Summary
Conference and Workshop on Apoptosis and Disease
Keywords: apoptosis, cancer, disease, signalling, tissue models
Introduction
Apoptosis is an evolutionarily conserved form of cell death that was first described by Kerr and colleagues in 1972 (Kerr et al, 1972). It is essential for successful embryonic development and maintains normal cellular homeostasis in adult organisms. Gain- and loss-of-function models of genes in the core apoptotic pathway suggest that perturbation of cellular homeostasis can be a primary pathological event that results in disease. There is now compelling evidence that insufficient apoptosis can result in cancer or autoimmunity, whereas accelerated cell death is evident in degenerative diseases, immunodeficiency and infertility. Not surprisingly, a huge endeavour aimed at unravelling this fundamental biological process has led to major advances in our understanding of the apoptotic process during the past 20 years.

The European Tissue Culture Society Conference and Workshop on 'Apoptosis and Disease' was held at the University of Bristol, between 26 and 27 November 2003, and was organized by A. Hague and C. Paraskeva.
The primary aim of this symposium was to provide “an overview of the regulation, signalling and execution of apoptosis in model systems relevant to human diseases”. In this report, we highlight recent findings from several laboratories that outline crucial control points in the apoptotic pathway, some of which could provide rational targets for therapeutic intervention.
Initiating the demolition phase of apoptosis
During apoptosis, the controlled destruction of the cell is coordinated, from within, by the caspase family of cysteine proteases. This meeting focused on two of the major apoptotic pathways: one initiated by the activation of death receptors and the other by stress-inducing stimuli (Fig 1; reviewed in Adams, 2003; Danial & Korsmeyer, 2004). Triggering of cell surface death receptors of the tumour necrosis factor (TNF) receptor superfamily, including TNF-R1, CD95 and TNF-related apoptosis-inducing ligand (TRAIL)-R1 and -R2, results in the rapid activation of the 'initiator', caspase 8, after it has been recruited through adaptor molecules to a trimerized receptor–ligand complex (known as the DISC). Fas-associated death domain protein (FADD) is reported to be the universal adaptor used by death receptors to recruit and activate the initiator caspase 8. Whereas CD95, TRAIL-R1 and -R2 bind to FADD directly, recruitment to TNF-R1 is indirect through another adaptor molecule, TNF-receptor-associated death domain protein (TRADD). However, G. Cohen (Leicester, UK) introduced another possibility: analysis of the native TNF signalling complex revealed that although caspase 8 and FADD are both obligatory for TNF-mediated apoptosis, they are not recruited to a TNF-induced membrane-bound receptor signalling complex as occurs with CD95 or TRAIL (Harper et al, 2003). This introduces a new concept: that FADD and caspase 8 must be localized in another activation scaffold elsewhere within the cell. Cohen also reported that primary B cells isolated from patients with chronic lymphocytic leukaemia (CLL) are resistant to the cytotoxic effects of TRAIL (MacFarlane et al, 2002). TRAIL is now undergoing evaluation as a potential cancer therapeutic agent; however, these data suggest that although this molecule is toxic to most tumour cell lines, many primary tumour cells might only be sensitive to TRAIL in combination with other agents.
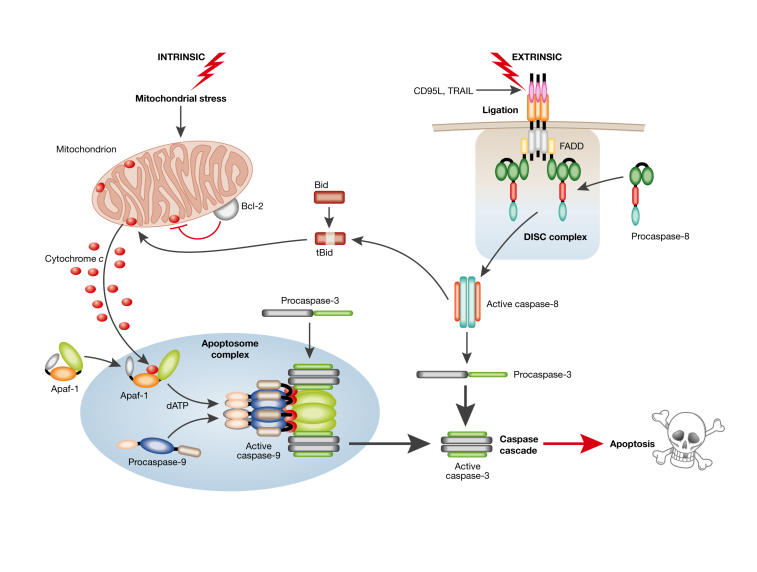
Figure 1.
Apoptosis: the 'extrinsic' and 'intrinsic' pathways to caspase activation. Two major apoptotic pathways are illustrated: one activated via death receptor activation ('extrinsic') and the other by stress-inducing stimuli ('intrinsic'). Triggering of cell surface death receptors of the tumour necrosis factor (TNF) receptor superfamily, including CD95 and TNF-related apoptosis-inducing ligand (TRAIL)-R1/-R2, results in rapid activation of the initiator caspase 8 after its recruitment to a trimerized receptor-ligand complex (DISC) through the adaptor molecule Fas-associated death domain protein (FADD). In the intrinsic pathway, stress-induced apoptosis results in perturbation of mitochondria and the ensuing release of proteins, such as cytochrome c, from the inter-mitochondrial membrane space. The release of cytochrome c, from mitochondria is regulated in part by Bcl2 family members, with anti-apoptotic (Bcl2/ Bcl-XL/Mcl1) and pro-apoptotic (Bax, Bak and tBid) members inhibiting or promoting the release, respectively. Once released, cytochrome c binds to apoptotic protease-activating factor 1 (Apaf1), which results in formation of the Apaf1–caspase 9 apoptosome complex and activation of the initiator caspase 9. The activated initiator caspases 8 and 9 then activate the effector caspases 3, 6 and 7, which are responsible for the cleavage of important cellular substrates resulting in the classical biochemical and morphological changes associated with the apoptotic phenotype (reviewed in Adams, 2003; Danial & Korsmeyer, 2004).
Stress-induced apoptosis caused by chemicals or growth factor deprivation results in the perturbation of mitochondria and the ensuing release of proteins, such as cytochrome c, from the mitochondrial intermembrane space. Once released, cytochrome c binds to apoptotic protease-activating factor 1 (Apaf1), which in the presence of dATP results in the formation of the Apaf1–caspase 9 'apoptosome' complex and in the activation of the caspase cascade (Fig 1). Therefore, in addition to their role in cellular energy metabolism, mitochondria are now recognized as central players in cell death. Crucial for the latter role are not only the Bax/Bak channel, which is open to direct regulation by Bcl2/Bcl-XL (reviewed in Danial & Korsmeyer, 2004), but also a nonspecific pore in the inner mitochondrial membrane, known as the mitochondrial permeability transition pore (MPTP). The opening of these pores uncouples mitochondria, which prevents them from providing energy for the cell and leads to necrotic cell death. MPTP opening is important in the injury to the heart and brain that follows an ischaemic episode such as a heart attack or stroke. Significantly, A. Halestrap (Bristol, UK) and colleagues have now shown that agents that inhibit pore opening can protect hearts and brains from ischaemia/reperfusion injury (Halestrap et al, 2004). Furthermore, although the release of cytochrome c from mitochondria is crucial for stress-induced caspase activation, other pro-apoptotic proteins such as apoptosis-inducing factor (AIF), Smac/DIABLO and Omi/HtrA2 are also released from the intermembrane space in response to an apoptotic stimulus. One mechanism by which these proteins are released might be through the opening of the MPTP: this causes mitochondrial swelling, rupture of the outer membrane and nonspecific release of intermembrane proteins. However, MPTP opening must be transient for apoptosis to occur, otherwise ATP would be depleted and cells would die by necrosis, even though caspase activation and other early changes that are characteristic of apoptosis have taken place. Therefore, according to the severity of the cell insult, Halestrap proposed that mitochondria could determine not only whether a cell should die, but also the nature of that death.
In most situations, apoptosis is coordinated by caspases, which dismantle the cell by targeting numerous proteins for limited proteolysis (Fig 1). The mammalian caspase family contains 13 members, a subset of which participate in apoptosis, whereas the remainder are probably involved in the processing of pro-inflammatory cytokines. S. Martin (Dublin, Ireland) discussed the hierarchical nature of the caspase activation cascade that is triggered by cellular stress ('intrinsic' pathway; Fig 1). Martin's team have shown that Apaf1, caspase 9, caspase 3 and the X-linked inhibitor of apoptosis (XIAP) are the main constituents of the native 'apoptosome', and that cytochrome c is not stably associated with the active complex (Hill et al, 2004). Martin also presented data obtained from global proteomic analyses of apoptotic cells and discussed the role of specific caspases within this cascade in targeting cellular proteins for degradation. The data suggest that more than 400 proteins are targeted for limited proteolysis during the terminal 'demolition' phase of apoptosis.
Tumour suppressor genes in the regulation of apoptosis
In response to various cellular stresses, p53 expression is increased and the protein is post-translationally modified, resulting in either arrest of the cell at G1 or commitment to death through apoptosis. However, until recently, it was not known how the decision to induce cell-cycle arrest or apoptosis was made. In an elegant presentation, X. Lu (London, UK) was able to shed some light on this conundrum. She showed that co-expression of two members of the apoptosis-stimulating protein for p53 (ASPP) family, ASPP1 and ASPP2, specifically stimulate the transactivation function of p53 on promoters of pro-apoptotic genes, such as Bax and p53-inducible gene 3 (PIG3), but not on the promoters of the cell-cycle arrest-associated genes, p21 or MDM2 (Bergamaschi et al, 2004). Therefore, ASPP1 and ASPP2 specifically stimulate the apoptotic function of p53 but do not enhance p53-induced cell-cycle arrest. Significantly, some tumours retain wild-type p53, but it is not known how the function of this protein is suppressed and, moreover, how it can be reactivated. Lu suggested that one mechanism by which wild-type p53 is tolerated in human breast cancers is through the loss of ASPP activity. In such a model, an inhibitory form of ASPP would allow cells to bypass the tumour suppressor functions of p53 and ASPP. Such an inhibitory protein is the third member of the ASPP family, iASPP, which is an evolutionarily conserved inhibitor of p53 (Bergamaschi et al, 2003). iASPP inhibits p53-induced apoptosis but not Bax-induced apoptosis, suggesting that it acts upstream of Bax. Moreover, iASPP is an oncoprotein that co-operates with Ras and E1A but not mutant p53, to transform cells in vitro. By contrast, ASPP1 and ASPP2 decrease Ras/E1A transformation. Increased expression of iASPP also confers resistance to ultraviolet (UV) radiation and to cisplatin-induced apoptosis. Whereas ASPP expression is frequently downregulated in human tumours, iASPP expression is upregulated in human breast carcinomas that express wild-type p53 and normal levels of ASPP. Hence, the ASPP family members both positively and negatively regulate the apoptotic function of p53.
p53 has a crucial role in apoptosis that is provoked by DNA damage (for example, by exposure to UV). However, G. Melino (Leicester, UK) illustrated the complexity of apoptosis regulation after DNA damage by describing another member of the p53 family, p73. p73, which has at least eight distinct isoforms, shares a high degree of sequence homology with p53, induces G1 arrest, transcriptionally activates some p53 target genes (including p21, MDM2 and Bax), and induces apoptosis irrespective of p53 status. Unlike p53, mutant p73 has rarely been found in human tumours, although expression of the recently cloned p73 isoform, ΔNp73, correlates with poor prognosis in neuroblastoma. However, the molecular mechanisms by which p73 induces apoptosis have not been elucidated. Melino and colleagues have directly addressed this question. They reported that p73-induced apoptosis is mediated by p53-upregulated modulator of apoptosis (PUMA), which in turn causes Bax translocation, cytochrome c release and activation of the apoptosome (Melino et al, 2004). Significantly, both Bax and its B-cell lymphoma 2 (Bcl-2)-family homologue, Bak, are required for p73-induced apoptosis. The p73 isoform, TAp73, can, however, induce apoptosis by additional mechanisms: through upregulation of CD95 and the autocrine activation of an active CD95 DISC or, alternatively, through induction of endoplasmic reticulum stress mediated by direct transactivation of the recently cloned p53 target gene, Scotin. Melino also reported that the p73 isoform, ΔNp73, inhibits apoptosis induced by either p53 or TAp73, whereas both p53 and TAp73 transcriptionally regulate ΔNp73. This pathway creates a regulatory 'dominant-negative' feedback loop, and highlights the role of p73 as both 'friend' and 'foe' in tumorigenesis.
Loss of the apoptotic response has been implicated in carcinogenesis owing to the increased survival of cells that have damaged DNA. A. Clarke and colleagues (Cardiff, UK) used mice deficient in a series of genes with either known or potential tumour suppressive activity to test this inference (Clarke & Sansom, 2003). For example, they analysed mice that were mutant for the methyl-binding domain (MBD) protein Mbd4, which has been shown to interact with Mlh1 and function as a thymine glycosylase. Their analysis shows Mbd4 to be essential for the normal apoptotic programme after DNA damage and that Mbd4 deficiency can confer increased clonogenic survival in vivo. The precise mechanism for this reliance is unclear, but probably derives from an interaction with the death molecule FADD. In addition, they showed that Mbd4 deficiency accelerates intestinal tumour development and alters the mutation spectrum in mice heterozygous for the ApcMin allele. Conversely, a second member of the MBD family, Mbd2, normally augments neoplasia in the intestine. Taken together, their results show that mutations in many genes can have an impact on apoptosis, but that the disruption of these genes cannot always be directly translated into increased clonogenic survival, mutation and cancer predisposition.
Bcl2 family members and related proteins in apoptosis
Mcl1, originally identified in myeloid cells, is an anti-apoptotic protein with close structural homology to the oncogenic Bcl-2 protein. Mcl1 has been shown to have a role in survival and is transcribed after the induction of differentiation. Enforced expression of Mcl1 promotes lymphomagenesis in the mouse, however the functional role of Mcl1 in human B-cell lymphoma remains unclear. G. Packham's group (Southampton, UK) showed that Mcl1 is expressed in malignant B cells and that a high level expression of Mcl1 is required for B-lymphoma cell survival as anti-sense ablation was sufficient to trigger apoptosis. Apoptosis was associated with decreased expression of the Mcl1 RNA and protein, and Mcl1 was efficiently cleaved by caspases at evolutionarily conserved aspartic acid residues both in vitro and during apoptosis. Significantly, transfection of the Mcl1 cleavage product that accumulates during apoptosis was sufficient to kill cells (Michels et al, 2004). Therefore, Mcl1 is an essential survival molecule for B-lymphoma cells and is downregulated and cleaved by caspases to a death-promoting molecule during apoptosis. Interfering with Mcl1 function appears to be an effective means of inducing apoptosis in Mcl1-positive B-cell lymphoma, and the unique sensitivity of Mcl1 to caspase-mediated cleavage suggests an attractive strategy for converting Mcl1 to a proapoptotic molecule. Packham thus highlighted the potential importance of Mcl1 as a therapeutic target, particularly as it has also been found to be expressed in other malignacies, including several solid tumours.
Recently, the retinoblastoma susceptibility gene, RB1, has been shown to have an anti-apoptotic role and at this meeting it was suggested that this could in part be due to the ability of retinoblastoma (Rb) to interact with the Bcl2-associated athanogene, Bag1 (Arhel et al, 2003). The Bag1 protein is an anti-apoptotic regulator, which is frequently deregulated in several malignancies including colorectal cancer (reviewed in Sharp et al, 2004). A. Williams (Bristol, UK) reported that Rb, through increasing the nuclear localization of Bag1, increases the resistance of colorectal epithelial cells to apoptosis. As apoptosis is one of the main mechanisms by which chemotherapy and radiotherapy induce killing of tumour cells, high levels of Bag1 in the nucleus may represent a mechanism by which colorectal carcinoma cells become insensitive to therapy.
Survival signalling pathways
Transformation of cells by Ras oncogenes leads to profound changes in their sensitivity to apoptotic stimuli. The nature of the effect of Ras transformation on apoptosis regulation varies depending on cell type and death trigger. For example, detachment-induced programmed cell death is blocked by activated Ras protein, acting principally through its ability to activate phosphatidylinositol-3-OH-kinase (PI(3)K) and protein kinase B (PKB)/Akt. In normal cells, matrix adhesion stimulates PI(3)K and hence the downstream kinase PKB/Akt to suppress death signals. J. Downward (London, UK) reported on several novel Akt substrates, which are also 14-3-3 binding proteins, that his team has identified using two-dimensional gel and tandem affinity purification (TAP) technology. One of these is the Yes-associated protein (YAP), which is a transcriptional co-activator that is required for efficient transcription by several families of transcription factors, including p73 (Basu et al, 2003). More recently, Downward's team have set out to identify novel signalling proteins that are regulated by Ras by performing a functional genomic RNAi library screen for inhibitors of Ras-induced senescence in human ovarian epithelial cells. Using this approach, three crucial Ras-activated kinases have been identified, namely PI(3)K p110α, p70 S6 kinase 1 and MINK. Significantly, MINK (a protein that activates p38 and c-Jun-N-terminal kinase (JNK)) has not previously been associated with Ras signalling, which raises the possibility that inhibitors of senescence might also have a crucial role in Ras-induced transformation. Downward advocated the power of RNAi libraries as functional genomic screening tools, but he also offered this cautionary note: although the Ras screens have produced several targeted hits, preliminary studies suggest that apoptosis screens might identify molecules that are components of parallel pathways.
Damaged cells can compromise the function of tissues, so social signals between cells must override any intrinsic mechanisms that might inappropriately promote their survival. In this context, insulin-like growth factors (IGFs) are the most prevalent and most potent survival factors for many cell types. As well as regulating cell survival through sequestering IGFs, the IGF-binding proteins (IGFBP1–IGFBP6) have been shown to have IGF-independent activity. The discussion at this meeting was centred on the apparent opposing roles for IGFBP3 and IGFBP5 (Perks & Holly, 2003). J. Holly's team (Bristol, UK) were able to demonstrate that IGFBP3 and IGFBP5 can either enhance an apoptotic response to cell stress or act as a survival factor, dependent on the context of the extracellular matrix. Therefore, the association of IGFs with IGFBPs not only controls the availability of IGFs and their survival signals, but also controls the availability of IGFBPs and their intrinsic effects on cell stress responses.
Apoptosis in tissue modelling and disease
Included in this meeting were several excellent talks covering aspects of apoptotic regulation in tissue modelling and disease. C. Watson (Cambridge, UK) gave a stimulating talk underlining the importance of apoptosis in the mammary gland (Clarkson et al, 2004). Mammary epithelial cells are induced to undergo extensive apoptosis during the first three days of post-lactational regression. Watson's group has used Affymetrix microarray analysis to compare transcriptional profiles at different stages of early mammary gland involution. Using conditional gene targeting and mammary epithelial cell-culture models, they identified several transcription factors that mediate this apoptotic process, including Stat3 and NF-κB. This revealed an unexpected association of the apoptotic response with the inflammatory response. Her talk highlighted the sequential induction of distinct apoptosis pathways in involution and the stimulation of immunomodulatory signals, which probably suppress the potentially damaging effects of a cellular inflammatory response during the transition from lactation to involution.
R. Grafstrom (Stockholm, Sweden) described the modelling of oral cancer development in vitro and concluded that epithelial cell transformation often couples with a loss of the ability to undergo turnover by programmed mechanisms. In this manner, impaired ability for 'death' contributes to mass expansion of proliferation-prone tumour cells. By contrast, T. Cotter (Cork, Ireland) described both in vitro and in vivo models of cell death in the eye as a model for disease and development. Apoptosis is the mode of cell death in retinitis pigmentosa (RP), which is a group of retinal degenerative disorders that primarily affect photoreceptors. The photoreceptor cell line 661W undergoes caspase-dependent apoptosis in response to staurosporine or serum starvation. Both inducers of apoptosis led to activation of caspases 3 and 9, but serum deprivation also led to activation of caspase 12 and calpain, which suggests the involvement of the endoplasmic reticulum stress pathway. By contrast, in vivo models of photoreceptor cell death and retinal degeneration showed that loss of photoreceptors is independent of the activation of caspases 9, 8, 7, 3 and 2. DNA fragmentation occurs in the absence of inhibitor of caspase-activated DNase (ICAD) proteolysis, which suggests that an alternative endonuclease is responsible for DNA cleavage in these models. Importantly, this group showed that apoptosome activation is prevented owing to an absence of mitochondrial cytochrome c release. Cotter suggested that the lack of caspase activation could potentially be a physiological process to protect post-mitotic cells from apoptosis (Doonan et al, 2003). J. Uney (Bristol, UK) also focused on apoptosis in post-mitotic cells, in his case neuronal cells, and showed that heatshock protein 70 (Hsp70), facilitated by Hsp40, can protect neurons from ischaemic stress. The protective effects of Hsp70 have generally been attributed to its ability to stabilize/refold damaged or nascent proteins. However, Uney's group have recently established that Hsp70 can protect against apoptosis through inhibiting the activation of JNK, which is potentially important in the pathogenesis of Alzheimer's disease.
Conclusions and future directions
A. Hague and C. Paraskeva assembled an exiting and stimulating group of speakers who described significant advances in our understanding of the mechanisms that regulate apoptosis in both normal and disease models. Overall, the meeting brought together researchers who work on specific areas of cell death but who are linked by the common theme of elucidating the 'directors' and 'executioners' of apoptosis. The array of techniques now being applied was highlighted in several talks and posters and discussed in a workshop entitled 'Apoptosis in Adherent Cells'. This clearly illustrates the importance of using complementary technologies to elucidate and understand such a fundamental but complex biological process. The clear message that emerged from this meeting was that the identification of crucial control points in the apoptotic pathway will provide rational targets for the development of a new generation of therapeutics. Indeed, some potential targets are already under active development, including inhibitors that block caspase activity, agents that target Ras signalling pathways, molecules that intervene at the Bcl-2 control point, and death-inducing ligands such as TRAIL. However, despite these exceptional advances, much remains to be discovered: we now look forward to future meetings in which some of these details will be revealed.


Acknowledgments
The meeting was generously supported by the European Tissue Culture Society, UK branch, with sponsorship from Alexis Platform, ECACC (European Collection of Cell Cultures), PAA Laboratories, Promega and New England BioLabs. We apologize to those speakers whose work has not been discussed in detail owing to space limitations.
References
- Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17: 2481–2495 [DOI] [PubMed] [Google Scholar]
- Arhel NJ et al. (2003) The retinoblastoma protein interacts with Bag-1 in human colonic adenoma and carcinoma derived cell lines. Int J Cancer 106: 364–371 [DOI] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-induced apoptosis. Mol Cell 11: 11–23 [DOI] [PubMed] [Google Scholar]
- Bergamaschi D et al. (2003) iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 33: 162–167 [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X (2004) ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol 24: 1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Sansom OJ (2003) Analyzing tumor suppressor activities in the murine small intestine. Oncol Res 13: 333–337 [DOI] [PubMed] [Google Scholar]
- Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ (2004) Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res 6: R92–R109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- Doonan F, Donovan M, Cotter TG (2003) Caspase-independent photoreceptor apoptosis in mouse models of retinal degeneration. J Neurosci 23: 5723–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res 61: 372–385 [DOI] [PubMed] [Google Scholar]
- Harper N, Hughes M, MacFarlane M, Cohen GM (2003) FADD and caspase-8 are not recruited to the TNF-R1 signalling complex during TNF-induced apoptosis. J Biol Chem 278: 25534–25541 [DOI] [PubMed] [Google Scholar]
- Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ (2004) Analysis of the composition, assembly kinetics and activity of the native Apaf-1 apoptosome. EMBO J 23: 2134–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane M, Harper N, Snowden RT, Dyer MJS, Barnett GA, Pringle JH, Cohen GM (2002) Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lympocytic leukaemia. Oncogene 21: 6809–6818 [DOI] [PubMed] [Google Scholar]
- Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, Knight R, Green DR, Thompson G, Vousden KH (2004) p73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem 279: 8076–8083 [DOI] [PubMed] [Google Scholar]
- Michels J, O'Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, Zhang KYJ, Craig RW, Marcusson EG, Johnson PWM, Packham G (2004) Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene (in press) [DOI] [PubMed] [Google Scholar]
- Perks C, Holly J (2003) Actions of IGFBP on epithelial cancer cells: potential for new therapeutic targets. Horm Metab Res 35: 828–835 [DOI] [PubMed] [Google Scholar]
- Sharp A, Crabb SJ, Cutress RI, Brimmell M, Wang XH, Packham G, Townsend PA (2004) BAG-1 in carcinogenesis. Expert Rev Mol Med 2004: 1–15 [DOI] [PubMed] [Google Scholar]