The origins of DNA replication were proposed in the replicon model to be specified genetically by replicator elements that coordinate the initiation of DNA synthesis with gene expression and cell growth. Recent studies have identified DNA sequences in mammalian cells that fulfil the genetic criteria for replicators and are beginning to uncover the sequence requirements for the initiation of DNA replication. Mammalian replicators are composed of non-redundant modules that cooperate to direct initiation to specific chromosomal sites. Conversely, replicators do not show strong sequence similarity, and their ability to initiate replication depends on the chromosomal context and epigenetic factors, as well as their primary sequence. Here, we review the properties of metazoan replicators, and discuss the genetic and epigenetic factors that determine where and when DNA replication is initiated.
Introduction
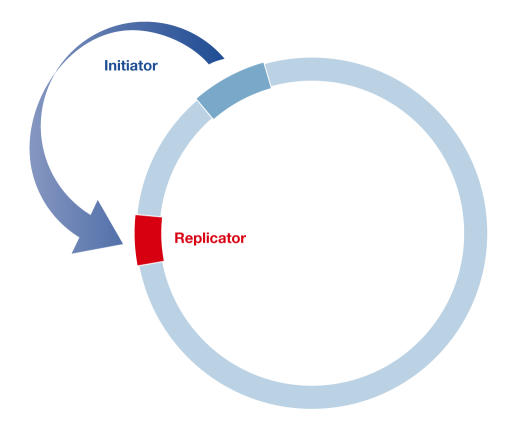
DNA replication is a highly orchestrated process that precisely duplicates the genome once during each cell-division cycle. The replicon model, which was formulated more than four decades ago, laid the foundation for our understanding of the control of DNA replication in the cell cycle (Fig 1; Jacob et al, 1963). Specific sequence elements, termed replicators, were postulated to genetically determine replication-initiation sites on DNA molecules. The interaction of replicators with trans-acting regulatory factors, called initiators, was proposed to activate initiation in response to specific signals from the cell-cycle machinery. Replicators were readily identified in prokaryotes, DNA viruses and lower eukaryotes by their ability to mediate the extrachromosomal replication of plasmids, and initiators were identified on the basis of their ability to bind to replicators. These findings validated the model and revealed the basic mechanisms by which eukaryotic cells regulate DNA replication (Bell & Dutta, 2002).
Figure 1.
The replicon model. A trans-acting protein encoded by the initiator gene was proposed to recognize a cis-acting sequence (the replicator) that controls the initiation of DNA replication in the replicon (Jacob et al, 1963).
In mammalian chromosomes, DNA replication begins at multiple initiation regions (IRs) or origins, with an average spacing of 100 kb (Huberman & Riggs, 1968). About two-dozen metazoan IRs have been physically or biochemically mapped so far (Gilbert, 2001; Bell, 2002; Gerbi et al, 2003). However, until recently, genetic evidence for metazoan replicators that specify initiation sites has been elusive. A handful of metazoan replicators, which are defined by their ability to trigger the initiation of DNA replication in cis when placed at ectopic chromosomal locations, have now been identified (Fig 2). Below, we review the properties of these replicators and discuss the roles of trans-acting factors and chromatin structure in regulating where and when replication is initiated.
Figure 2.
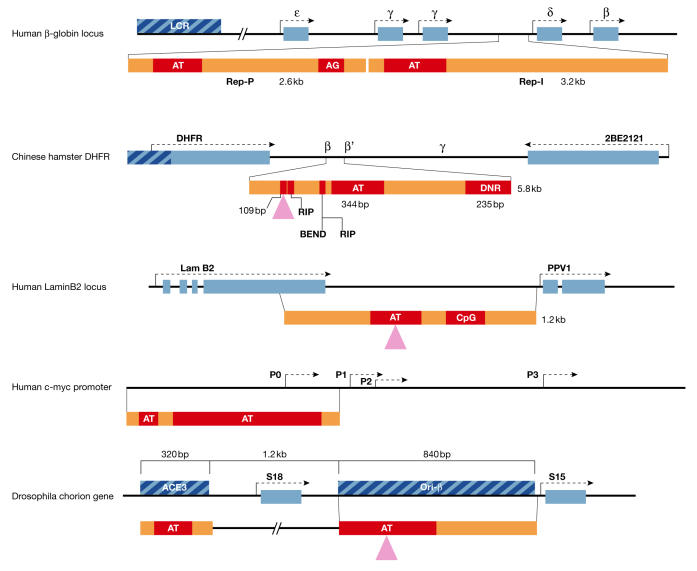
Sequence features of metazoan replicators that are active at ectopic chromosomal sites. The genes surrounding the six replicators in five native loci are shown in blue (not to scale), and the elements required for initiation in the native locus are shown as hatched blue boxes. Mapped initiation regions in each replicator are indicated by purple triangles. In each ectopic replicator (yellow), the sequence elements in red have been shown to be required for full initiation activity. AG, asymmetric purine:pyrimidine regions; AT, AT-rich element; BEND, a sequence-directed bent DNA region; DHFR, dihydrofolate reductase; DNR, dinucleotide repeat; CpG, CpG island; LamB, lamin B; LCR, locus-control region; RIP60, replication-initiation region protein 60 kDa.
Will the real metazoan replicator please stand up?
Because the chromosomal context of a metazoan replicator might influence its ability to initiate replication at ectopic sites (see below), various strategies have been used to overcome position effects and reveal replicator activity. These measures include placing a series of replicator candidates at constant genomic sites (Aladjem et al, 1998; Malott & Leffak, 1999), placing them between chromatin insulators (Lu et al, 2001) and using pools of uncloned stably transfected cells to study replicator activity (Altman & Fanning, 2001; Paixao et al, 2004). The length of DNA shown to have ectopic replicator activity ranges from 1.2 kb (lamin B2) to 5.8 kb (dihydrofolate reductase (DHFR) ori-β).
Mutational analyses of these replicators identified DNA sequence elements that are necessary for replicator activity (Liu et al, 2003; Altman & Fanning, 2004; Paixao et al, 2004; Wang et al, 2004), but none of these crucial elements are able to specify initiation by themselves. For example, the initiation of DNA replication from the Drosophila chorion locus requires two distinct sequences: ori-β which is a segment that overlaps the replication origin and includes two essential regions (140 and 226 base pairs (bp), respectively); and ACE3, a replication enhancer that contains an essential 142-bp sequence (Lu et al, 2001; Zhang & Tower, 2004; Fig 2). The Chinese hamster DHFR ori-β requires at least four elements for the initiation of DNA replication at ectopic loci (Altman & Fanning, 2001, 2004), and an additional 109-bp element cooperates with other sequences to initiate replication at ectopic sites and from the endogenous locus (G. Wahl & J.L. Kolman, personal communication). Similarly, the human c-MYC replicator requires several elements (Liu et al, 2003), and the lamin B2 replicator requires a 290-bp region and is enhanced by a separate element (Paixao et al, 2004). The human β-globin IR contains two non-overlapping independent replicators; initiation within each of these requires at least two distinct sequence elements (Wang et al, 2004). No consensus sequence has emerged from a sequence comparison of essential replicator elements.
These observations suggest that metazoan replicators are composed of several non-redundant sequence-specific modules that cooperate to direct local initiation. This apparently combinatorial organization of metazoan replicators is reminiscent of the control of metazoan transcription: a diverse collection of modules, which are dispersed up to several kilobases from the initiation site, collectively dictate where replication will initiate (Hochheimer & Tjian, 2003; Levine & Tjian, 2003).
Nuts and bolts: how do the replicator modules work?
The absence of a consensus sequence common even to a few metazoan replicators raises the question of how these replicators are recognized for the assembly of pre-replication complexes. In budding yeast, replicators exhibit a consensus binding site for the origin-recognition complex (ORC), which acts as a landing pad for pre-replication proteins. This site is an asymmetric AT-rich region (mostly A on one strand and mostly T on the other). No consensus was identified in fission yeast, but AT-rich stretches also serve as ORC-binding sites (Bell, 2002; Takahashi et al, 2003). Both symmetric (alternating A and T) and asymmetric AT-rich sequences are found in the DHFR ori-β, c-MYC and β-globin replicators (Fig 2). In the DHFR ori-β deletion of a 344-bp AT-rich region, which contains both symmetric and asymmetric stretches, compromises ectopic initiation (Altman & Fanning, 2001). Other AT-rich sequences that are crucial for ectopic ori-β replicator activity include an intrinsically bent DNA that is dictated by five A3–4 stretches spaced at ten-nucleotide intervals, binding sites for the conserved polydactyl zinc-finger protein RIP60 formed by (TTA)4–5 (Altman & Fanning, 2004) and a 109-bp region that cooperates with the other modules to dictate initiation (G. Wahl, personal communication). Similarly, AT-rich regions are necessary for ectopic initiation at the Drosophila chorion gene locus (Lu & Tower, 2001) and at the human c-MYC replicator (Liu et al, 2003). A long stretch of asymmetric AT-rich sequences is required for initiation at the human β-globin Rep-P replicator, but an evolutionarily conserved alternating (A and T) sequence is not (Wang et al, 2004).
Previously, AT-rich sequences were thought to serve primarily as DNA unwinding elements, but emerging evidence indicates a range of functions for these regions. For example, AT-rich sequences in the lamin B2 replicator bind ORC (Abdurashidova et al, 2003; Stefanovic et al, 2003) and can facilitate the loading of the Mcm4/6/7 helicase and the unwinding of duplex DNA in vitro (You et al, 2003). The two AT-rich elements in the chorion replicator (Bell, 2002) and the Sciara puff II/9A origin (Bielinsky et al, 2003) also bind ORC. The DHFR ori-β has two ORC-binding regions in vivo: one in the AT element and one at the start site for replication (A. Patten and E.F., unpublished observations). Interestingly, the ORC-binding region from the lamin B2 replicator can partially or fully substitute for the DHFR AT-rich element, but initiation occurs only at the ori-β start site (Altman & Fanning, 2004). Nevertheless, purified metazoan ORC shows no detectable binding specificity to lamin B2 and DHFR ori-β DNA in vitro (Vashee et al, 2003), which indicates that ORC recruitment to these AT-rich elements in vivo must require additional proteins and/or DNA elements. This idea is confirmed by the finding that ORC binds randomly to mammalian episomes that do not contain specific replicators, with some preference for AT-rich sequences (Schaarschmidt et al, 2004). The affinity of ORC for negatively supercoiled DNA is an order of magnitude greater than its affinity for linear or relaxed DNA, which points to an additional mechanism for ORC selection of DNA-binding sites (Remus et al, 2004).
Architectural roles for intrinsically bent AT-rich DNA can also be envisioned (Fig 2). The AT-hook DNA-binding motifs of fission yeast ORC4, which resemble those of the high mobility group protein HMG-I/Y, could have an architectural role (Bell, 2002). In the Sciara puff II/9A origin, the adjacent bent DNA region could act as a 'histone magnet' to attract histones to form nucleosomes over the bent DNA, leaving the origin free to bind ORC (S. Gerbi, personal communication). AT-rich bent DNA is commonly found in promoter regions and replicators of yeast, in SV40 and in prokaryotes, in which it is thought to facilitate duplex opening. Protein-mediated bending analogous to the HMG-I/Y-mediated DNA bending that facilitates V(D)J recombination, and the assembly and stabilization of transcription complexes at enhancers and promoters in eukaryotes, might also occur (Levine & Tjian, 2003). Replicators also contain binding sites for other proteins, such as RIP60 in DHFR ori-β (Altman & Fanning, 2004), DNA unwinding element-binding protein (DUE-B) and transcription factors in c-MYC (Liu et al, 2003; M. Leffak, personal communication), Hox proteins in lamin B2 (Stefanovic et al, 2003), and a MYB/p120-containing complex in the chorion replicator (Beall et al, 2002). Mutational analysis indicates that these binding sites contribute to replicator function, but their roles are not yet understood.
Asymmetric stretches of purines on one strand and pyrimidines on the other strand are found in several metazoan replicators (Fig 2). A TC/AG-rich dinucleotide-repeat region that can form triplex and Z-DNA in vitro is essential for initiation at the ectopic DHFR ori-β (Altman & Fanning, 2001). AG-rich sequences are present in the regions that are required for initiation activity of the two human β-globin replicators, and one of these asymmetric purine:pyrimidine stretches is essential for initiation (Wang et al, 2004). Similar, but not identical, AG-rich stretches (>75% AG) are present in the human lamin B2 replicator (Stefanovic et al, 2003). Whether the requirement for asymmetric purine:pyrimidine sequences is a universal feature of replicators, and its molecular significance, remain to be discovered.
The potential role of CpG islands in metazoan replicator function also remains poorly understood (Delgado et al, 1998; Rein et al, 1999; Paixao et al, 2004). Methylation of CpG islands is part of an epigenetic regulatory programme that leads to the silencing of specific genes in development and in the aetiology of diseases, such as cancer (Jaenisch & Bird, 2003). CpG methylation inhibits local ORC binding to chromatin and replication in Xenopus egg extracts (Harvey & Newport, 2003). However, CpG methylation in the intergenic initiation zone downstream of the hamster DHFR locus enhances initiation at ori-β (Rein et al, 1999). CpG islands are not essential for initiation in the human β-globin (Wang et al, 2004) and the lamin B2 replicators, but inclusion of a CpG island enhances ectopic initiation from the lamin B2 replicator (Paixao et al, 2004). Further work will be required to understand how CpG elements and their methylation affect metazoan replicator activity.
Flexibility in origin choice
The wide range of elements that cooperate to determine replicator activity in genetic assays shows that many sequence combinations can serve as replicators. Why should the sequence requirements be satisfied in more than one way? Perhaps evolution has favoured a relaxed code for metazoan replicators to promote regulatory versatility. Analysis of single nascent DNA molecules from the Chinese hamster GNAI3 locus provides strong evidence for such flexibility in metazoan origin usage (Anglana et al, 2003). The GNAI3 locus contains a frequently used origin located 0.3-kb downstream of the gene, but it also contains some cryptic less-frequent initiation sites. Increasing the nucleotide pool led to a higher frequency of initiation from the primary origin, whereas reducing the nucleotide pool slowed replication forks and increased initiation at the secondary sites. The cell can therefore sense the rate of replication-fork progression and compensate by activating dormant origins in regions that otherwise would be replicated passively by forks originating at the primary origin. This versatility might also come into play when DNA damage stalls replication forks. Therefore, the Jesuit dictum that “many [origins] are called and few are chosen” (DePamphilis, 1993) provides some insurance against incomplete replication in metazoans, as well as in yeast, which could otherwise culminate in genetic instability (Bielinsky, 2003).
Interplay between replication and transcription
The initiation of DNA replication in the human β-globin locus requires a 40-kb region upstream of the globin gene cluster—known as the locus-control region (LCR)—that regulates globin gene expression (Aladjem et al, 1995, 2002, and references therein). Similarly, deletion of the transcriptional promoter at the native hamster DHFR locus prevents initiation in the downstream initiation zone, whereas replacement of the promoter by an inducible Drosophila promoter restores the original pattern of origin usage (Kalejta et al, 1998; Saha et al, 2004). These data strongly indicate that a distal transcriptional control element or transcription itself restricts origin usage in the downstream chromatin. Given that the distal sequences that are needed for initiation in native loci are dispensable for initiation in the ectopic DHFR ori-β or the β-globin replicator (Aladjem et al, 1998; Altman & Fanning, 2001), it seems likely that the requirement for distal elements can be satisfied by a range of sequences that can substitute for one another.
The pattern of replication start sites at a given locus can be programmed by development or tissue differentiation in coordination with changes in transcription. For example, in mitotic cells of Sciara larvae, replication in the II/9A locus initiates in a zone of 7–8 kb. When the II/9A locus undergoes developmentally programmed amplification and increased gene expression in salivary glands, the 3′ boundary of the initiation region contracts, which results in an initiation region of less than 2 kb. RNA polymerase associates with the new 3′ boundary and might help to define it (Lunyak et al, 2002). In another example, replication at the immunoglobulin heavy-chain locus in mouse non-B cells initiates at the extreme boundary of the locus. A unidirectional fork moves more than 500 kb to the 5′ boundary. In developing pro-B and pre-B cells, which are active in transcription and gene rearrangement, replication initiates earlier from latent origins that are not used in non-B cells (Zhou et al, 2002).
Replication through a locus might create a time window between replication-fork passage and nucleosomal packaging, which facilitates changes in higher order chromatin organization that might allow developmental and differentiation programmes to unfold. An example of developmentally programmed gene expression that requires DNA replication for its activation was recently elucidated in the HoxB locus of the mouse and Xenopus (Fisher & Mechali, 2003). Replication-fork progression through the HoxB locus seems to contribute to the collinearity of gene position and expression timing during development.
Global genomic analysis of metazoan replication timing and gene transcription indicates that early-replicating regions generally correlate with expressed genes, whereas late-replicating regions usually correlate with silent genes (Schuebler et al, 2002). Consistent with this correlation, the replication timing of monoallelically expressed immunoreceptor genes, olfactory genes, imprinted genes and X-linked genes in females is asynchronous: it is early for the active allele and late for the silent one (reviewed in Goren & Cedar, 2003). However, the genetic and epigenetic programmes that regulate replication timing remain poorly defined. Early-replicating chromatin is generally associated with acetylated histones, correlating with active gene expression, and experimental targeting of histone acetyltransferase activity to an origin advances its replication timing (Vogelauer et al, 2002). Consistent with this, an expressed transgene inserted into late-replicating mouse heterochromatin became hyperacetylated and advanced the replication timing of the flanking mouse sequences, whereas silencing of the transgene reverted to late replication (Lin et al, 2003). By contrast, histone hyperacetylation did not correlate with early replication at three of the four origins in the chicken β-globin locus, which shows that hyperacetylation is not necessary for early replication in all cells (Prioleau et al, 2003). DNA methylation, which is another epigenetic modification that often correlates with the gene-expression status of chromatin, also does not correlate with the timing of DNA replication (Gribnau et al, 2003). The picture emerging from these and other studies is that replication timing and origin choice are likely to determine, and be determined by, global chromatin remodelling coordinated with gene expression.
Gene expression is regulated in part by insulator elements that define chromatin domains that are looped out and held at their base by insulator-binding proteins anchored to nuclear lamins (Labrador & Corces, 2002). Similarly, 'replication factories' containing active replication enzymes at stationary sites on a nuclear scaffold in metazoan cells spool the template DNA through the factory, extruding the nascent strands into loops that are anchored at their base (reviewed in Frouin et al, 2003; Laskey & Madine, 2003). This higher order chromatin organization is also thought to facilitate the sequential assembly and disassembly of hundreds of replication foci in S-phase mammalian nuclei (Sporbert et al, 2002). The similar spatial organization of gene expression and replication indicates that replicons might exist in chromatin loops that are defined by insulators. Consistent with this idea, Drosophila chorion gene amplification is position-independent when transcriptional insulators flank the locus, but amplification fails when the insulator is inserted between the two elements of the chorion replicator (Lu et al, 2001).
Cell-proliferation control and programmes of gene expression are intimately related to the spatial organization of replicons in the nucleus. Changes in the subnuclear spatial organization of replication foci occur in cells preparing to exit the cell cycle, and coincide with the recruitment of retinoblastoma tumour suppressor protein (RB) and histone deacetylases to the foci (Barbie et al, 2004). Consistent with this, binding of RB to the chorion locus in Drosophila is important in limiting developmentally regulated amplification at this locus (Bosco et al, 2001). Interestingly, RB recruitment to replication origins in human cells occurs in the same temporal order in which the origins are activated and is crucial in preventing endoreduplication after DNA damage in mammalian cells (Avni et al, 2003). These observations indicate that replication in the proper subnuclear location and temporal order might help to direct higher order chromatin structure, and to execute transcriptional programmes in proliferating and differentiating cells.
Conclusion
The replicator elements and initiator proteins proposed in the original replicon model are now recognizable in metazoan cells, but the range of sequence modules in metazoan replicators is much greater than anticipated. How these modules cooperate to recruit pre-replication complexes is a challenging question for future work. Histone modifications, DNA methylation, higher order chromatin organization and subnuclear position seem to regulate replication both temporally and spatially. However, the mechanisms that link replication patterns to programmes for development and differentiation remain to be elucidated.


Acknowledgments
We thank G. Biamonti, M. Botchan, A. Falaschi, S. Gerbi, M. Giacca, J. Hamlin, O. Hyrien, S.-J. Jun, R. Knippers, J. L. Kolman, M. Leffak, M. Mechali, D. Remus, S. Saha, J. Tower, G. Wahl and M. Zannis-Hadjopoulos for sharing unpublished data, and E. M. Warren for help with the figures. We apologize to those whose work could not be cited directly owing to space restrictions. Our research is supported by the National Institutes of Health (NIH) Intramural Program (M.I.A.), NIH grants GM52948 and CA09385, the Army Breast Cancer Program (BC980907), Howard Hughes Medical Institute (52003905), Merck-United Negro College Fund and Vanderbilt University (E.F.).
References
- Abdurashidova G, Danailov B, Ochem A, Triolo G, Djeliova V, Radulescu S, Vindigni A, Riva S, Falaschi A (2003) Localization of proteins bound to a replication origin of human DNA along the cell cycle. EMBO J 22: 4294–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aladjem MI, Groudine M, Brody LL, Dieken ES, Fournier RE, Wahl GM, Epner EM (1995) Participation of the human β-globin locus control region in initiation of DNA replication. Science 270: 815–819 [DOI] [PubMed] [Google Scholar]
- Aladjem MI, Rodewald LW, Kolman JL, Wahl GM (1998) Genetic dissection of a mammalian replicator in the human β-globin locus. Science 281: 1005–1009 [DOI] [PubMed] [Google Scholar]
- Aladjem MI, Rodewald LW, Lin CM, Bowman S, Cimbora DA, Brody LL, Epner EM, Groudine M, Wahl GM (2002) Replication initiation patterns in the β-globin loci of totipotent and differentiated murine cells: evidence for multiple initiation regions. Mol Cell Biol 22: 442–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman AL, Fanning E (2001) The Chinese hamster dihydrofolate reductase replication origin β is active at multiple ectopic chromosomal locations and requires specific DNA sequence elements for activity. Mol Cell Biol 21: 1098–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman AL, Fanning E (2004) Defined sequence modules and an architectural element cooperate to promote initiation at an ectopic mammalian chromosomal replication origin. Mol Cell Biol 24: 4138–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglana M, Apiou F, Bensimon A, Debatisse M (2003) Dynamics of DNA replication in mammalian somatic cells: nucleotide pool modulates origin choice and interorigin spacing. Cell 114: 385–394 [DOI] [PubMed] [Google Scholar]
- Avni D, Yang H, Martelli F, Hofmann F, ElShamy W, Ganesan S, Scully R, Livingston DM (2003) Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Mol Cell 12: 735–746 [DOI] [PubMed] [Google Scholar]
- Barbie DA, Kudlow BA, Frock R, Zhao J, Johson BR, Dyson N, Harlow E, Kennedy BK (2004) Nuclear reorganization of mammalian DNA synthesis prior to cell cycle exit. Mol Cell Biol 24: 595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EL, Manak JR, Zhou S, Bell M, Lipsick JS, Botchan MR (2002) Role for a Drosophila Myb-containing protein complex in sitespecific DNA replication. Nature 420: 833–837 [DOI] [PubMed] [Google Scholar]
- Bell SP (2002) The origin recognition complex: from simple origins to complex functions. Genes Dev 16: 659–672 [DOI] [PubMed] [Google Scholar]
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 [DOI] [PubMed] [Google Scholar]
- Bielinsky AK, Blitzblau H, Beall EL, Ezrokhi M, Smith HS, Botchan MR, Gerbi SA (2001) Origin recognition complex binding to a metazoan replication origin. Curr Biol 11: 1427–1431 [DOI] [PubMed] [Google Scholar]
- Bielinsky AK (2003) Replication origins: why do we need so many? Cell Cycle 2: 307–309 [PubMed] [Google Scholar]
- Bosco G, Du W, Orr-Weaver TL (2001) DNA replication control through interaction of E2F-RB and the origin recognition complex. Nat Cell Biol 3: 289–295 [DOI] [PubMed] [Google Scholar]
- Delgado S, Gomez M, Bird A, Antequera F (1998) Initiation of DNA replication at CpG islands in mammalian chromosomes. EMBO J 17: 2426–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis ML (1993) Origins of DNA replication in metazoan chromosomes. J Biol Chem 268: 1–4 [PubMed] [Google Scholar]
- Fisher D, Mechali M (2003) Vertebrate HoxB gene expression requires DNA replication. EMBO J 22: 3737–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frouin I, Montecucco A, Spadari S, Maga G (2003) DNA replication: a complex matter. EMBO Rep 4: 666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbi SA, Strezoska Z, Waggener JM (2003) Initiation of DNA replication in multicellular eukaryotes. J Struct Biol 140: 17–30 [DOI] [PubMed] [Google Scholar]
- Gilbert DM (2001) Making sense of eukaryotic DNA replication origins. Science 294: 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren A, Cedar H (2003) Replicating by the clock. Nat Rev Mol Cell Biol 4: 25–32 [DOI] [PubMed] [Google Scholar]
- Gribnau J, Hochedlinger K, Hata K, Li E, Jaenisch R (2003) Asynchronous replication timing of imprinted loci is independent of DNA methylation, but consistent with differential subnuclear localization. Genes Dev 17: 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KJ, Newport J (2003) CpG methylation of DNA restricts replication complex assembly in Xenopus egg extracts. Mol Cell Biol 23: 6769–6779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochheimer A, Tjian R (2003) Diversified transcription initiation complexes expand promoter selectivity and tissuespecific gene expression. Genes Dev 17: 1309–1320 [DOI] [PubMed] [Google Scholar]
- Huberman JA, Riggs AD (1968) On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol 32: 327–341 [DOI] [PubMed] [Google Scholar]
- Jacob F, Brenner S, Cuzin F (1963) On the regulation of DNA synthesis in bacteria: the hypothesis of the replicon. Cold Spring Harbor Symp Quant Biol 256: 298–300 [PubMed] [Google Scholar]
- Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33: 245–254 [DOI] [PubMed] [Google Scholar]
- Kalejta RF, Li X, Mesner LD, Dijkwel PA, Lin HB, Hamlin JL (1998) Distal sequences, but not ori-β/OBR-1, are essential for initiation of DNA replication in the Chinese hamster DHFR origin. Mol Cell 2: 797–806 [DOI] [PubMed] [Google Scholar]
- Labrador M, Corces VG (2002) Setting the boundaries of chromatin domains and nuclear organization. Cell 111: 151–154 [DOI] [PubMed] [Google Scholar]
- Laskey RA, Madine MA (2003) A rotary pumping model for helicase function of MCM proteins at a distance from replication forks. EMBO Rep 4: 26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Tjian R (2003) Transcriptional regulation and animal diversity. Nature 424: 147–151 [DOI] [PubMed] [Google Scholar]
- Lin CM, Fu H, Martinovsky M, Bouhassira E, Aladjem MI (2003) Dynamic alterations of replication timing in mammalian cells. Curr Biol 13: 1019–1028 [DOI] [PubMed] [Google Scholar]
- Liu G, Malott M, Leffak M (2003) Multiple functional elements comprise a mammalian chromosomal replicator. Mol Cell Biol 23: 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang HJ, Tower J (2001) Functionally distinct, sequencespecific replicator and origin elements are required for Drosophila chorion gene amplification. Genes Dev 15: 134–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunyak VV, Ezrokhi M, Smith HS, Gerbi SA (2002) Developmental changes in the Sciara II/9A initiation zone for DNA replication. Mol Cell Biol 22: 8426–8437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malott M, Leffak M (1999) Activity of the c-myc replicator at an ectopic chromosomal location. Mol Cell Biol 19: 5685–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixao S et al. (2004) Modular structure of the human lamin B2 replicator. Mol Cell Biol 24: 2958–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioleau MN, Gendron MC, Hyrien O (2003) Replication of the chicken β-globin locus: early-firing origins at the 5′ HS4 insulator and ρ- and βA-globin genes show opposite epigenetic modifications. Mol Cell Biol 23: 3536–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein T, Kobayashi T, Malott M, Leffak M, DePamphilis ML (1999) DNA methylation at mammalian replication origins. J Biol Chem 274: 25792–25800 [DOI] [PubMed] [Google Scholar]
- Remus D, Beall EL, Botchan MR (2004) DNA topology, not DNA sequence, is a critical determinant for Drosophila ORC-DNA binding. EMBO J 23: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Shan Y, Mesner LD, Hamlin JL (2004) The promoter of the Chinese hamster ovary dihydrofolate reductase gene regulates the activity of the local origin and helps define its boundaries. Genes Dev 18: 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt D, Baltin J, Stehle IM, Lipps HJ, Knippers R (2004) An episomal mammalian replicon: sequence-independent binding of the origin recognition complex. EMBO J 23: 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuebler D, Scalzo D, Kooerpberg C, van Steensel B, Delrow J, Groudine M (2002) Genome-wide DNA replication profile for Drosophila melanogaster: a link between transcription and replication timing. Nat Genet 32: 438–442 [DOI] [PubMed] [Google Scholar]
- Sporbert A, Gahl A, Ankershold R, Leonhardt H, Cardoso MC (2002) DNA polymerase clamp shows little turnover at established replication sites but sequential de novo assembly at adjacent origin clusters. Mol Cell 10: 1355–1365 [DOI] [PubMed] [Google Scholar]
- Stefanovic D, Stanojcic S, Vindigni A, Ochem A, Falaschi A. (2003) In vitro protein–DNA interactions at the human lamin B2 replication origin. J Biol Chem 278: 42737–42743 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ohara E, Nishitani H, Masukata H (2003) Multiple ORC binding sites are required for efficient mcm loading and origin firing in fission yeast. EMBO J 22: 964–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashee S, Cvetic C, Lu WY, Simancek P, Kelly TJ, Walter JC (2003) Sequence-independent DNA binding and replication initiation by the human origin recognition complex. Genes Dev 17: 1894–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelauer M, Rubbi L, Lucas I, Brewer BJ, Grunstein M (2002) Histone acetylation regulates the time of replication origin firing. Mol Cell 10: 1223–1233 [DOI] [PubMed] [Google Scholar]
- Wang L, Lin C, Brooks S, Cimbora D, Groudine M, Aladjem MI (2004) The human β-globin replication initiation region consists of two modular independent replicators. Mol Cell Biol 24: 3373–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Ishimi Y, Mizuno T, Sugasawa K, Hanaoka F, Masai H (2003) Thymine-rich singlestranded DNA activates Mcm4/6/7 helicase on Y-fork and bubble-like substrates. EMBO J 22: 6148–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tower J (2004) Sequence requirements for function of the Drosophila chorion gene locus ACE3 replicator and ori-β origin elements. Development 131: 2089–2099 [DOI] [PubMed] [Google Scholar]
- Zhou J, Ashouian N, Delepine M, Matsuda F, Cheillard C, Riblet R, Schildkraut CL, Birshtein BK (2002). The origin of a developmentally regulated Igh replicon is located near the border of regulatory domains for Igh replication and expression. Proc Natl Acad Sci USA 99: 13693–13698 [DOI] [PMC free article] [PubMed] [Google Scholar]