Abstract
Ubiquitylation, the modification of cellular proteins by the covalent attachment of ubiquitin, is critical for diverse biological processes including cell cycle progression, signal transduction and stress response. This process can be reversed and regulated by a group of proteases called deubiquitylating enzymes (DUBs). Otubains are a recently identified family of DUBs that belong to the ovarian tumour (OTU) superfamily of proteins. Here, we report the first crystal structure of an OTU superfamily protein, otubain 2, at 2.1 Å resolution and propose a model for otubain–ubiquitin binding on the basis of other DUB structures. Although otubain 2 is a member of the cysteine protease superfamily of folds, its crystal structure shows a novel fold for DUBs. Moreover, the active-site cleft is sterically occluded by a novel loop conformation resulting in an oxyanion hole, which consists uniquely of backbone amides, rather than the composite backbone/side-chain substructures seen in other DUBs and cysteine proteases. Furthermore, the residues that orient and stabilize the active-site histidine of otubain 2 are different from other cysteine proteases. This reorganization of the active-site topology provides a possible explanation for the low turnover and substrate specificity of the otubains.
Introduction
In eukaryotes, the modification of cellular proteins by ubiquitin is one of the most important regulatory mechanisms determining protein stability and function. Almost as common as protein phosphorylation, ubiquitylation is critical for diverse biological processes including cell cycle progression, signal transduction and stress response (Hershko & Ciechanover, 1998; Ben-Neriah, 2002; Pickart, 2004). The covalent attachment of ubiquitin to target proteins involves a cascade of enzymatic reactions catalysed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) (Hershko & Ciechanover, 1998; Pickart, 2004). Ubiquitin is linked by an isopeptide bond formed between its carboxy-terminal glycine and the ɛ-amino group of lysine of the target protein. Deubiquitylation is catalysed by a heterogeneous group of cysteine proteases called deubiquitylating enzymes (DUBs; Wilkinson, 1997; Kim et al, 2003). On the basis of their sequences and activities, DUBs have been divided into two principal families of cysteine peptidases: ubiquitin C-terminal hydrolases (UCHs) and ubiquitinspecific proteases (USP/UBPs). Furthermore, several novel cysteine protease families with DUB activity have been identified, including cylindromatosis tumour suppressor (CYLDs), ataxins and josephines (Borodovsky et al, 2002; Burnett et al, 2003; Scheel et al, 2003). Additionally, a new deubiquitylating activity has been associated with the proteasomal Rnp11/POH1 protein (Maytal-Kivity et al, 2002; Verma et al, 2002; Yao & Cohen, 2002). The inhibition profile of this ‘cryptic' protease suggests that Rnp11, along with its signalosome analogue JAB1 (Cope et al, 2002), defines a new class of DUB—the JAMM, Jab1/MPN domain metalloenzyme family. This has been strongly supported by crystal structures of the JAMM motif, which show a fold that resembles that of cytidine deaminase and thermolysin, and places JAMM in a superfamily of metal-dependent hydrolases (Tran et al, 2003; Ambroggio et al, 2004).
Only two families of DUBs have been well characterized structurally and biochemically—UCHs (Johnston et al, 1997, 1999) and USP/UBPs (Hu et al, 2002)—both of which are cysteine proteases (Wilkinson, 1997). Despite having different overall structures, topologies and mechanisms of substrate recognition, they show a nearly identical papain-like geometry at the active site, including a characteristic organization of the catalytic triad and highly conserved positioning of the oxyanion hole. This similarity implies that DUBs in general might use a common catalytic mechanism for ubiquitin cleavage.
We have described the ubiquitin isopeptidase activity of two new proteins called otubain 1 and 2 that have no sequence homology to known DUBs, but which belong to the ovarian tumour (OTU) superfamily of proteins (Balakirev et al, 2003). Otubain 1 has also been shown to interact with the specific DUB inhibitor Ub1–75-bromoethylamide (Borodovsky et al, 2002). The OTU superfamily comprises more than 100 proteins from different organisms, including other proteins with isopeptidase activities such as Cezanne (Evans et al, 2003) and A20 (Evans et al, 2004). Both A20 and otubain 1 are involved in the regulation of cell immune response: A20 protein is an inhibitor of the NF-κB signalling pathway (Beyaert et al, 2000), whereas otubain 1 is a key regulator of T-cell anergy through the RING-type ubiquitin ligase GRAIL (Soares et al, 2004). Thus far, no biological role has been attributed to otubain 2. Although it has been demonstrated that OTU domain DUBs interact with a number of cellular proteins (Beyaert et al, 2000; Soares et al, 2004), the molecular mechanisms underlying their function are largely unknown. The low activity of otubains, their partnership with other DUBs and ubiquitin ligases, and their apparent specificity for isopeptide bonds all imply a different role from that of previously characterized DUBs (Balakirev et al, 2003; Soares et al, 2004). To identify possible determinants of otubain activity, we have determined the crystal structure of otubain 2 to 2.1 Å resolution (Fig 1).
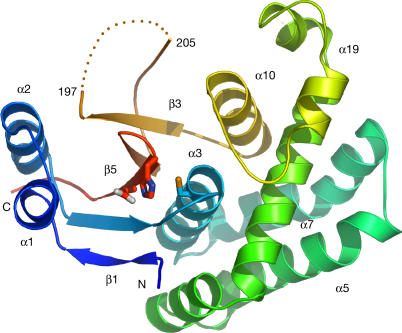
Figure 1.
Structure of otubain 2. Ribbon diagram of otubain 2, coloured from dark blue (N terminus) to red (C terminus). The catalytic residues Cys 51, His 224 and Asn 226 are shown in a stick representation. Selected secondary structural elements are labelled.
Results And Discussion
Otubain 2 has an overall dimension of 48 Å × 30 Å × 50 Å, and consists of a five-stranded β-sheet sandwiched in between a small helical amino-terminal region consisting of α1 and α2, and a large helical region comprised of α3–α10 (Fig 1). The active site is formed at the interface of α3 and the loop connecting strands 4 and 5 of the βsheet, and contains the catalytic triad, Cys 51, His 224 and Asn 226 (Figs 1, 2). The structure of the active site of otubain 2 confirms that OTU superfamily proteins in general, and otubains in particular, are members of the cysteine protease superfamily of folds. This is in accordance with previous studies that have shown that Cys 51 and His 224, as well as their homologous residues in otubain 1, are required for catalysis (Balakirev et al, 2003; Soares et al, 2004). However, the overall three-dimensional structure of otubain 2 is distinct from that of previously characterized DUBs and other cysteine proteases, with no similarity to any known structures, as verified using the DALI algorithm (Holm & Sander, 1995). To elucidate the similarities and differences between otubain 2 and other cysteine proteases, we performed a systematic structural comparison of otubain 2 with 18 other catalytic triad-containing cysteine protease structures from the MEROPS database (Rawlings et al, 2002). From this analysis, we were able to determine that the catalytic cysteine (Cys 51) and histidine (His 224) are structurally conserved with other cysteine proteases, and that otubain 2 has a novel sterically restricted activesite cleft (Fig 2). Additionally, we have created a model of ubiquitin interaction on the basis of the structures of Yuh1 and HAUSP, DUBs from the UCH and UBP families, respectively.
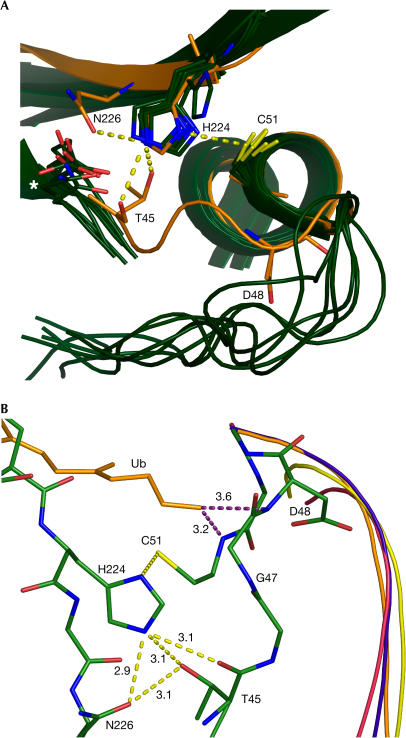
Figure 2.
Architecture of the otubain activesite. (A) The active-site cysteine, histidine and helix are structurally conserved between otubain (orange) and other cysteine proteases. Cysteine proteases from clans 1 (MEROPS database classification: 1pip, 8pch), 5 (1hav), 12 (1cmx), 19 (1nbf), 28 (1qmy) and 48 (1euv) are shown in green, with active-site residues shown in a stick representation. The spatial locations of the active-site residues T45 and N226 in otubain 2 are novel compared with previous cysteine protease structures (white asterisk on the left). Additionally, the conformation of the loop leading to the active site (44–48) is shifted towards C51 and H224 compared with other cysteine proteases. Also note that the distance between D48 and H224 is too far for a direct interaction. (B) Reorganization of the otubain 2 active site. Otubain is shown in green, UCH in yellow, the UCH Yuh1 in complex with ubiquitin in purple, HAUSP in red and HAUSP in complex with ubiquitin in orange. The ubiquitin chain from the HAUSP–ubiquitin complex is shown in an orange stick representation and labelled ‘Ub'. The side chain of K46 has been omitted for clarity. An oxyanion hole comprised solely of main-chain amides, rather than main-chain/side-chain interactions found in other cysteine proteases, is seen. Also note that between their bound and unbound states, no conformations from UCH/Yuh1 or HAUSP resemble that of the 44–48 loop in otubain 2.
The active-site cysteine and histidine residues and the core helix (α3) and strands (β4 and β5) are structurally well conserved with other cysteine proteases (Fig 2A). The similarity in the side-chain orientations of Cys 51 and His 224 in otubain 2 to those of other cysteine protease structures implies that they are in a catalytically productive geometry in otubain 2. In many cysteine proteases, a third residue is postulated to orient the activesite histidine or stabilize the imidazolium ion that is generated by deprotonation of the active-site cysteine. On the basis of sequence analysis, it was previously assumed that Asp 48 fulfilled this role (Makarova et al, 2000). However, in otubain 2, the side chain of Asp 48 is more than 8 Å from the activesite histidine, and thus too far to interact directly with His 224 (Fig 2). Instead, a hydrogen-bonding network is formed between Oδ of Asn 226, Oγ of Thr 45, the carbonyl oxygen of Thr 45, and Nɛ of His 224 (Fig 2). Both Thr 45 and Asn 226 (Asp in otubain 1) are highly conserved in all known otubain sequences, further underlining their importance (Fig 3). This hydrogen-bonding network is not seen in other DUBs and cysteine proteases. The involvement of Asn 226 in catalysis is supported by observations that mutation of Asn 226 to alanine inhibits the cleavage of ubiquitin C-terminal 7-amido-4-methylcoumarin (Ub-AMC) by otubain 2 (supplementary Fig S1 online). Although the position of Asn 226 is novel, it is likely that it orients and stabilizes His 224 in the same fashion as in other cysteine proteases.
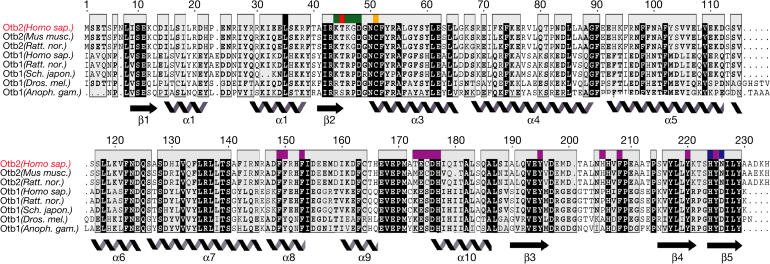
Figure 3.
Sequence alignment of otubains. Conserved residues are blocked in black. Colours above the sequence alignment are indicated as follows: Cys 51 (orange), His 224/Asn 226 (blue), the activesite occluding loop Lys 44–Asp 48 (green), Thr 45 (red) and proposed ubiquitin-binding region (pink). The N-terminal extensions of otubain 1 were removed for clarity. Anoph. gam., Anopheles gambiense; Dros. mel., Drosophila melanogaster; Homo. sap., Homo sapiens; Ratt. nor., Rattus norvegicus; Sch. japon., Schizosaccharomyces japonicus.
The loop preceding the active-site helix (residues 44–48) is in a conformation that is substantially different from all other structurally characterized cysteine proteases (Fig 2). The loop is shifted towards Cys 51 and His 224, generating a much more spatially restricted active site. As a result of this, it is possible that this structure represents a self-inhibited state. This novel conformation is not a consequence of crystal packing, as symmetry-related protomers do not make contacts near this loop region, and it is in the same conformation in a second crystal form (unpublished data). It has previously been shown that reorganization of activesite residues and secondary structural elements occurs in UCHL3/Yuh1 and HAUSP on ubiquitin binding. Similarly, conformational changes occur on release of proenzyme regions in other cysteine proteases. However, the structure of the 44–48 loop is distinct from UCHL3/Yuh1 and HAUSP in both their unbound and ubiquitin-bound forms (Fig 2B). Notably, the positioning of the 44–48 loop is structurally distinct from the L9 loop of UCHL3, which has previously been implicated in autoinhibition (Johnston et al, 1997). Indeed, in the region of the catalytic triad, both HAUSP and Yuh1 show a slightly more open active site in their unbound forms than in their ubiquitin-bound forms. However, in contrast to HAUSP, the geometry of the otubain 2 active site, as determined by the orientations of the activesite triad, is in a catalytically productive conformation in the absence of bound ubiquitin. Thus, if otubain 2 is inhibited by the conformation of the 44–48 loop, it could represent a novel mechanism of autoinhibition. Alternatively, the spatially restricted active site may explain the observed specificity of otubains towards an isopeptide bond, because this type of linkage should present much less sterical hindrance at the P′ position compared with a peptide bond.
Besides inducing a more spatially restricted active-site cleft, the 44–48 loop also induces a novel structure for the oxyanion hole. In most cysteine proteases, a glutamine located 5–7 residues before the active-site cysteine and the backbone amide group of the catalytic cysteine stabilize the oxyanion reaction intermediate (Fig 2B; Rawlings et al, 2002). Although Cys 51 is structurally homologous to other cysteine proteases, no known otubain sequences have a glutamine or an asparagine immediately preceding the activesite cysteine. Additionally, the 44–48 loop is positioned closer to the active-site residues, which leaves no room for an asparagine or a glutamine to stabilize the oxyanion reaction intermediate. These two facts make a novel oxyanion hole organization necessary. In looking for hydrogen bond donors in the vicinity of Cys 51, it becomes apparent that otubains use the amide of Cys 51 in combination with the backbone amide of Asp 48 to form the oxyanion hole. The amide of Asp 48 mimics the hydrogen-bonding potential of the terminal nitrogen of the canonical oxyanion glutamine (Fig 2B). Replacement of the oxyanionstabilizing side chain with a main-chain amide is unknown in cysteine proteases, but is well established in serine proteases (Blevins & Tulinsky, 1985) and acetylcholinesterases (Sussman et al, 1991). This model of the oxyanion hole is also well supported by our model of ubiquitin interaction (Fig 4).
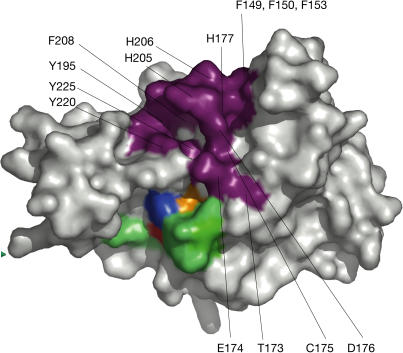
Figure 4.
Ubiquitin interaction and activesite. Colouring is the same as for Fig 3. Proposed ubiquitin-binding residues are shown in purple and are individually labelled.
To create a structural model of otubain–ubiquitin interactions, we overlaid the otubain 2 structure with ubiquitin-bound structures of Yuh1 (Johnston et al, 1999) and HAUSP (Hu et al, 2002) on the basis of their active sites. The activesite cysteine, histidine and secondary structure elements align well with those of otubain 2. Despite very different contacting residues, the C-terminal residues of ubiquitin aldehyde from Yuh1 and HAUSP are largely superimposable, lending further credence to the idea of a common structural geometry for ubiquitin binding. We identified a hydrophobic pocket similar to that found in HAUSP, which is formed by residues Tyr 195, Phe 208, Tyr 220 and Tyr 225 (Fig 4). Nearer to the activesite, a loop that is structurally conserved among HAUSP, Yuh1 and otubain 2 consisting of residues 173–177 also contacts ubiquitin in our model. Interestingly, we identified a potential metal-binding site, formed by His 205, His 206 and His 177, which is in a region that contacts ubiquitin in the HAUSP complex (Fig 4). Despite having side chains oriented in a geometry that could coordinate a metal ion, no metal ion density was seen, and initial enzymatic assays were unaffected by the addition of zinc, EDTA or phenanthroline. This motif is structurally unrelated to the recently structurally characterized NZF module (Alam et al, 2004). Finally, a surface-exposed hydrophobic patch consisting of Phe 149, Phe 150 and Phe 153 on α8 is well positioned and at the correct distance from the C terminus of Ub to form a hydrophobic pocket for residues His 68, Leu 69 and Val 70 of ubiquitin. Together, these regions define a likely ubiquitin-binding region. Sequence information supports the importance of these residues. Specifically, His 205 and His 206 are conserved within otubain 2, and Phe 149, Phe 150, Phe 153, His 177, Tyr 195, Phe 208, Tyr 220 and Tyr 225 are highly conserved across all otubains (Fig 3).
Recently, several members of a large superfamily of proteins containing OTU domains have been shown to possess deubiquitylating activity (Balakirev et al, 2003; Evans et al, 2003, 2004). However, no structural information on this intriguing class of DUBs has been available until now. We have determined the structure of otubain 2, a new type of DUB containing an OTU domain. The structure shows that otubains differ structurally from other DUBs in both their overall structure and activesite topology. Furthermore, several compensatory changes, including a novel hydrogen-bonding network to the active-site histidine and an oxyanion hole geometry that is unknown in cysteine proteases, are observed. The structural similarity between the active-site cysteine and histidine and other cysteine protease structures suggests a catalytically active state, whereas the reduction in the volume of the active-site loop points to a self-inhibited conformation. Alternatively, the active-site loop conformation could be implicated in the specificity of otubains for isopeptide bond (Balakirev et al, 2003; Soares et al, 2004). The elucidation of the significance of the novel conformation of the activesite loop awaits further structural and biochemical studies.
Methods
Protein purification and crystallization. N-terminally His-tagged otubain 2 was cloned into a pET20 expression vector. A mutation from G49 to an arginine was induced during cloning. It is unlikely that this mutation introduces any structural rearrangements, as there is no visible electron density for the side chain of this residue, implying that the side chain is disordered. More importantly, this mutant is catalytically active and hydrolyses Ub-AMC with a kc about one-third of that of wild-type enzyme (supplementary Fig S1 online). This decrease in catalytic activity could result from sterical hindrance of the active-site cleft by the arginine side chain and/or from the introduction of a positive charge in the neighbourhood of the active site. Finally, it should be noted that other otubain family members also have an arginine at this position. Protein was expressed in Escherichia coli BL21 (DE3) cells and purified on Ni NTA agarose (Qiagen) by a single elution step of 200 mM imidazole in buffer A (50 mM Tris (pH 8.0), 500 mM NaCl, 0.5 mM dithiothreitol (DTT)). Eluted samples were pooled, concentrated and dialysed against buffer A in the presence of 1,000 U of TEV protease. The cleaved and dialysed sample was subsequently depleted on a Ni NTA column to remove any uncleaved product. Protein was subsequently concentrated to 1 ml and loaded onto a Superdex 200 HiLoad 16/60 column (Amersham Pharmacia) gel filtration column in 50 mM NaCl, 5 mM DTT and 20 mM Tris (pH 8.0). Peak fractions were pooled and concentrated to 20–30 mg/ml and stored at −80°C. Selenomethionine protein production was performed by the methionine pathway inhibition method (Doublié, 1997). Sample purity and selenomethionine substitution (not detectably less than 100%) were confirmed by ESI-MS. Crystals of otubain 2 in the space group P212121 with one protomer in the asymmetric unit were obtained by the hanging-drop vapour diffusion method at 15°C using 2 μl drops and reservoir conditions of 14% PEG 4000, 0.1 M HEPES (pH 7.0), 12% isopropanol and 5 mM DTT. Although crystals formed overnight, none was of suitable quality for diffraction experiments. Initial crystals were used to generate larger crystals by microseeding. Crystals were transferred to a cryoprotection solution containing 20% PEG 4000, 0.1 M HEPES (pH 7.0) and 5 mM DTT; subsequently, PEG concentration was increased to 25% after a 20 min soak. Most crystals diffracted anisotropically with poor spot shape, but about one crystal in 30 diffracted well.
Structure determination. Phases were obtained from a four-wavelength MAD experiment (supplementary Table S1 online). Oscillation data were processed in Denzo/Scalepack (Otwinowski & Minor, 1997) and imported into SOLVE (Terwilliger & Berendzen, 1999), which identified the two ordered selenium atoms. Data were imported into the CCP4 (Collaborative Computational Project) package, and all three non-remote wavelengths were scaled to the remote wavelength using SCALEIT (Collaborative Computational Project). Selenium positions were then refined in SHARP (La Fortelle & Bricogne, 1997), and the map was improved by solvent flipping using the SOLOMON (Collaborative Computational Project) and DM packages. The resulting map was of excellent quality, and ARP/WARP (Perrakis & Lamzin, 1999) was used to automatically build and assign sequence to ∼80% of the model. The remaining model was built manually in O (Jones et al, 1991) and refined in REFMAC (supplementary Table S2 online; Collaborative Computational Project). Out of the 234 residues of otubain 2, 221 residues are visible in the structure. The remaining residues are in disordered regions at the N-terminus (M1–F6) and a seven-residue loop consisting of E198–N204. Final refinement statistics are shown in supplementary Table S2 online. The structure has been deposited in the PDB with code 1TFF.
Structural alignments. For all clans with an active-site triad and an available structure in MEROPS (Rawlings et al, 2002), structural alignments were performed with LSQKAB (Collaborative Computational Project). Structures were aligned on the basis of main-chain atoms at positions n−2 to n+1 and m−2 to m+1, where n is the position of the catalytic cysteine and m the position of the catalytic histidine.
Molecular graphics. All structural figures were generated by PyMOL v.93 for OS X (Delano, 2002).
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref.7400201s1.pdf).
Supplementary Material
Supplementary Figure and Tables
Acknowledgments
We thank D. Lemaire for the mass spectrometry data, J.L. Ferrer, R. Kahn, A. McCarthy and R. Ravelli for data collection time and beamline assistance, J. Doudna for TEV protease and C. Zubieta for critical reading of the manuscript. S.O.T. was partly funded by an FEBS fellowship.
References
- Alam SL, Sun J, Payne M, Welch BD, Blake BK, Davis DR, Meyer HH, Emr SD, Sundquist WI (2004) Ubiquitin interactions of NZF zinc fingers. EMBO J 23: 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggio XI, Rees DC, Deshaies RJ (2004) JAMM: a metalloprotease-like zinc site in the proteasome and signalosome. PLoS Biol 2: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakirev MY, Tcherniuk SO, Jaquinod M, Chroboczek J (2003) Otubains: a new family of cysteine proteases in the ubiquitin pathway. EMBO Rep 4: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y (2002) Regulatory functions of ubiquitination in the immune system. Nat Immunol 3: 20–26 [DOI] [PubMed] [Google Scholar]
- Beyaert R, Heyninck K, Van Huffel S (2000) A20 and A20-binding proteins as cellular inhibitors of nuclear factor-κB-dependent gene expression and apoptosis. Biochem Pharmacol 60: 1143–1151 [DOI] [PubMed] [Google Scholar]
- Blevins RA, Tulinsky A (1985) The refinement and the structure of the dimer of α-chymotrypsin at 1.67-Å resolution. J Biol Chem 260: 4264–4275 [DOI] [PubMed] [Google Scholar]
- Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM (2002) Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol 9: 1149–1159 [DOI] [PubMed] [Google Scholar]
- Burnett B, Li F, Pittman RN (2003) The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet 12: 3195–3205 [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–763 [DOI] [PubMed] [Google Scholar]
- Cope GA, Suh GS, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, Deshaies RJ (2002) Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science 298: 608–611 [DOI] [PubMed] [Google Scholar]
- Delano WL (2002) The PyMOL Molecular Graphics System. San Carlos, CA: Delano Scientific [Google Scholar]
- Doublié S (1997) Preparation of selenomethionyl proteins for phase determination. In Methods in Enzymology, Carter CW, Sweet RM (eds) Vol 276, pp 523–530. San Diego, CA: Academic [PubMed] [Google Scholar]
- Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL, Kilshaw PJ (2003) A novel type of deubiquitinating enzyme. J Biol Chem 278: 23180–23186 [DOI] [PubMed] [Google Scholar]
- Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS (2004) A20, a regulator of inflammation and cell survival, has deubiquitinating activity. Biochem J 378: 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479 [DOI] [PubMed] [Google Scholar]
- Holm L, Sander C (1995) Dali: a network tool for protein structure comparison. Trends Biochem Sci 20: 478–480 [DOI] [PubMed] [Google Scholar]
- Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y (2002) Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111: 1041–1054 [DOI] [PubMed] [Google Scholar]
- Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP (1997) Crystal structure of a deubiquitinating enzyme (human UCH-L3) at 1.8 Å resolution. EMBO J 16: 3787–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SC, Riddle SM, Cohen RE, Hill CP (1999) Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J 18: 3877–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Kim JH, Park KC, Chung SS, Bang O, Chung CH (2003) Deubiquitinating enzymes as cellular regulators. J Biochem (Tokyo) 134: 9–18 [DOI] [PubMed] [Google Scholar]
- La Fortelle ED, Bricogne G (1997) Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. In Methods in Enzymology, Carter CW, Sweet RM (eds) Vol 276, pp 472–494. San Diego, CA: Academic [DOI] [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Koonin EV (2000) A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem Sci 25: 50–52 [DOI] [PubMed] [Google Scholar]
- Maytal-Kivity V, Reis N, Hofmann K, Glickman MH (2002) MPN+, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. In Methods in Enzymology, Carter CW, Sweet RM (eds) Vol 276, pp 307–326. San Diego, CA: Academic [DOI] [PubMed] [Google Scholar]
- Perrakis AMR, Lamzin VS (1999) Automated protein model building combined with iterative structure refinement. Nat Struct Biol 6: 458–463 [DOI] [PubMed] [Google Scholar]
- Pickart CM (2004) Back to the future with ubiquitin. Cell 116: 181–190 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, O'Brien E, Barrett AJ (2002) MEROPS: the protease database. Nucleic Acids Res 30: 343–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel H, Tomiuk S, Hofmann K (2003) Elucidation of ataxin-3 and ataxin-7 function by integrative bioinformatics. Hum Mol Genet 12: 2845–2852 [DOI] [PubMed] [Google Scholar]
- Soares L, Seroogy C, Skrenta H, Anandasabapathy N, Lovelace P, Chung CD, Engleman E, Fathman CG (2004) Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol 5: 45–54 [DOI] [PubMed] [Google Scholar]
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (1991) Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 253: 872–879 [DOI] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J (1999) Automated MAD and MIR structure solution. Acta Crystallogr D 55: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HJ, Allen MD, Lowe J, Bycroft M (2003) Structure of the Jab1/MPN domain and its implications for proteasome function. Biochemistry 42: 11460–11465 [DOI] [PubMed] [Google Scholar]
- Verma R, Aravind L, Oania R, McDonald WH, Yates JR III, Koonin EV, Deshaies RJ (2002) Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298: 611–615 [DOI] [PubMed] [Google Scholar]
- Wilkinson KD (1997) Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 11: 1245–1256 [DOI] [PubMed] [Google Scholar]
- Yao T, Cohen RE (2002) A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419: 403–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure and Tables