Summary
First in Molecular Medicine Review Series
Keywords: ubiquitin ligase, unfolded protein stress, parkinsonism, dopamine, neurodegeneration
Recessive mutations in the human PARKIN gene are the most common cause of hereditary parkinsonism, which arises from the degeneration of dopaminergic neurons in the substantia nigra. However, the molecular mechanisms by which the loss of parkin causes dopaminergic neurodegeneration are not well understood. Parkin is an enzyme that ubiquitinates several candidate substrate proteins and thereby targets them for proteasomal degradation. Hypothesis-driven searches have led to the discovery of aggregation-prone protein substrates of parkin. Moreover, the enzyme is upregulated when under unfolded protein stress. Thus, loss-of-function mutations of parkin might impair the removal of potentially toxic protein aggregates. However, the limited neuropathological information that is available from parkin-proven patients, as well as the recent knockout of the parkin gene in fruit flies and mice, may indicate a more complex disease mechanism, possibly involving the misfolding of parkin itself or of additional substrates. The risk factors that predispose dopaminergic neurons to degenerate on parkin failure are yet to be identified.
Introduction
Parkinson's disease (PD) is the most common neurodegenerative movement disorder in the elderly and affects approximately 1% of the population who are over 60 years old. PD symptoms include resting tremor, rigor and a generalized reduction of locomotor performance. The loss of dopaminergic neurons that project from the midbrain substantia nigra to the striatum underlies parkinsonian symptoms. Dopamine replacement therapy can ameliorate the locomotor dysfunction in patients for some time, but PD relentlessly proceeds until the patient dies.
The majority of idiopathic PD cases occur sporadically, but in some patients parkinsonism is inherited (Dawson & Dawson, 2003). Several gene loci are associated with familial parkinsonism. Of particular note is αsynuclein (α-SYN), which is not only genetically linked to rare cases of PD (Golbe & Mouradian, 2004), but also comprises the main constituent of Lewy bodies (LBs). These are the hallmark inclusions that form the basis of the neuropathological diagnosis of all PD patients (Goedert, 2001). LBs are often ubiquitin-immunoreactive, and failure of the ubiquitin-proteasome system is implicated in PD (McNaught & Olanow, 2003).
A logical link between parkinsonism and the ubiquitin-proteasome system can be deduced from the finding that the PD gene product parkin has ubiquitin ligase activity (Shimura et al, 2000). The gene that encodes parkin was originally associated with autosomal-recessive juvenile parkinsonism (AR-JP) in Japan (Kitada et al, 1998). A great variety of mutations in the parkin gene have subsequently been found worldwide in families with early-onset parkinsonism. It is estimated that up to 50% of hereditary parkinsonism cases are due to parkin mutations (for a review, see Mata et al, 2004). Although their phenotypes vary, these 'parkin patients' tend to develop parkinsonism at an early age with slow disease progression, and they respond very well to the standard PD drug levodopa, which increases the supply of dopamine.
What are the molecular and cellular consequences of parkin mutations, how do they impinge on dopaminergic neuron viability, and what does this tell us about the pathological mechanisms of sporadic PD? Here, we review recent progress in the investigation of parkin mutations and protein misfolding and of parkin substrates, with special emphasis on the recently developed knockout animal models.
Parkin ubiquitinates a diverse set of substrates
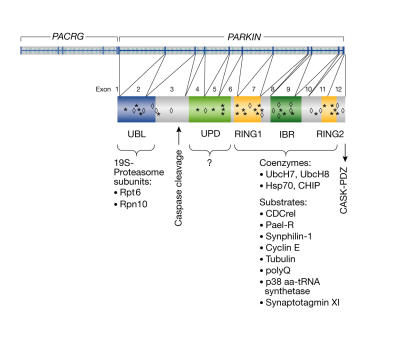
Parkin belongs to the 'really interesting new gene' (RING) finger class of E3 ubiquitin ligases (Shimura et al, 2000). The carboxy-terminal half of parkin comprises a specific arrangement of three zinc-finger domains: two RING fingers flank a domain known as the in-between RING (IBR) domain (Fig 1). This RING–IBR–RING configuration defines a protein superfamily, which includes dorfin and ariadne ubiquitin ligases (Marín & Ferrús, 2002). The extreme amino terminus of parkin is structurally related to ubiquitin (Sakata et al, 2003). This ubiquitin-like (UBL) domain is separated from the unique parkin domain (UPD; Kahle et al, 2000) and the RING–IBR–RING region by a linker (Fig 1) that contains cleavage sites for the pro-apoptotic caspases 1 and 8 (Kahns et al, 2003). The extreme C-terminus of parkin binds to the postsynaptic density 95, discs large, zona occludens (PDZ) motif of the calcium/calmodulin-dependent serine protein kinase, thereby promoting the synaptic transport of parkin (Fallon et al, 2002). All of these structural elements are essential for the functional integrity of parkin, because point mutations cluster in the UBL, UPD, RING–IBR–RING and the C-terminus (Fig 1).
Figure 1.
Genomic and domain arrangement of parkin. Within the human chromosome band 6q23, PARKIN and the parkin co-regulated gene (PACRG) are located in a head-to-head configuration. The 12 exons of PARKIN are spliced and translated into a 457-amino-acid protein. The positions of disease-associated mutations are indicated using asterisks for missense mutations and diamonds for nonsense/frameshift mutations. Functional domains within the parkin protein include an amino-terminal ubiquitin-like (UBL) domain and the cysteine-rich unique parkin domain (UPD) followed by two really interesting new gene (RING) fingers flanking an in-between RING (IBR) domain. The UBL binds to 19S proteasome subunits, the RING–IBR–RING domain binds to specific co-enzymes and substrates. The extreme carboxyl terminus binds to the PDZ motif of calcium/calmodulin-dependent serine protein kinase and targets a proportion of parkin to the postsynaptic density.
The most straightforward mechanism by which the recessive loss of parkin could eliminate dopaminergic neurons would involve some neurotoxic substrate proteins, which would accumulate when there is insufficient parkin for its ubiquitin-proteasome system-dependent degradation (Kahle et al, 2000). Several proteins have been identified as parkin substrates (Fig 1), but the molecular mechanism of dopaminergic neuron loss in AR-JP patients remains obscure.
One of the best-characterized parkin substrates is the parkin-associated endothelin receptor-like receptor (Pael-R), which is a multipass endoplasmic reticulum (ER) transmembrane protein, the function of which is unknown (Imai et al, 2001). Folding of Pael-R is a formidable challenge to cells and is chaperoned by the interaction of the nascent Pael-R polypeptide with the 70-kDa heatshock protein (Hsp70). Overexpression of Pael-R elicits a marked ER stress response (Imai et al, 2001). Parkin is then upregulated (Imai et al, 2000) and directly suppresses Pael-R-mediated toxicity through the ubiquitination and proteasomal degradation of Pael-R (Imai et al, 2001). When Pael-R misfolding exceeds the cellular chaperone capacity, a protein known as C-terminus of Hsp70-interacting protein (CHIP) is upregulated, which sequesters Hsp70 and facilitates parkin-mediated Pael-R ubiquitination (Imai et al, 2002). Thus, parkin contributes to the suppression of ER stress due to proteasomal targeting of the highly aggregative Pael-R protein. It remains to be shown to what extent misfolding of Pael-R contributes to the specific loss of dopaminergic neurons in AR-JP patients. Moreover, it should be established whether Pael-R is a unique parkin substrate, or whether other difficult-to-fold polytopic membrane proteins are subject to parkin-mediated ubiquitination.
Parkin has been generally implicated in the elimination of aggregation-prone cytosolic proteins, including polyglutamine polypeptides (Tsai et al, 2003). Ataxin 3 or synthetic green fluorescent protein fused to a stretch of 79 glutamines confers cytosolic protein-misfolding stress. The cell reacts to this condition with an upregulation of Hsp70, similar to the ER stress mediated by Pael-R. When the polyglutamine expansion of cytosolic proteins exceeds the chaperone capacity of Hsp70, the latter recruits parkin through its interaction with the RING–IBR–RING domain (Fig 1), which ubiquitinates the polyglutamine protein and thereby labels it for proteasomal degradation (Tsai et al, 2003). In fact, proteasomal delivery of polyglutamine proteins and other parkin substrates might be further facilitated by direct binding of the UBL domain of parkin to the regulatory 19S proteasome (Sakata et al, 2003; Tsai et al, 2003).
If parkin functions to ubiquitinate aggregation-prone proteins, α-SYN would be an obvious candidate substrate. Using a particular combination of immunoprecipitating/immunoblotting antibodies, a rather unusual cytosolic O-linked glycosylated α-SYN species (α-Sp22) has been detected. This modification was reported to be a prerequisite for parkin binding, eventually leading to the accumulation of soluble α-Sp22 in AR-JP brains (Shimura et al, 2001). In contrast to all other substrates (Fig 1), αsp22 interacts with the UBL of parkin (Shimura et al, 2001). However, little is known about αsp22, and its existence could not be confirmed by other groups (Giasson & Lee, 2001). It has been suggested that the interaction between parkin and αsYN is bridged by synphilin 1, a protein of unknown function that can bind to parkin and α-SYN individually (Chung et al, 2001; Engelender et al, 1999). Co-transfection of αsYN and synphilin 1 in human embryonic kidney 293 cells leads to the formation of inclusion bodies, which were found to become ubiquitinated in the presence of wild-type, but not AR-JP mutant parkin (Chung et al, 2001). These results support the view that parkin ubiquitinates protein aggregates. However, the impact on cell death was not resolved in these studies.
Parkin substrates that may exert a direct cytotoxic effect on accumulation include α/β-tubulins (Ren et al, 2003), which are toxic in free, monomeric form (Burke et al, 1989), and cyclin E (Staropoli et al, 2003), which might force postmitotic neurons into abortive cell cycling, a process known to promote apoptosis. A more generalized toxic effect might arise from the aberrant turnover of the aminoacyl-tRNA synthetase subunit p38 (Corti et al, 2003). Dysregulation of synaptic vesicle transport might be caused by the accumulation of the septins CDCrel-1 and CDCrel-2, the steadystate levels of which are regulated by parkin-mediated ubiquitination (Choi et al, 2003; Zhang et al, 2000). Synaptotagmin XI is another synaptic protein substrate for parkin (Huynh et al, 2003). Taken together, the cell-culture models have provided clues, but have not resolved the toxic mechanism of parkin deficiency.
Dopaminergic neuron loss without Lewy body formation?
Autopsies have been performed on only four parkin-proven cases of PD, so we should be cautious in drawing conclusions from neuropathological examinations. Patients with homozygous parkin deletions showed depigmentation and loss of dopaminergic neurons in the substantia nigra, accompanied by gliosis (Hayashi et al, 2000; Mori et al, 1998). Dopaminergic neuron loss was also reported for compound heterozygous patients with exon 3 deletion and parkin missense mutations (Farrer et al, 2001; van de Warrenburg et al, 2001).
Despite massive neurodegeneration in the substantia nigra and also in the locus coeruleus of AR-JP patients, LBs were conspicuously absent from the brains of patients with homozygous parkin deletions (Hayashi et al, 2000; Mori et al, 1998; Takahashi et al, 1994). These observations give rise to two interpretations. The first is that the aetiology of AR-JP is distinct from idiopathic PD, which means that the loss of parkin causes the loss of dopaminergic neurons by a distinct pathway that differs from the classical αsynucleinopathy course. Alternatively, parkin expression might be a prerequisite for LB formation (Chung et al, 2001). The examination of compound heterozygous patients bearing exon 3 deletions and point mutations is revealing in this context. Whereas a patient with a K211N parkin allele had no LBs (van de Warrenburg et al, 2001), an individual expressing R275W parkin did display brainstem LBs (Farrer et al, 2001). In marked contrast to the non-aggregating K211N mutant, R275W parkin localized to aggresome-like structures in cultured cells (Cookson et al, 2003). Thus, the R275W parkin expressed in the only AR-JP patient with LBs is not a simple loss-of-function mutant, but might instead mediate protein misfolding. In fact, there have been several recent reports of aggresome formation elicited by specific parkin mutants (Table 1). Aggresome-forming parkin mutations reside in the RING fingers, the many disulphide bonds of which depend on well-controlled protein-folding trajectories. Moreover, proteasomal inhibition led to the recruitment of wild-type parkin to aggresomes (Muqit et al, 2004; Zhao et al, 2003), and a fraction of LBs contained parkin (Schlossmacher et al, 2002). Although aggresome formation is a fascinating cellular defence mechanism against unfolded protein stress, the relevance of this process in LB formation remains to be discovered. It will be interesting to investigate whether conditions that impair the folding of parkin actually cause LB formation, whereas mutations in other domains are not permissive.
Table 1.
Aggresome-forming parkin mutants
| Mutation | Domain | Aggresomes | Cell type | Reference |
|---|---|---|---|---|
| A82E | Linker | − | HEK293 | Cookson et al, 2003 |
| K161N | UPD | − | COS7 | Gu et al, 2003 |
| SH-SY5Y | Muqit et al, 2004 | |||
| R256C | RING1 | + | HEK293 | Cookson et al, 2003 |
| − | COS7 | Gu et al, 2003 | ||
| R275W | RING1 | + | HEK293, primary neurons | Cookson et al, 2003 |
| C289G | RING1 | + | COS7 | Gu et al, 2003 |
| G328E | IBR | − | HEK293 | Cookson et al, 2003 |
| SH-SY5Y | Muqit et al, 2004 | |||
| C418R | RING2 | + | COS7 | Gu et al, 2003 |
| C431F | RING2 | − | HEK293 | Cookson et al, 2003 |
| W453stop | C-terminus | + | N2a, SH-SY5Y | Winklhofer et al, 2003 |
The R275W mutation was validated in post-mortem human tissue. IBR, in-between RING domain; RING, really interesting new gene domain; UPD, unique parkin domain.
Parkin knockout animal models
Parkin-null mutants have been generated in Drosophila by P-element insertion and transposon mutagenesis (Greene et al, 2003; Pesah et al, 2004). parkin mutant flies showed a reduced lifespan, as well as defects in spermatogenesis and muscle degeneration. Furthermore, these flies were sensitized to oxidative stress (Pesah et al, 2004). Mitochondrial dysfunction probably contributes to the phenotype (Greene et al, 2003). For example, mitochondria in the main flight muscles were grossly swollen and the myofibrillar arrangement was dispersed. Mitochondrial swelling is associated with apoptosis, and indeed, TUNEL staining revealed apoptotic nuclei in the indirect flight muscles. Consequently, wing posture and flight was significantly impaired in parkin-null mutants. Transgenic expression of parkin restored muscle integrity.
However, the nervous system was not affected in parkin mutant Drosophila and the dopaminergic neurons in the dorsomedial cluster were not impaired (Greene et al, 2003; Pesah et al, 2004). These neurons selectively degenerate upon expression of the PD gene αsYN (Feany & Bender, 2000) as well as the parkin substrate Pael-R (Yang et al, 2003). Importantly, co-expression of parkin ameliorated dopaminergic neuron loss in the dorsomedial cluster of αsYN and Pael-R transgenic flies (Yang et al, 2003). Conversely, knockdown of Drosophila parkin by RNA interference enhanced the toxicity of Pael-R expressed specifically in dopaminergic neurons. Primary neurons derived from such flies showed an accumulation of Pael-R (Yang et al, 2003). Similarly, parkin knockdown combined with unfolded protein stress (caused by proteasome inhibition and αsYN overexpression) was neurotoxic to mammalian neurons (Petrucelli et al, 2002). Conversely, wild-type but not mutant parkin rescued primary dopaminergic neurons from proteasome inhibitor toxicity (Petrucelli et al, 2002). Thus, parkin may preserve cellular viability under conditions of unfolded protein stress.
This concept was further evaluated in mice with the targeted disruption of parkin exon 3. Parkin-deficient mice showed reduced weight gain (Itier et al, 2003; Palacino et al, 2004), but as in flies, no loss of dopaminergic neurons was observed (Goldberg et al, 2003; Itier et al, 2003). Parkin−/− mice had elevated levels of extracellular dopamine (Goldberg et al, 2003), which might originate from the limbic system rather than the striatum (Itier et al, 2003). Dopamine metabolism appeared to be enhanced, as indicated by elevated amounts of 3,4-dihydroxyphenylacetic acid (Itier et al, 2003), which is produced by monoamine oxidase. This enzymatic reaction produces hydrogen peroxide, which might confer oxidative stress in the parkin−/− mice. Indirect markers of oxidative damage that appeared in ageing parkin−/− mice included protein carbonyls and 4-hydroxynonenal (Palacino et al, 2004). Although the mitochondrial ultrastructure appeared intact in parkin−/− mice, a proteomic analysis revealed reduced levels of several protein subunits of the respiratory chain, and the respiratory capacity of mitochondria isolated from the striatum of parkin−/− mice was decreased (Palacino et al, 2004).
Surprisingly, none of the known parkin substrates (CDCrel-1, synphilin 1, α-Sp22 and α-SYN) were found to accumulate in the brains of parkin−/− mice, when measured specifically (Goldberg et al, 2003) or through proteomic analysis (Palacino et al, 2004). Furthermore, no accumulation of any αsYN species was detected in quakingviable mice (Lorenzetti et al, 2004). This spontaneous mouse mutant bears a deletion that spans PARKIN and the parkin co-regulated gene (PACRG; Fig 1), both of which encode enzymes that might act synergistically to suppress unfolded protein stress (Imai et al, 2003). Therefore, the possible explanations of these results are that redundant ubiquitination pathways for parkin substrates may exist in the mouse brain, that parkin-mediated ubiquitination does not target substrate proteins for proteasomal degradation, or that the parkin substrates identified so far are erroneous.
Conclusions and outlook
Several parkin substrate proteins have been identified and validated to variable extents. Unfortunately, no straightforward common denominator of parkin substrates has emerged. An attractive hypothesis states that aggregation-prone proteins are cleared after parkin-mediated polyubiquitination followed by proteasomal degradation. Although most authors could detect their parkin substrates in LBs of sporadic PD patients, parkin-mutant cases tend to lack LBs. Rather than forming protein aggregates, parkin substrates showed only some increase in steady-state levels in the small number of AR-JP brains available. Thus, the question arises whether parkin mutations cause dopaminergic neuron loss by a distinct pathway that does not involve idiopathic PD neuropathology. If so, AR-JP might be considered as a separate disease entity.
The widespread expression of parkin and its substrates raises the question why dopaminergic neurons selectively fall prey to parkin mutations in human patients. The pigmented dopaminergic neurons in the human substantia nigra sustain a high basal level of oxidative stress. Although the disruption of the parkin gene in laboratory animals was not sufficient to elicit dopaminergic neuron loss, mitochondrial deficiencies were consistently observed. Thus, parkin might protect against mitochondrial failure. It will be interesting to study the effects of parkin on mitochondrial integrity. It will also be crucial to investigate whether the loss of parkin sensitizes mice to PD-relevant stressors, such as dopaminergic neurotoxins or protein folding stress due to α-SYN fibrillogenesis.
Finally, one should bear in mind the emerging complexity of ubiquitin biology (Schwartz & Hochstrasser, 2003). Does parkin monoubiquitinate key substrates and/or does it require accessory factors (such as the E4 enzyme CHIP) for polyubiquitination? Are the classical K48-linked polyubiquitin chains the primary product of the enzymatic activity of parkin, or can K63- or K29-linked polyubiquitin chains be attached to substrate proteins? Could parkin even act as an E3 ligase for members of the emerging family of small ubiquitin-like modifiers? And if multiple E3 ligase activities are exerted by parkin, how are they regulated in cells, especially the nigral dopaminergic neurons, in health and disease? After a highly productive phase that led to the discovery of several parkin-interacting proteins, the time might have come to take a fresh look at parkin beyond its putative function to ubiquitinate proteins for proteasomal targeting.
Acknowledgments
The authors would like to thank A. Yamamoto and W. Springer for their helpful discussions. This work is supported by the German National Genome Research Network (NGFN) and the European Union project (N)EUROPARK.
References
- Burke D, Gasdaska P, Hartwell L (1989) Dominant effects of tubulin overexpression in Saccharomyces cerevisiae. Mol Cell Biol 9: 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P et al. (2003) SEPT5_v2 is a parkin-binding protein. Mol Brain Res 117: 179–189 [DOI] [PubMed] [Google Scholar]
- Chung KKK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM (2001) Parkin ubiquitinates the αsynuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7: 1144–1150 [DOI] [PubMed] [Google Scholar]
- Cookson MR, Lockhart PJ, McLendon C, O'Farrell C, Schlossmacher M, Farrer MJ (2003) RING finger 1 mutations in Parkin produce altered localization of the protein. Hum Mol Genet 12: 2957–2965 [DOI] [PubMed] [Google Scholar]
- Corti O et al. (2003) The p38 subunit of the aminoacyl–tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet 12: 1427–1437 [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL (2003) Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J Clin Invest 111: 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S et al. (1999) Synphilin-1 associates with αsynuclein and promotes the formation of cytosolic inclusions. Nat Genet 22: 110–114 [DOI] [PubMed] [Google Scholar]
- Fallon L, Moreau F, Croft BG, Labib N, Gu W-J, Fon EA (2002) Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem 277: 486–491 [DOI] [PubMed] [Google Scholar]
- Farrer M et al. (2001) Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol 50: 293–300 [DOI] [PubMed] [Google Scholar]
- Feany MB, Bender WW (2000) A Drosophila model of Parkinson's disease. Nature 404: 394–398 [DOI] [PubMed] [Google Scholar]
- Giasson BI, Lee VM-Y (2001) Parkin and the molecular pathways of Parkinson's disease. Neuron 31: 885–888 [DOI] [PubMed] [Google Scholar]
- Goedert M (2001) Alphasynuclein and neurodegenerative diseases. Nat Rev Neurosci 2: 492–501 [DOI] [PubMed] [Google Scholar]
- Golbe LI, Mouradian MM (2004) Alphasynuclein in Parkinson's disease: light from two new angles. Ann Neurol 55: 153–156 [DOI] [PubMed] [Google Scholar]
- Goldberg MS et al. (2003) Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 278: 43628–43635 [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 100: 4078–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W-J et al. (2003) The C289G and C418R missense mutations cause rapid sequestration of human Parkin into insoluble aggregates. Neurobiol Dis 14: 357–364 [DOI] [PubMed] [Google Scholar]
- Hayashi S, Wakabayashi K, Ishikawa A, Nagai H, Saito M, Maruyama M, Takahashi T, Ozawa T, Tsuji S, Takahashi H (2000) An autopsy case of autosomal-recessive juvenile parkinsonism with a homozygous exon 4 deletion in the parkin gene. Mov Disord 15: 884–888 [DOI] [PubMed] [Google Scholar]
- Huynh DP, Scoles DR, Nguyen D, Pulst SM (2003) The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet 12: 2587–2597 [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem 275: 35661–35664 [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105: 891–902 [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama K-I, Takahashi R (2002) CHIP is associated with parkin, a gene responsible for familial Parkinson's disease, and enhances its ubiquitin ligase activity. Mol Cell 10: 55–67 [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Murakami T, Shoji M, Abe K, Takahashi R (2003) A product of the human gene adjacent to parkin is a component of Lewy bodies and suppresses Pael receptor-induced cell death. J Biol Chem 278: 51901–51910 [DOI] [PubMed] [Google Scholar]
- Itier J-M et al. (2003) Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet 12: 2277–2291 [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Leimer U, Haass C (2000) Does failure of parkin-mediated ubiquitination cause juvenile parkinsonism? Trends Biochem Sci 25: 524–527 [DOI] [PubMed] [Google Scholar]
- Kahns S, Kalai M, Jakobsen LD, Clark BFC, Vandenabeele P, Jensen PH (2003) Caspase-1 and caspase-8 cleave and inactivate cellular parkin. J Biol Chem 278: 23376–23380 [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608 [DOI] [PubMed] [Google Scholar]
- Lorenzetti D, Antalffy B, Vogel H, Noveroske J, Armstrong D, Justice M (2004) The neurological mutant quakingviable is Parkin deficient. Mamm Genome 15: 210–217 [DOI] [PubMed] [Google Scholar]
- Marín I, Ferrús A (2002) Comparative genomics of the RBR family, including the Parkinson's disease-related gene Parkin and the genes of the Ariadne subfamily. Mol Biol Evol 19: 2039–2050 [DOI] [PubMed] [Google Scholar]
- Mata IF, Lockhart PJ, Farrer MJ (2004) Parkin genetics: one model for Parkinson's disease. Hum Mol Genet 13 (Suppl 1): R127–R133 [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Olanow CW (2003) Proteolytic stress: a unifying concept for the etiopathogenesis of Parkinson's disease. Ann Neurol 53 (Suppl 3): S73–S84 [DOI] [PubMed] [Google Scholar]
- Mori H, Kondo T, Yokochi M, Matsumine H, Nakagawa-Hattori Y, Miyake T, Suda K, Mizuno Y (1998) Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology 51: 890–892 [DOI] [PubMed] [Google Scholar]
- Muqit MMK, Davidson SM, Payne Smith MD, MacCormac LP, Kahns S, Jensen PH, Wood NW, Latchman DS (2004) Parkin is recruited into aggresomes in a stressspecific manner: over-expression of parkin reduces aggresome formation but can be dissociated from parkin's effect on neuronal survival. Hum Mol Genet 13: 117–135 [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279: 18614–18622 [DOI] [PubMed] [Google Scholar]
- Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G (2004) Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 131: 2183–2194 [DOI] [PubMed] [Google Scholar]
- Petrucelli L et al. (2002) Parkin protects against the toxicity associated with mutant αsynuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36: 1007–1019 [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhao J, Feng J (2003) Parkin binds to α/β tubulin and increases their ubiquitination and degradation. J Neurosci 23: 3316–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata E et al. (2003) Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep 4: 301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmacher MG et al. (2002) Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am J Pathol 160: 1655–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DC, Hochstrasser M (2003) A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci 28: 321–328 [DOI] [PubMed] [Google Scholar]
- Shimura H et al. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25: 302–305 [DOI] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ (2001) Ubiquitination of a new form of αsynuclein by parkin from human brain: implications for Parkinson's disease. Science 293: 263–269 [DOI] [PubMed] [Google Scholar]
- Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A (2003) Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron 37: 735–749 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ohama E, Suzuki S, Horikawa Y, Ishikawa A, Morita T, Tsuji S, Ikuta F (1994) Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology 44: 437–441 [DOI] [PubMed] [Google Scholar]
- Tsai YC, Fishman PS, Thakor NV, Oyler GA (2003) Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J Biol Chem 278: 22044–22055 [DOI] [PubMed] [Google Scholar]
- van de Warrenburg BPC, Lammens M, Lücking CB, Denèfle P, Wesseling P, Booij J, Praamstra P, Quinn N, Brice A, Horstink MWIM (2001) Clinical and pathologic abnormalities in a family with parkinsonism and parkin gene mutations. Neurology 56: 555–557 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Henn IH, Kay-Jackson PC, Heller U, Tatzelt J (2003) Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J Biol Chem 278: 47199–47208 [DOI] [PubMed] [Google Scholar]
- Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B (2003) Parkin suppresses dopaminergic neuronselective neurotoxicity induced by Pael-R in Drosophila. Neuron 37: 911–924 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KKK, Huang H, Dawson VL, Dawson TM (2000) Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA 97: 13354–13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ren Y, Jiang Q, Feng J (2003) Parkin is recruited to the centrosome in response to inhibition of proteasomes. J Cell Sci 116: 4011–4019 [DOI] [PubMed] [Google Scholar]