Abstract
UV irradiation causes inflammatory and proliferative cellular responses. We have proposed previously that these effects are, to a large extent, caused by the ligand-independent activation of several receptor tyrosine kinases due to the inactivation of their negative control elements, the protein tyrosine phosphatases (PTPs). We examined the mechanism of this inactivation and found that, in addition to reversible oxidation of PTPs, UV triggers a novel mechanism: induced degradation of PTPs by calpain, which requires both calpain activation and substrate PTP oxidative modification. This as yet unrecognized effect of UV is irreversible, occurs predominantly with UVA and UVB, the range of wavelengths in sunlight that reach the skin surface, and at physiologically relevant doses.
Keywords: UV irradiation, PTP1B, SHP1, LAR-PTP, calpeptin
Introduction
The high-energy spectrum of sunlight exerts a number of adverse effects on human skin, ranging from sunburn to whole-body immune reactions to skin cancer. For some of these, reasonably well-documented mechanisms have been proposed: for example, UV-induced growth arrest and apoptosis through p53 stabilization and/or the FasR/Fas ligand system (e.g. Maltzman & Czyzyk, 1984; Yamaizumi & Sugano, 1994; Leverkus et al, 1997; Aragane et al, 1998). The primary steps and signalling components involved in most responses are, however, still obscure. It is clear that UV energy is absorbed by numerous cellular molecules, and a correspondingly large number of molecular alterations are to be expected. We and others discovered a non-nuclear action of UV, which causes the ligand-independent activation of receptor tyrosine kinases (RTKs; Sachsenmaier et al, 1994; Coffer et al, 1995) on the basis of the inhibition of negatively acting protein tyrosine phosphatases (PTPs; Knebel et al, 1996; Gross et al, 1999). Until now, about 90 PTPs have been identified in human cells (Andersen et al, 2001). If PTPs were sensitive to UV irradiation in general, a whole spectrum of cellular consequences could arise. As the majority of active PTPs exert negative control on signalling pathways (Long, 1999; Östman & Böhmer, 2001), PTP inhibition may contribute significantly to the inflammatory and tumour-promoting effects of sunlight on the skin.
The inactivation of PTPs is in part reversible and is caused by oxidation of the common cysteine in the active centre of all PTPs (Barrett et al, 1999; Gross et al, 1999; Meng et al, 2002; Salmeen et al, 2003; van Montfort et al, 2003; P.G., B.M., M.G., F.-D.B. and P.A.H., unpublished data). During the study of the inactivation process in response to UV irradiation, we discovered a novel mechanism induced by UVB and UVA: PTP degradation by calpain.
Results
Inactivation of PTP1B by UVA irradiation of cells
Inactivation of PTPs was determined using a number of enzymatic assays and one of these, the hydrolysis of p-nitrophenyl phosphate (pNPP) by immunoprecipitated PTP1B, is shown in Fig 1A. These assays revealed an amazing hypersensitivity of PTPs in comparison to inactivation of other thiol-dependent enzymes (shown for UVA irradiation of A431 cells). PTP inactivation by UVA occurred with doses at least in a range that would occur during human skin exposure to sunlight (Cole, 2001; Mckenzie et al, 2003). Also, UVB and UVC inactivate PTPs (shown for PTP1B in Fig 1B). Part of the inactivation was reversible in that activity could be regained by the addition of a reducing agent, N-acetylcysteine (NAC; Fig 1B). Very high doses of these UV qualities are, however, required for PTP inactivation.
Figure 1.
Loss of protein tyrosine phosphatase activity and protein from A431 cells by UV irradiation. (A) Hypersensitivity of PTP1B to UV irradiation. Confluent A431 cells were irradiated with different doses of UVA. PTP1B was immunoprecipitated from lysates, and its activity was measured using the pNPP assay. Alkaline phosphatase (AP) and alcohol dehydrogenase (ADH) activities were assayed as described in Methods, and the activity of caspase 3 was assayed using a commercial kit (Calbiochem). (B) Reversible inactivation of PTP1B. PTP1B immunoprecipitates obtained as in (A) were split, either mock-treated or treated with 50 mM NAC at 25°C for 10 min under agitation, and activity was determined by the pNPP assay. (C) The data shown in (B) were normalized for the protein amounts of PTP1B in the immunoprecipitates as detected by immunoblotting. (D) Loss of PTP protein on UV irradiation. Within 1–2 min after irradiation, cells were lysed and subjected to western blotting using antibodies directed against PTP1B, SHP1, LAR-PTP and actin. (E) No loss of PP-2A, pro-caspase 8, actin or Hsp90 occurred on UV irradiation. Immunoblots are as in (D).
In parallel to the enzymatic assays, we determined the amount of PTP1B by immunoblot and found, to our surprise, that part of the enzyme had disappeared, most predominantly after UVA or UVB irradiation of cells. These losses account for only the partial recovery by NAC treatment. If the data of Fig 1B were normalized for immunodetectable PTP1B, nearly complete recovery of activity for the remaining protein was detectable (Fig 1C).
UVA and UVB trigger proteolytic degradation of PTPs
Comparison of immunoblots for several PTPs after irradiation of A431 cells with doses leading to PTP inactivation revealed different degrees but substantial dose-dependent losses of PTP1B, LAR-PTP and SHP1 induced by UVA and UVB (Fig 1D). Disappearance of immunodetectable PTP occurred with amazingly brief kinetics, that is, within 10 min of UVA and UVB irradiation (at 12–17°C). The losses were less pronounced or even absent after irradiation of cells with shorter wavelengths (Fig 1D). In contrast to PTPs, the levels of actin in UV-treated cells remained unchanged. Also, levels of several other proteins tested including the serine/threonine phosphatase PP2A, pro-caspase 8 and heatshock protein 90 (Hsp90) were unchanged in UVA- or UVB-treated cells (Fig 1E). Subsequent to UVA irradiation with 25 kJ/m2, PTP1B levels remained low for at least 1 h and then gradually recovered to control levels at 24 h, presumably by new synthesis (supplementary Fig 1 online).
Non-recoverable PTP could either be the result of a modification, which leads to loss of recognition by the antibodies used, or the result of degradation. We tested several specific anti-PTP1B antibodies in the immunoblotting assay to decrease the chance of non-recognition by epitope modifications. The losses were, however, observed with all antibodies used (not shown). Indeed, several lines of evidence support the existence of UV-induced PTP degradation. As a first indication, the PTP in-gel assay (Burridge & Nelson, 1995) revealed, in the UVA- and UVB-treated samples, faster migrating fragments of PTP1B, which retained enzymatic activity (Fig 2A), suggesting stepwise degradation.
Figure 2.
Calpain-dependent protein tyrosine phosphatase degradation. (A) Active fragments of PTP1B were detected by in-gel assay (Burridge & Nelson, 1995; Markova et al, 2003). (B,C) The calpain inhibitor calpeptin prevents UV-induced degradation of PTPs. PTP1B was immunoprecipitated before western blotting, and LAR-PTP was blotted directly from the lysate. Before UV irradiation, A431 cells were mock-treated or were treated with calpeptin (1 μM) at 37°C for 4 h. Cell lysates of all samples were generated in the presence of calpeptin (5 μM).
With the notion of induced proteolytic degradation, we tested whether protease inhibitors could counteract the UV-induced loss of PTPs. Two inhibitors of the Ca2+-dependent protease calpain, E64d and calpeptin, blocked the induced disappearance of the full-length enzymes PTP1B and LAR-PTP (shown for calpeptin in Fig 2B,C). Other inhibitors, for example, those acting on the proteasome or caspase-3 (MG132; z-DEVD-fmk), could not prevent the loss of PTP (not shown).
Requirement for PTP modification and calpain activation
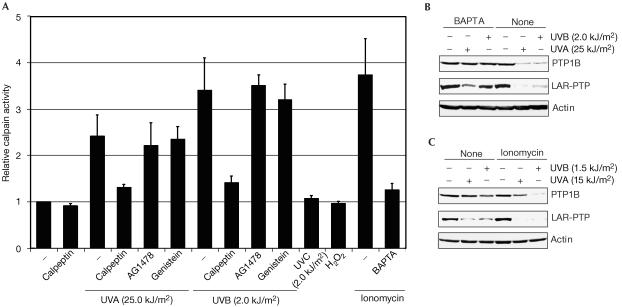
How could the calpain-dependent degradation of PTPs be induced by UV? We considered two possibilities: a UV-induced activation of calpain and a UV-induced modification of PTPs, which renders them susceptible to calpain. Irradiation of cells with UVA or UVB, but not UVC, led to an elevation of calpain activity (Fig 3A). Calpain is a calcium-dependent enzyme, and addition of ionomycin also caused an increase of calpain activity (Fig 3A). We therefore considered whether UVA or UVB could cause an increase in intracellular calcium. To this end, we treated cells with calcium-chelating agents. The intracellular calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester (BAPTA) efficiently blocked the UVA- or UVB-induced PTP degradation (Fig 3B), whereas the extracellular calcium chelator EGTA did not (not shown). Conversely, ionomycin enhanced PTP degradation and acted additively with limiting doses of UVB or UVA (shown for PTP1B and LAR-PTP in Fig 3C). BAPTA prevented the ionomycin-induced activation of calpain (Fig 3A). The tentative conclusion is that UV irradiation leads to an increase in intracellular calcium concentration.
Figure 3.
UV induces calcium-dependent calpain activation and protein tyrosine phosphatase degradation. (A) Relative calpain activity after various types of treatment. A431 cells were pretreated with calpeptin as in Fig 2B,C. AG1478 (100 nM), genistein (100 μM) or H2O2 (5 mM) was present for 15 min, ionomycin (2 μM) for 2 min or BAPTA (2 μM) for 30 min before UV irradiation. Calpain activity of lysates was determined using a commercially available kit (Calbiochem). The activity of untreated cells, set at 1, represents cleavage of 53 pmol/min of substrate at 37°C. (B,C) Modulation of intracellular calcium levels affects UV-induced PTP degradation. (B) Cell-permeable calcium chelator blocks UV-induced PTP degradation. A431 cells were cultivated in calcium-free medium for 30 min, and then treated with BAPTA (2 μM) for 30 min before irradiation. Immunoblots of lysates are shown. (C) Ionomycin enhances UV-induced PTP degradation. Ionomycin treatment is as in (A); immunoblots of lysates are shown.
Calcium release occurs in response to activation of RTKs. However, neither the treatment with the general tyrosine kinase inhibitor genistein nor treatment with the epidermal growth factor receptor (EGFR)-specific inhibitor AG1478 (Fig 3A; Levitzki & Gazit, 1995), or with the MEKselective inhibitor UO126 (supplementary Fig 2 online) interfered with UV-induced calpain activation. It is therefore improbable that calpain activation is downstream of the UV-induced activation of RTKs.
Importantly, ionomycin treatment alone, without UV, had no effect on PTP levels (Fig 3C) despite three- to fourfold elevation of calpain activity (Fig 3A). Thus, calpain activation was possibly required but clearly not sufficient for the induction of PTP degradation, strongly suggesting that a modification of PTP was required to make it a calpain substrate.
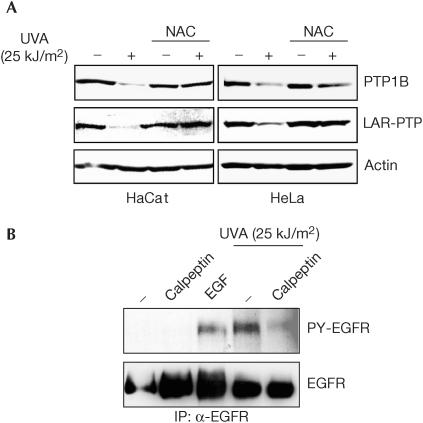
The obvious modification triggered by irradiation and possibly relevant for recognition by calpain is the UV-mediated PTP oxidation. This was proven to be true by the use of the cell-permeable reducing agent NAC. As shown in Fig 4A, pretreatment of cells with NAC significantly reduced UVA- and UVB-induced PTP1B degradation.
Figure 4.
UV- and calpain-dependent protein tyrosine phosphatase degradation requires previous PTP oxidation. (A) Antioxidant treatment attenuates UV-induced degradation. A431 cells were treated with NAC (30 mM) for 2 min before irradiation, and the lysates were immunoblotted. (B) Reversible oxidation converts PTP1B to a calpain substrate in vitro. Left panel: Cells were treated with H2O2 (5 mM) at 37°C for 15 min and PTP1B was immunoprecipitated. The precipitates were divided into two parts, one of which was digested with 0.4 U of calpain at 37°C for 30 min. Right panel: PTP1B was immunoprecipitated from an untreated cell lysate, and either reversibly or irreversibly oxidized by treatment with 50 μM of H2O2 for 4 or 16 h, respectively (Salmeen et al, 2003; supplementary Fig 3 online) and exposed to calpain as in the left panel. (C) Reversible oxidation of PTP1B increases the susceptibility to calpain but not to other proteinases. PTP1B was immunoprecipitated and reversibly oxidized (as in Fig 3B) or mock-treated. The immunoprecipitates were then left untreated or were treated with 0.4 U of the indicated proteinases at 37°C for different periods. PTP1B protein digestion was measured by quantification of the PTP1B band in immunoblots. The ratio of the amount of reversibly oxidized versus non-oxidized PTP1B remaining after digestion is depicted. (D) Combined treatment with H2O2 (as in (B)) and ionomycin (as in Fig 3A) leads to PTP degradation. Immunoblots of lysates are shown.
An observation made with H2O2 helped us to dissociate and distinguish calpain activation from substrate oxidation. In all, 50% immunoprecipitated PTP1B is cleaved once by calpain in vitro during the incubation time of 30 min at 37°C, yielding a 42 kDa cleavage product, which has been described previously (Frangioni et al, 1993). To test whether oxidation renders PTPs susceptible to calpain-dependent complete degradation, we treated A431 cells with 5 mM H2O2 under conditions that lead to the oxidation of PTPs. This treatment neither induced calpain activity (Fig 3A) nor caused PTP degradation (Fig 4B, left panel). Then, PTP1B was immunoprecipitated (supplementary Fig 3A,B online) and exposed to calpain in vitro. PTP1B from H2O2-treated cells but not from mock-treated cells was readily degraded by calpain within 30 min at 37°C (Fig 4B, left panel). Further, treatment of immunoprecipitated PTP1B with H2O2 in vitro under conditions leading to reversible oxidation (4 h at 20°C; Salmeen et al, 2003; supplementary Fig 3C online) increased its susceptibility for digestion by calpain but not by other proteinases (Fig 4B,C). Interestingly, treatment of PTP1B under conditions leading to largely irreversible oxidation (16 h at 20°C; Salmeen et al, 2003; supplementary Fig 3C online) led to lesser sensitivity for calpain degradation (Fig 4B, right panel). Thus, both reversible substrate modification, perhaps as the major determinant, and enzyme activation are needed to trigger degradation of the PTPs. H2O2 apparently causes substrate modification but not sufficiently the activation of calpain. Ionomycin induces calpain activity but does not modify the substrate. The combination should, however, cause degradation, if the hypothesis were correct. To this end, we tested PTP oxidation by H2O2, on the one hand, combined with calpain activation by ionomycin, on the other, for an effect on PTP stability. Both agents alone were insufficient to trigger degradation. The combined treatment, however, led to pronounced degradation of PTP1B to a similar extent as by UVA treatment (Fig 4D).
Physiological significance
UV-induced PTP degradation can also be detected in HeLa and HaCat cells (Fig 5A), demonstrating the generality of this effect. This raises the question as to what extent the novel pathway contributes to growth factor receptor activation. If the loss of PTP were relevant, a calpain inhibitor should suppress UV-induced and ligand-independent growth factor receptor activation. This is indeed the case. As shown in Fig 5B, calpeptin treatment significantly reduced UVA-induced phosphorylation of the EGFR. This finding indicates that calpain-mediated PTP degradation may even be the dominant mechanism of EGFR activation and of UV-induced signal transduction in a wavelength range (UVA) most relevant for sun exposure. Still, some elevation of EGFR phosphorylation occurred in the presence of calpeptin, probably reflecting the contribution of the inactivating reversible PTP oxidation.
Figure 5.
Physiological significance of UVA induced PTP degradation. (A) Generality of UVA-induced PTP degradation. HaCat and HeLa cells were left untreated or were treated with NAC as in Fig 4A, before mock treatment or UVA irradiation, and the lysates were immunoblotted. (B) UVA-induced activation of epidermal growth factor receptor (EGFR) requires calpain activity. A431 cells were treated with calpeptin before irradiation as in Fig 2 or stimulated with EGF (50 ng/ml) at 37°C for 5 min, where indicated. EGFR was immunoprecipitated from lysates and its tyrosine phosphorylation was determined by immunoblotting.
Discussion
Here we show that, in addition to reversible oxidation, UV irradiation of cells induces degradation of PTPs. It occurs with UVA at physiologically relevant doses and also with very high doses of UV of shorter wavelength (UVB≫UVC). The degradation is calpain-dependent, and requires the activation of the enzyme as well as the oxidative alteration of the substrates: PTPs.
The activation of calpain occurs independently of tyrosine kinase signalling; thus, it must be an early event like PTP oxidation. It involves an increase of intracellular calcium. It is yet unclear how UV can cause an immediate increase of intracellular calcium before receptor-induced signal transduction.
Oxidative PTP modification by UV is the second requirement for calpain-mediated degradation. Calpain is known to generate one major cut close to the carboxyl terminus of PTP1B (Frangioni et al, 1993), producing the 42 kDa cleavage product spontaneously visible in our cell lysates (Fig 2B) or on in vitro digestion of non-oxidized PTP1B (Fig 4B). UV-induced modification seems to expose additional cleavage sites such that the bulk of PTP1B or LAR disappears. The sulphenyl amide modification, which is probable also on UV-induced oxidation, distorts the catalytic site significantly including conformational changes, which expose previously buried parts of the molecule (Salmeen et al, 2003; van Montfort et al, 2003). One consequence may be the exposure of novel calpain cleavage sites, and in turn PTP degradation. The precise position of cleavage sites remains to be investigated. In in vitro studies with H2O2 under conditions that create only the single sulphenyl amide modification of the active centre cysteine (Salmeen et al, 2003), we observed elevated susceptibility of PTP1B to calpain. Irreversibly oxidized PTP1B, which again assumes the conformation of native enzyme (Salmeen et al, 2003), was less sensitive to calpain degradation, further supporting that reversible oxidation and the associated structural changes may suffice to convert PTPs to calpain substrates.
The biological relevance of UV-induced PTP degradation is supported by our observations that it occurs in several cell types, including the human keratinocyte cell line HaCat, leads to growth factor receptor activation and can be achieved at physiologically relevant doses of UVA. Whether the loss of PTP activity and subsequent receptor tyrosine kinase activation translates into tumour promotion or progression and/or it constitutes a major factor in the development of inflammation, is plausible, nevertheless still subject to speculation. It should be testable in suitable cell and mouse models. For example, cultured cells equipped with an excess of a relevant PTP should be less sensitive to UV, and disruption of the PTP gene should promote tumorigenesis in mice.
Methods
Cells and reagents. A431, HaCat and HeLa cells were grown at 37°C and 7% CO2 in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Monoclonal anti-PTP1B (clone FG6-1G) antibody, calpeptin, E64d, proteasome inhibitor MG132, caspase 3 inhibitor z-DEVD-fmk, BAPTA, genistein, AG1478, human erythrocyte calpain, recombinant caspase 3, pepsin, trypsin and agarose A/G plus beads were from Calbiochem; monoclonal anti-SHP1, anti-LAR-PTP, anti-EGFR, anti-PP2A and anti-phosphotyrosine (HRP conjugate) antibodies were from Transduction Laboratories; rabbit polyclonal anti-PTP1B (H-135), goat polyclonal anti-caspase 8 and goat polyclonal anti-actin antibodies were from Santa Cruz; monoclonal anti-EGFR antibodies 425 was a gift from Dr A. Luckenbach; goat polyclonal anti-Hsp90 antibodies was provided by Dr O. Kassel; complete protease inhibitor cocktail was from Roche, Mannheim. Various other chemicals were obtained from Sigma.
UV treatments. UVB and UVC irradiations were performed with lamps from Vetter, Wiesloch, with the following half-maximal wavelengths and power: UVB (UVM 30), 285–330 nm, 18 W/m2 at a distance of 8 cm; UVC (UVK 15), 253–255 nm, 10 W/m2 at a distance of 8 cm; UVA (UVA 1), irradiation with a Sellasol-1200 lamp from Sellas Sunlight Systems, Gevelsberg, Germany, 340–440 nm, 22 W/m2 at a distance of 8 cm. The UV dose was monitored with a Black-Ray Ultraviolet meter equipped with a UV detector (Ultraviolet Products Inc.). Control samples were kept in the dark under the same conditions.
For UV irradiation, A431 cells were cultured to confluence in 5–10 cm Petri dishes and washed two times with ice-cold phosphate-buffered saline (PBS). A layer of about 1 mm PBS in height was left on the cells before UV irradiation (on ice to avoid heating by UVA lamp).
PTP1B immunoprecipitation. A431 cells were washed twice with ice-cold PBS and lysed in ice-cold degassed lysis buffer (degassing of buffer at 4°C for 6 h) containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1% Nonidet-P40 (NP40), 1 mM EDTA, 20 μg/ml leupeptin, 1 μg/ml pepstatin, 20 μg/ml aprotinin, 1X complete protease inhibitor cocktail and 1 mM phenylmethylsulphonyl fluoride. Immunoprecipitation (IP) was performed with anti-PTP1B antibody (clone FG6-1G) and Sepharose A beads at 4°C for 4 h. The immunoprecipitates were washed three times in lysis buffer.
pNPP assay for PTP1B activity. Immunoprecipitated PTP1B was resuspended in 50 μl of ice-cold degassed assay buffer, containing all constituents of lysis buffer except 1% NP40, and transferred to a 96-well plate. The assay was started by adding 24 mM (final concentration) of pNPP and incubating the immunoprecipitate at 37°C in the dark. Absorbance at 405 nm was measured at periods between 0 and 120 min. The slope of the resulting graph was used to calculate PTP activity.
Other enzyme assays. The activity of alcohol dehydrogenase was measured by incubating an aliquot of lysates with an equal volume of assay buffer (1 mM NAD, 0.2% ethanol and 50 mM Tris (pH 8.8)) at 37°C. The kinetics of NAD reduction was measured at 340 nm and was plotted as percentage activity. Alkaline phosphatase activity was measured according to Reese et al (1981) by incubating an aliquot of the lysates with an equal volume of assay buffer (0.64 M 2-amino-2-methyl-1-propanol (pH 10.3) and 1 mM MgCl2) containing 24 mM pNPP at 37°C. The absorbance of p-nitrophenol liberated was measured at 405 nm at periods between 0 and 120 min.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v5/n8/extref.7400190s1.pdf).
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Dr B. Fusenig (Heidelberg) for the kind provision of HaCat cells. This work was supported by grants from the Deutsche ForschungsgemeinSchaft (BO1043-4 and SFB604/A1 to F.D.B. and DFG HE551/13-1 to P.H.).
References
- Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Moller NP (2001) Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol 21: 7117–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger TA, Schwarz T (1998) Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol 140: 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB (1999) Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705 [DOI] [PubMed] [Google Scholar]
- Burridge K, Nelson A (1995) An in-gel assay for protein tyrosine phosphatase activity: detection of widespread distribution in cells and tissues. Anal Biochem 232: 56–64 [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Burgering BM, Peppelenbosch MP, Bos JL, Kruijer W (1995) UV activation of receptor tyrosine kinase activity. Oncogene 11: 561–569 [PubMed] [Google Scholar]
- Cole C (2001) Sunscreen protection in the ultraviolet A region: how to measure the effectiveness. Photodermatol Photoimmunol Photomed 17: 2–10 [DOI] [PubMed] [Google Scholar]
- Frangioni JV, Oda A, Smith M, Salzman EW, Neel BG (1993) Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO J 12: 4843–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S, Knebel A, Tenev T, Neininger A, Gaestel M, Herrlich P, Böhmer FD (1999) Inactivation of protein-tyrosine phosphatases as mechanism of UV-induced signal transduction. J Biol Chem 274: 26378–26386 [DOI] [PubMed] [Google Scholar]
- Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P (1996) Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J 15: 5314–5325 [PMC free article] [PubMed] [Google Scholar]
- Leverkus M, Yaar M, Gilchrest BA (1997) Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp Cell Res 232: 255–262 [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A (1995) Tyrosine kinase inhibition: an approach to drug development. Science 267: 1782–1788 [DOI] [PubMed] [Google Scholar]
- Long E (1999) Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 17: 875–904 [DOI] [PubMed] [Google Scholar]
- Maltzman W, Czyzyk L (1984) UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol 4: 1689–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova B, Herrlich P, Ronnstrand L, Böhmer FD (2003) Identification of protein tyrosine phosphatases associating with the PDGF receptor. Biochemistry 42: 2691–2699 [DOI] [PubMed] [Google Scholar]
- McKenzie RL, Bjorn LO, Bais A, Ilyasad M (2003) Changes in biologically active ultraviolet radiation reaching the Earth's surface. Photochem Photobiol Sci 2: 5–15 [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9: 387–399 [DOI] [PubMed] [Google Scholar]
- Östman A, Böhmer FD (2001) Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol 11: 258–266 [DOI] [PubMed] [Google Scholar]
- Reese DH, Fiorentino GJ, Claflin AJ, Malinin TI, Politano VA (1981) Rapid induction of alkaline phosphatase activity by retinoic acid. Biochem Biophys Res Commun 102: 315–321 [DOI] [PubMed] [Google Scholar]
- Sachsenmaier C, Radler PA, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ (1994) Involvement of growth factor receptors in the mammalian UVC response. Cell 78: 963–972 [DOI] [PubMed] [Google Scholar]
- Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D (2003) Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423: 769–773 [DOI] [PubMed] [Google Scholar]
- van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H (2003) Oxidation state of the activesite cysteine in protein tyrosine phosphatase 1B. Nature 423: 773–777 [DOI] [PubMed] [Google Scholar]
- Yamaizumi M, Sugano T (1994) U.V.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene 9: 2775–2784 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures