal cycling consisted of 1 min denaturing at 94°C, 1 min annealing at 54°C and 1 min extension at 72°C for 21 cycles. A final extension period at 72°C was performed. Thermal cycling for Pgk1 consisted of 45 s denaturing at 94°C, 45 s annealing at 52°C and a 1 min extension at 72°C for 18 cycles. A final extension period at 72°C was performed. The primers for the Bmp4-SV40pA transgene were 5′-TGGCCCCATTTCATCAAGT-3′ (forward) and 5′-GATCACCTCAACTCAACCAACC-3′ (reverse). The primers for Pgk1 were 5′-TTCCGAGCCTCACTGTCCAAACTA-3′ (forward) and 5′-AAGCCCATCCAGCCAGCAGGTAT-3′ (reverse). The expected sizes of the PCR products were 443 and 494 base pair (bp), respectively.
In situ hybridization, immunohistochemistry and confocal microscopy. Whole-mount in situ hybridization was performed as described (Wilkinson, 1992). Immunostaining was performed on 20 μm cryosections and imaged by confocal microscopy as described (Wang et al, 2001). The monoclonal antibodies MSX1/2, PAX7, NKX2.2 and HNF3-β were obtained from the National Institute of Child Health and Human Development (NICHD) Developmental Studies Hybridoma Bank (www.uiowa.edu/~dshbwww). The secondary antibody was Alexa 488, conjugated with anti-mouse IgG (Molecular Probes, Oregon). Four wild-type Bmp4 and four mutant Bmp4 bigenic embryos were examined for all the four markers.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v5/n7/extref.7400184s1.pdf).
Supplementary Material
Supplementary Fig. 1. Identification of bigenic embryos with comparable levels of transgene expression by semi-quantitative RT-PCR analysis. The posterior regions of 11 dpc bigenic embryos were analyzed. Primers were designed to amplify transcripts expressed from both types of UAS transgenes. Pgk1 was used as a positive control. Two different bigenic embryo samples for each type of UAS transgene are shown. Each of these bigenic embryos expressed approximately the same levels of Bmp4-SV40pA and mBmp4-SV40pA transcripts. M, 100 bp DNA marker; W/B, Wnt1/Bmp4; W/mB, Wnt1/mBmp4.
Acknowledgments
We thank A. Bradley for the Hprt R1V6.0I OLIGO vector, AB1 ES and SNL 76/7 STO cell lines, J. Deng for assistance with tissue culture, A. McMahon for Wnt1-GAL4 transgenic mice, S. Wang and H. Adams for assistance with confocal microscopy, and C. Cretekos and K.M. Kwan for helpful comments on the manuscript. DNA sequencing and veterinary resources were supported by the National Institutes of Health (NIH) Cancer Center Support Grant. This work was supported by NIH grant AR42919 to R.R.B.
References
- Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW (1999) Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development 126: 4977–4987 [DOI] [PubMed] [Google Scholar]
- Bendall AJ, Abateshen C (2000) Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 247: 17–31 [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M (1999) Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777 [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J (2001) Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol 11: 43–49 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J (1999) Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398: 622–627 [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383: 407–413 [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ (1997) Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208: 349–362 [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, Van Heyningen V, Jessell TM, Briscoe J (1997) Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90: 169–180 [DOI] [PubMed] [Google Scholar]
- Forsberg E, Kjellen L (2001) Heparan sulfate: lessons from knockout mice. J Clin Invest 108: 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann U, Maurer P (2001) Proteoglycans in the nervous system—the quest for functional roles in vivo. Matrix Biol 20: 23–35 [DOI] [PubMed] [Google Scholar]
- Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A (1995) Control of neuronal diversity by the floor plate: contact-mediated induction of midbrain dopaminergic neurons. Cell 80: 95–101 [DOI] [PubMed] [Google Scholar]
- Koenig BB, Cook JS, Wolsing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Ventura F, Grant RA (1994) Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol 14: 5961–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM (1998) Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev 12: 3394–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Roelink H, Jessell TM (1995) Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82: 969–979 [DOI] [PubMed] [Google Scholar]
- Liem KF Jr, Jessell TM, Briscoe J (2000) Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development 127: 4855–4866 [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A (1999) Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22: 361–365 [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP (1998) Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12: 1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC (2000) Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development 127: 1209–1220 [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Iemura S, ten Dijke P, Ueno N (2002) Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol 12: 205–209 [DOI] [PubMed] [Google Scholar]
- Rowitch DH, S-Jacques B, Lee SM, Flax JD, Snyder EY, McMahon AP (1999) Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci 19: 8954–8965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert R, Hoffmann E, Sebald W (1996) Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem 237: 295–302 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL (1993) Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development 118: 47–59 [DOI] [PubMed] [Google Scholar]
- Vincent JP, Briscoe J (2001) Morphogens. Curr Biol 11: 851–854 [DOI] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L (2001) Requirement for math5 in the development of retinal ganglion cells. Genes Dev 15: 24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG (1992), in In Situ Hybridization: A Practical Approach, (ed. Wilkinson DG) 75–83. IRL Press: Oxford, UK [Google Scholar]
- Zhang H, Hasty P, Bradley A (1994) Targeting frequency for deletion vectors in embryonic stem cells. Mol Cell Biol 14: 2404–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Identification of bigenic embryos with comparable levels of transgene expression by semi-quantitative RT-PCR analysis. The posterior regions of 11 dpc bigenic embryos were analyzed. Primers were designed to amplify transcripts expressed from both types of UAS transgenes. Pgk1 was used as a positive control. Two different bigenic embryo samples for each type of UAS transgene are shown. Each of these bigenic embryos expressed approximately the same levels of Bmp4-SV40pA and mBmp4-SV40pA transcripts. M, 100 bp DNA marker; W/B, Wnt1/Bmp4; W/mB, Wnt1/mBmp4.