Summary
A generally accepted model for assessing the environmental risk of GM organisms would not only help regulators but also address fears about this technology
If there ever was a major improvement in human life in the last millennium, it may well have been the green revolution of the twentieth century. The massive use of fertilizers, pesticides and improved seeds and livestock brought an enormous increase in agricultural production, more than enough to feed the population in the developed regions of the world such as Europe and North America. For the people on these continents, famine and hunger almost ceased to exist, thanks to the application of science and technology to food production. But all of these developments have benefited only agriculture. Fish, an important protein source for the majority of people on the planet, is still primarily gathered from the wild, with serious consequences. Heavy investments into fishing fleets and technology, and ever-increasing yields, put the ocean's fisheries under increasing stress. Many fishing grounds are already overfished to the point that their future viability is threatened. Fish consumption increased by 31% from 1990 to 1997 but the supply from marine fisheries grew by only 9% (FAO, 1999). And the unfettered growth of the human population will put them under even more stress.
Meeting the increasing demand for fish while protecting marine fish supplies can only be achieved by applying the experience from the agricultural revolution to increase the contribution of aquaculture
Meeting the increasing demand for fish while protecting marine fish supplies can only be achieved by applying experience from the agricultural revolution to increase the contribution of aquaculture (Tidwell & Allan, 2001). In fact, worldwide production of farmed fish is now at about 30% of global fish production and is expected to reach 50% in 2030 (FAO, 2000), but the expansion of aquaculture has met with growing resistance due to environmental concerns. And although there is much room for improvement (Tidwell & Allan, 2001), it will not be enough.
Genetically modified (GM) fish have considerable potential to further increase the yield of fish farms but have prompted serious concerns both in Europe and in the USA about the possible environmental impact on wild species. To overcome these concerns and address public resistance to biotechnology, it is therefore important to develop a sound, reliable and widely accepted method of estimating the potential for harm caused by GM fish escaping into the wild. We have developed such a method, based on population genetics, that investigates the effects of the transgene itself on wild populations. Although it has its limitations—as any mathematical model of nature has—it could provide regulators with a tool to assess such risk and make recommendations to improve and control the use of GM fish in aquaculture.
Companies and public research institutions have developed various transgenic fish, but none of them have been approved for aquaculture. The fish that has been mainly caught in the nets of criticism is an Atlantic salmon with a growth hormone gene from Chinook salmon. According to Aqua Bounty Technologies, Inc. (Waltham, MA, USA)—its developer and producer—it grows 4–6 times faster as a juvenile than wild-type salmon. By transferring a growth hormone gene from one carp species to another, Zuoyan Zhu of the Hydrobiology Institute of the Academia Sinica in Wuhan, China, has also created a fast-growing yellow river carp. Researchers in Cuba and the UK have engineered tilapia to grow and put on weight up to 300% faster (Rahman et al, 1998). Perhaps the most extraordinary example of the power of this approach was demonstrated with a mud loach developed in Korea that grows up to 35-fold faster than normal (Nam et al, 2001). Other genetic modifications, made in various fish species, provide better resistance to bacterial diseases or make the fish more tolerant to cold temperatures. Improving nutrient use is another important area of research. In addition, GM fish are being developed for biocontrol of invasive species. One way to target a specific species is to engineer a 'Trojan horse' gene into GM fish and release them, so the transgene will find its way into and affect the invading population. Research to control introduced carp, which have become a major problem in Australian rivers and lakes, is now close to being implemented (Nowak, 2002).
The only transgenic fish that is commercially available today is not designed to be eaten. A zebrafish that glows when illuminated is now available for aquarium owners under the brand name GloFish™. As it is not meant for human consumption but for aquariums, the US Food and Drug Administration saw no need to regulate it and allowed Yorktown Technologies LP (Austin, TX, USA), the licensee for GloFish, to market and sell it.
In addition to commercial GM fish, transgenic fish are widely developed and used in many laboratories all over the world as models for understanding the mechanisms of growth and development, and disease resistance, or for studying human diseases. GloFish originally started its career at the National University of Singapore as a living indicator for environmental pollution.
Proponents of GM fish point to the global problem of overfishing and depletion of fisheries when arguing for the use of any such GM fish in aquaculture. The Food and Agriculture Organization (FAO) of the United Nations estimates that worldwide demand for food fish will increase to 110 million tons in 2010 (FAO, 1999), mainly because of the growing populations in Asia, Africa and South America. Most of this fish will have to come from aquaculture.
More intense aquaculture, however, can create environmental problems of its own, mainly through runoff wastes and population concentrations, which can be hotbeds for the development of viral diseases and parasites, such as sea lice, and may also threaten wild fish. To partially address problems of waste runoff, cold-extrusion floating feeds have been developed. These feeds neither break apart before they can be consumed, nor do they sink straight to the bottom. They now dominate the market, combined with computer-and-video-linked feeding systems to monitor feed intake in the water and shut off the feeding system when pellets begin to drop below the water level at which the fish are feeding. In addition, most jurisdictions require 1–2 dives a year, during which regulators monitor the benthos and, if sludge is building up, also require adaptive management. Similarly, antibiotic use has largely been replaced by vaccines; during the 1990s, antibiotic use in Norwegian aquaculture dropped by 98% (J. McGonigle, Aqua Bounty Technologies, Waltham, MA, USA, personal communication).
But GM fish, proponents argue, could further ease some of these problems by providing better disease resistance, faster growth and improved food use. Clearly, some modifications aim mainly at increasing the economics of fish farms. Using faster growing fish allows facilities to produce more fish per year with less cost (Fig 1). Similarly, cold tolerance would allow farmers to expand aquaculture into colder and less populated areas, such as northern Canada or northern Norway, but would not necessarily improve the environmental impact of aquaculture. Other transgenes are more promising in that respect. Disease resistance would allow farmers to cut down further on antibiotics, insecticides or fungicides. Improved nutrient use would lower the impact of undigested food on the nearby environment. Furthermore, the aim of improving nutrient utilization is to use previously indigestible nutrients, such as phytic acid as a phosphorous source, to further reduce pollution while lowering costs for fish farmers. But no matter which transgene is used, the main benefit would still be to the native species—provided we can keep our farmed fish from escaping—as any improvements in aquaculture would take the pressure away from ocean fisheries.
Figure 1.
Effect of growth hormone in domestic and wild salmonids. Pairs of transgenic and non-transgenic rainbow trout produced from wild and domesticated strains reared at 8 °C. Reprinted with permission from Devlin et al, 2001 © (2001) Nature Publishing Group www.nature.com.
Opponents, even while acknowledging this argument, believe that GM fish nevertheless pose a serious threat to wildlife. If GM fish escaped from fish farms, they could further upset the oceans' delicate ecology, causing ecological disruption or species extinction. Transgenes that increase cold-, salt- or heat-tolerance could allow GM fish to expand into new territories. GM fish with higher disease resistance and better use of nutrients could outcompete wild relatives and change predator–prey relationships, and they could therefore occupy new ecological niches where wild species would usually not survive. Finally, by mating with wild fish, escaped GM fish could spread the transgene among the wild population, which could cause conflicting effects on mating success, viability in natural habitats and other fitness factors required for the species to survive.
...no matter which transgene is used, the main benefit would still be to the native species—provided we can keep our farmed fish from escaping—as any improvements in aquaculture would take the pressure away from ocean fisheries
In this light, I would also like to point out that the escape of non-transgenic domesticated fish may cause as great a harm as the escape of GM fish. Domesticated fish have been bred and selected for growth in captivity where predators are kept out and food is abundant, and have thus lost their ability to find food and avoid predators in the wild. If these fish breed with wild fish, their genes may pollute the wild gene pool and cause a general decrease in fitness of the entire population (Lynch & O'Hely, 2001). In the worst case—and this may have happened already in some places—the gene pool becomes so polluted that the 'natural' population depends on commercial releases to be viable (Naylor et al, 2001). This is in contrast to well-managed hatcheries, which try to avoid any artificial selection to circumvent this problem. However, the principal reason for hatchery-based supplementation or restoration programmes is to mitigate habitat loss, which mostly resulted from dam building (and which is why the Bonneville Power Administration shoulders a big part of the US$100 million restoration effort in the Pacific northwest). The second reason was to offset decades of overfishing and keep commercial fishers at work by introducing a surplus of fish.
...GM fish, once escaped into the open ocean, are obviously much harder to control and can spread much faster than GM plants do on land
Clearly, caution is valid, particularly as GM fish, once escaped into the open ocean, are obviously much harder to control and can spread much faster than GM plants do on land. Even if GM fish are kept only in safe pens, it cannot be ruled out that they might escape due to human error or natural disasters, such as storms that have enough power to destroy fish farms. But this concern may be overstated because escaped salmon tend to starve before they learn to seek natural prey rather than feed pellets. The first step in assessing whether the potential environmental risk of GM fish would outrank the possible benefits is therefore to develop a reliable and effective risk-assessment methodology. That is actually easier said than done due to the manifold factors that determine whether escaped transgenic animals can cause harm in the wild.
Richard Howard and I (Muir, 2001; 2002; Muir & Howard, 2002a,b) have developed such a method, termed net-fitness methodology, on the basis of standard risk assessment methodologies and biological modelling. Our model does not include all factors that define risk of harm for any given GM organism but concentrates on those that are more accessible to scientific research. Although we are able to define risk with a precise mathematical formula (see sidebar), quantifying the various parts of this formula is a formidable—or even impossible—task. Nevertheless, it allows us to understand the relationships between individual risk factors. Because risk results from a chain of events—escape, followed by spread, followed by harm—the analogy of a chain suggests that it is only as strong as its weakest link. It is therefore not necessary to quantify all aspects of risks if the probabilities of any of the links can be shown to be close to zero. The weakest link therefore defines the upper limit of risk.
The first link in the formula given below, the probability of harm if the transgene spreads—sometimes called the 'so what' question—is the most difficult to determine. Given the near infinite number of possible interactions within and between ecosystems, it is clearly not realistic to anticipate all possible harm from exposure. Even the probability of harm that can be anticipated, such as species displacement or extinction, cannot be determined reliably with our current state of science.
The second link, the probability of escape, spread and becoming feral, largely depends on the species. The US National Research Council (NRC, 2002) therefore considered aquatic organisms and insects as those with the highest probability. Fish and insects can escape easily, especially in juvenile form, spread quickly and both have wild counterparts with which to mate. Thus escaped GM fish could easily become feral and, as our waterways are interconnected, spread is likely. This link is therefore strong for aquatic organisms.Box 1
The last link, the possibility that the transgene spreads after escape, depends on the forces of natural selection, which are universal for all organisms. It is the easiest to quantify because the ability of a transgene to spread is based on the rules of population genetics. This factor therefore represents the part of the risk formula that science can address. Thus, if we concentrate our efforts on this link and, where possible, reduce its probability towards zero, then the overall risk would approach zero as well.
Predicting the outcome of natural selection is a two-step process. First, it involves estimating the net fitness components of the altered genotype. In the second step, these parameters are fed into a model that predicts changes in gene frequency and population size. Prout (1971) first described the relationship between net fitness components and population predictions. He stressed a general approach with a small set of components that encompasses the entire life cycle of the transgenic organism and can be verified by experimental validation. We expanded Prout's original approach to include six components: juvenile viability, age to sexual maturity, mating success, female fecundity, male fertility and adult viability. The advantage of this approach is that the specific mechanisms that cause a difference in net fitness, such as physiology, behaviour and immune processes, need not be identified, thus saving considerable time and expense. This also solves the problem that measurement of such subcomponents is hard to interpret in terms of risk; that is, would a higher metabolic rate result in higher or lower risk? GM fish may swim faster but would also need more food. Scientists can argue over such details until the cows come home but if they cannot agree on how to interpret results, the lay public may throw up their hands in disgust and conclude that they should err on the side of caution and reject the product in question. This is where our method can contribute, as it provides a model on which scientists can agree. In the end, it is the bottom line that counts, and most scientists can agree on what the net fitness components mean for risk. Our approach thus combines estimates of the net fitness parameters described above into a mathematical model to determine the fate of a transgene in the affected wild population (Muir & Howard, 2001; Howard et al, 2004).
Because risk results from a chain of events–escape, followed by spread, followed by harm–the analogy of a chain suggests that it is only as strong as its weakest link
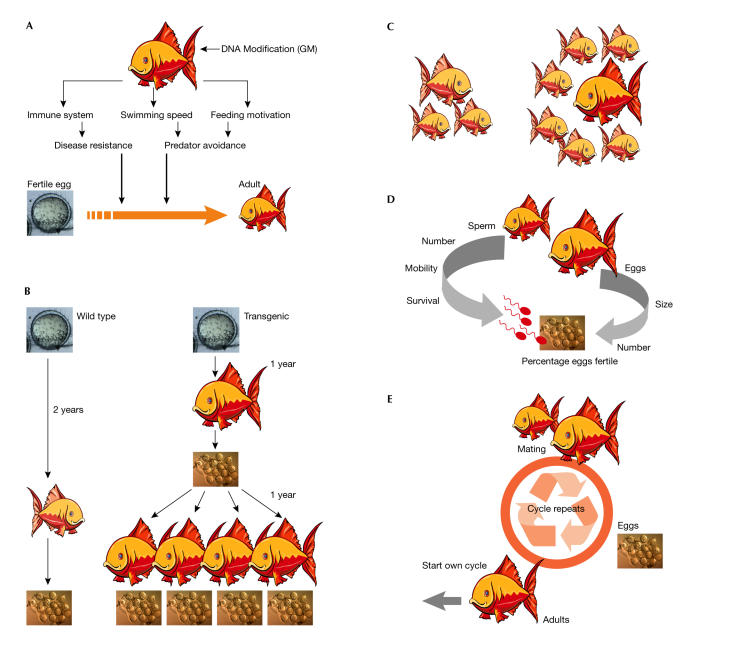
In the specific case of GM fish, juvenile viability describes the ability of young transgenic fish to reach adulthood and reproductive age (Fig 2A). Modifications to increase disease resistance would clearly influence this factor, as would transgenes that increase nutrient use, such as phytase, or cause an increase in environmental tolerance against cold, heat or salinity. A transgene, such as a growth hormone gene, that lowers the age of sexual maturity would also be a crucial factor for population expansion. Fish that reach maturity faster will expand in number more rapidly over the course of several generations (Fig 2B). Another important factor is mating success or sexual selection, which is often stronger than viability. If GM males are more attractive to wild-type females, then the prospects for a Trojan gene increase (Fig 2C). In nature, the ability to mate is a much more important force in evolution than Darwin's survival of the fittest. What good does it do for the species to survive to sexual maturity if it cannot then pass on its genes? Relative fecundity—the number of eggs produced by female fish and the number, mobility and survival chances of sperm produced by males—determines the number of offspring per mating pair (Fig 2D). Finally, adult viability describes how often a mating pair can repeat the mating process, again producing more offspring per individual (Fig 2E). These factors are interconnected and even if one of them is low, GM fish could still harm wild populations. If, for instance, juvenile viability is low but mating success is high, GM fish—if they survive to reproductive age—would still be able to spread the transgene among wild-type fish and thus lower the survival chances of both populations (Muir & Howard, 1999).
Figure 2.
Net fitness components for risk assessment. (A) All impacts of genetic modification on physiology and survival rate can be simply measured as the proportion of juvenile fish surviving to sexual maturity. (B) Age at sexual maturity determines the number of possible generations per time. (C) Differential mating success (sexual selection) is a strong selective force of evolution in natural populations. (D) Relative fecundity and fertility determine the number of offspring per mating pair. (E) Adult viability determines how often a pair can repeat the mating process
If such an assessment finds that the probability of transgene spread in the wild population is high, then our efforts should concentrate on biological methods to contain the transgene, such as by using sterile fish. It would also give regulators some idea of whether all transgenic fish should be tested for sterility or only samples, and how large these samples should be. The 2004 NRC report on Biological Confinement of Genetically Engineered Organisms remarks that “the net fitness method (Muir & Howard, 2001; Howard et al, 2004) provides a means to estimate—in a secure setting—the probability of severity of the harmful consequences from such transgenic spread. This estimate would help decision makers determine whether to screen all or only a subsample of the population lot” (NRC, 2004). Alternatively, the method could also be used to manage risk when designing GM organisms. By answering the question “What aspects of the life history of the organism result in spread of the transgene?” scientists can devise measures to change or mitigate them.
However, our model has its limitations. In its current form, it is deterministic; that is, the input parameters absolutely determine the predictions. Two workshops convened by Information Systems for Biotechnology (www.isb.vt.edu), a biological impact assessment programme managed by the US Department of Agriculture, discussed the model and made two main recommendations to improve it (Hallermann, 2002; ISB, 2004). It should incorporate stochasticity to include random effects, such as failure to mate or gene swamping if a large number of domesticated fish escape into a relatively small natural gene pool. Furthermore, the model predictions should reflect any uncertainty in both the fitness estimates and the outcome of natural selection.
By allowing regulators to define and use a consistent set of criteria, our model could provide a general way of evaluating GM animals as part of a standard risk-assessment procedure
Another problem with estimating fitness components is the genetic background. If the transgene is inserted next to polymorphic genes that have an impact on fitness in some way, the joint effect of these genes must be considered. Because transgenes are usually inserted into domesticated animals, which are poorly adapted to natural environments, the transgene will probably be linked to maladaptive genes under natural conditions. Although linkage effects between the transgene and other genes disappear over time through recombination, they are high in the short term and present a true challenge for net fitness estimates. Such problems could be addressed by crossing GM fish into a wild background and randomly mating for a few generations to dissipate linkage effects before estimating fitness components. Finally, our approach does not eliminate the need to assess for other events, such as natural disaster or human error. But such catastrophes are rare and cannot be demonstrated on normal timescales. The emphasis must therefore remain on cautious predictions of transgene spread as a first step in risk assessment. To relate this to the analogy of 'Trojan horse' genes that may seep into wild populations, we can take the horse apart and examine it before we take it in; that is, before we allow the commercial use of GM fish.
Risk assessment for transgenic organisms has become an increasingly important tool for regulators and policymakers to ascertain potential environmental harm and draft prevention measures. By allowing regulators to define and use a consistent set of criteria, our model could provide a general way of evaluating GM animals as part of a standard risk-assessment procedure. In light of the overall resistance against GM organisms in general, such a generalized model, if widely accepted by regulators and scientists, could make the risk-assessment process more streamlined and effective and allow regulators to address specific concerns. Furthermore, as the political fight over the approval and regulations of GM crops has shown (Moore, 2003), a widely accepted risk-assessment model for regulators could prevent or solve trade conflicts over the use and marketing of foods made from GM organisms. Improving risk-assessment procedures would also help to ensure public trust in this potentially beneficial technology, something that is sorely needed to counter the concern and growing resistance against GM organisms in Europe and the USA. Given the declining stocks of wild fish worldwide and the increasing stress on natural resources, GM fish should not be dismissed so easily.
The mathematics of risk assessment.
In general, risk is the likelihood of harm done to the environment resulting from exposure under environmentally relevant conditions. Thus, in the case of GM organisms, the risk is the product of the probability of exposure, that is, whether the transgene spreads, P(Exposure), and harm if the transgene spreads, P(Harm/Exposure). The probability of exposure is again the product of at least two parts, one conditional on the other. The first one is the probability of the organism escaping into the wild, dispersing and becoming feral, P(Escape). The second factor is the ability of the transgene itself to spread in the wild population once it has been introduced by an escaped animal, P(Transgene spread/Escape). Thus, the overall formula for assessing risk of harm from a GM animal is: risk = P(Harm/Exposure) × P(Escape) × P(Transgene spread/Escape).
Acknowledgments
GloFish™ images from www.glofish.com (courtesy of Yorktown Technologies LP).
References
- Devlin RH, Biagi CA, Yesaki TY, Smailus DE, Byatt JC (2001) Growth of domesticated transgenic fish. Nature 409: 781–782 [DOI] [PubMed] [Google Scholar]
- FAO (1999) The state of world fisheries and aquaculture 1998. FAO, Rome, Italy
- FAO (2000) The state of world fisheries and aquaculture 2000. FAO, Rome, Italy
- Hallermann EM (2002) ISB workshop suggests strengthening and broadening net fitness model. ISB News Report, August 2002. www.isb.vt.edu
- Howard RD, deWoody JA, Muir WM (2004) Transgenic male mating advantage provides opportunity for Trojan gene effect in a fish. Proc Natl Acad Sci USA 101: 2934–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISB (2004) Extending the net fitness model to consideration of crop gene flow. ISB News Report, January 2004. www.isb.vt.edu
- Lynch M, O'Hely M (2001) Captive breeding and the genetic fitness of natural populations. Conserv Genet 2: 363–378 [Google Scholar]
- Moore A (2003) Food fights. EMBO reports 4: 647–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir WM (2001) Potential environmental risks and hazards of biotechnology. I. Risks and hazards. ISB News Report, November 1. www.isb.vt.edu
- Muir WM (2002) Potential environmental risks and hazards of biotechnology. II. Methods to estimate risks and hazards. ISB News Report, February 1. www.isb.vt.edu
- Muir WM, Howard RD (1999) Possible ecological risks of transgenic organisms release when transgenes affect mating success: sexual selection and the Trojan gene hypothesis. Proc Natl Acad Sci USA 96: 13853–13856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir WM, Howard RD (2001) Fitness components and ecological risk of transgenic release: a model using Japanese medaka (Oryzias latipes). Amer Nat 158: 1–16 [DOI] [PubMed] [Google Scholar]
- Muir WM, Howard RD (2002a) in Genetically Engineered Organisms: Assessing Environmental and Human Health Effects (eds Letourneau DK, Burrows BE) 355–383. CRC Press, Boca Raton, FL, USA [Google Scholar]
- Muir WM, Howard RD (2002b) Environmental risk assessment of transgenic fish with implications for other diploid organisms. Transgenic Res 11: 101–114 [DOI] [PubMed] [Google Scholar]
- Nam YK, Noh JK, Cho YS, Cho HJ, Cho KN, Kim CG, Kim DS (2001) Dramatically accelerated growth and extraordinary gigantism of transgenic mud loach Misgurnus mizolepis. Transgenic Res 10: 353–362 [DOI] [PubMed] [Google Scholar]
- Naylor RL, Williams SL, Strong DR (2001) Aquaculture—a gateway for exotic species. Science 294: 1655–1656 [DOI] [PubMed] [Google Scholar]
- Nowak R (2002) Gene warfare to be waged on invasive fish. New Scient online 8 May. www.newscientist.com [Google Scholar]
- NRC (2004) Biological Confinement of Genetically Engineered Organisms. National Academic Press, Washington, DC, USA [Google Scholar]
- NRC (2002) Animal Biotechnology: Science Based Concerns. National Academic Press, Washington, DC, USA [PubMed] [Google Scholar]
- Prout T (1971) The relation between fitness components and population predictions. Genetics 68: 127–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Mak R, Ayad H, Smith A, Maclean N (1998) Expression of a novel piscine growth hormone gene results in growth enhancement in transgenic tilapia (Oreochromis niloticus). Transgenic Res 7: 357–369 [DOI] [PubMed] [Google Scholar]
- Tidwell JH, Allan GL (2001) Fish as food: aquaculture's contribution. EMBO Rep 2: 958–963 [DOI] [PMC free article] [PubMed] [Google Scholar]