Abstract
Immune responses against pathogens require that microbial components promote the activation of antigen-presenting cells (APCs). Autoimmune diseases and graft rejections occur in the absence of pathogens; in these conditions, endogenous molecules, the so-called ‘innate adjuvants', activate APCs. Necrotic cells contain and release innate adjuvants; necrotic cells also release high-mobility group B1 protein (HMGB1), an abundant and conserved constituent of vertebrate nuclei. Here, we show that necrotic HMGB1−/− cells have a reduced ability to activate APCs, and HMGB1 blockade reduces the activation induced by necrotic wild-type cell supernatants. In vivo, HMGB1 enhances the primary antibody responses to soluble antigens and transforms poorly immunogenic apoptotic lymphoma cells into efficient vaccines.
Keywords: HMGB1, apoptosis, necrosis, innate immunity, immune adjuvants
Introduction
Signals produced by microbes favour the establishment of adaptive immune responses. The presence of microbial structures reveals an infection, and their recognition is achieved by receptors on cells of the innate immune system, in particular Toll-like receptors (TLRs). The activation of TLRs on antigen-presenting cells (APCs) triggers a coordinated series of events, including the upregulation of molecules involved in antigen presentation and T-cell costimulation, known as ‘maturation'.
The maturation of APCs is also necessary for immune responses that occur in the absence of pathogens (Steinman & Nussenzweig, 2002). However, the adjuvant signals involved are less characterized. Tissue damage per se activates immune responses (Kurts et al, 1998; Srivastava et al, 1998; Gallucci et al, 1999; Savill et al, 2002). Injured tissue evokes acute but generally transient immune responses against ‘self' constituents (Savill et al, 2002; Bondanza et al, 2003). Antigens from dying cells are preferentially recognized in vivo (Kurts et al, 1998), and dying cells release adjuvant factors that amplify and sustain T-cell-dependent immune responses, in situ and at a distance (Shi & Rock, 2002). Recently, uric acid has been identified as an endogenous immune adjuvant (Shi et al, 2003), but it is not likely to be the only one.
The high-mobility group B1 protein (HMGB1) is a nuclear constituent loosely bound to chromatin, and a mediator of inflammation in the extracellular environment (Wang et al, 1999; 2004). Damaged and necrotic cells release HMGB1 (Scaffidi et al, 2002); in contrast, the chromatin of apoptotic cells sequesters HMGB1. Extracellular HMGB1 is responsible for the inflammatory response to cell necrosis, as shown in a model of acute liver toxicity (Scaffidi et al, 2002). RAGE (receptor for advanced glycation end products) is a surface receptor for HMGB1 (Huttunen & Rauvala, 2004), but others may exist (Park et al, 2004). RAGE activation results in nuclear factor-κB (NF-κB) translocation (Andersson et al, 2002), and the NF-κB pathway is responsible for most events elicited by necrotic cells (Li et al, 2001).
In this study, we have identified HMGB1 as an innate adjuvant that favours immune responses in vivo against soluble and cell-associated antigens.
Results And Discussion
T-cell priming and initiation of adaptive immune responses require the maturation of the most potent APCs, the dendritic cells (DCs). As expected (Gallucci et al, 1999; Sauter et al, 2000), human monocyte-derived DCs matured when challenged with necrotic HeLa cells (Fig 1A). Cell constituents released into the supernatant were sufficient to cause DC maturation (Fig 1A). In contrast, DCs challenged with low numbers of early apoptotic cells or their supernatant did not mature (Rovere et al, 1998; Gallucci et al, 1999; Sauter et al, 2000) (data not shown).
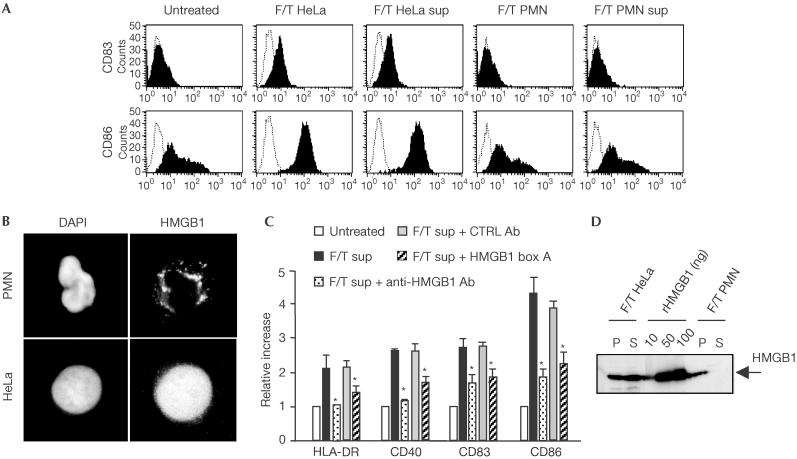
Figure 1.
The supernatants of necrotic cells that cause dendritic cell maturation contain HMGB1. (A) The expression of the CD83 and CD86 surface molecules was assessed by flow cytometry on untreated immature human DCs or DCs challenged with freeze/thawed (F/T) HeLa and polymorphonuclear cells (PMNs) or with their supernatants (sup). Only DCs treated with necrotic HeLa cells or their supernatants had significantly higher expression of the markers (P<0.001). (B) The intracellular distribution of HMGB1 was assessed by immunohistochemistry in PMNs and HeLa cells. Nuclei were revealed by staining with DAPI. (C) HLA-DR, CD40, CD83 and CD86 surface molecules were assessed by flow cytometry on immature DCs untreated or treated with the supernatant of necrotic HeLa cells (F/T sup) in the absence or in the presence of anti-HMGB1 antibodies (Ab), of irrelevant control antibodies or the HMGB1 inhibitory fragment box A. Results are expressed as relative increase (y-axis) in surface expression. DCs treated with F/T sup and anti-HMGB1 antibodies had significantly lower expression of the markers (*P<0.005). Experiments were repeated at least three times with DCs from different donors. (D) HMGB1 was assessed by western blotting in the pellet (P) or in the supernatant (S) of necrotic F/T HeLa or PMNs. As a control, purified recombinant HMGB1 (rHMGB1; 10, 50 or 100 ng/lane) was used.
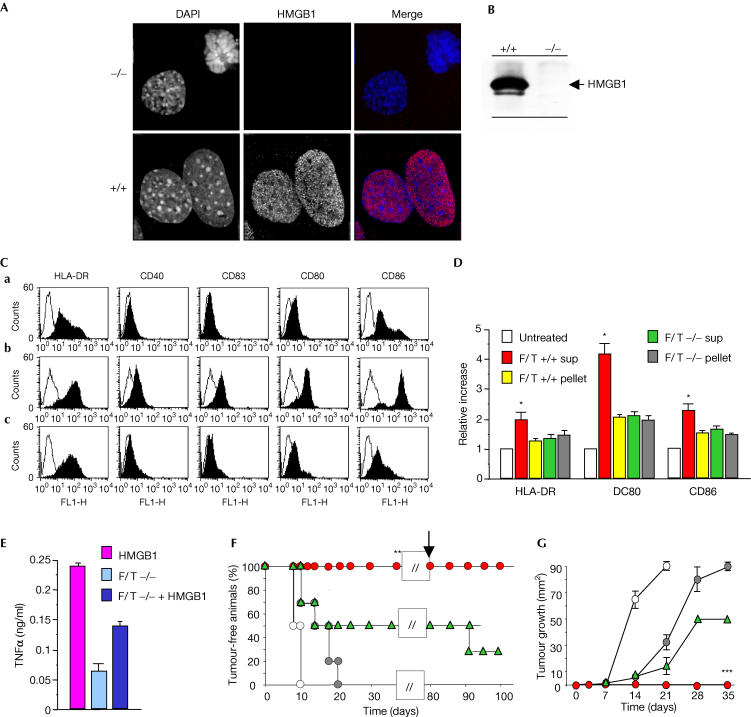
We verified whether HMGB1 in necrotic cell supernatants was required to trigger maturation. The addition of HMGB1-specific antibodies or of the HMGB1 inhibitory fragment box A (Palumbo et al, 2004) to the supernatants of necrotic HeLa cells abrogated DC maturation (Fig 1C). Fibroblasts from wild-type or Hmgb1−/− mice (Fig 2A) were necrotized; supernatants from wild-type cells contained HMGB1, whereas those from Hmgb1−/− cells did not (Fig 2B). Only supernatants containing HMGB1 activated the DCs, as shown by the upregulation of membrane markers (HLA-DR, CD40, CD83, CD80 and CD86) and the induction of TNF-α secretion; the insoluble fraction from wild-type or −/− necrotic cells was not active (Fig 2C–E). The addition of recombinant HMGB1 to the supernatant of necrotic −/− fibroblasts only partly reconstituted the effect of the endogenous counterpart, suggesting a possible role for intracellular regulatory activities (Fig 2E). Necrotic wild-type cell supernatants complemented with anti-HMGB1 antibodies and necrotic −/− cell supernatants had similar effects on the DC activation state.
Figure 2.
HMGB1 is required for the in vivo adjuvant activity of necrotic cells. (A) HMGB1 is expressed in the nuclei of wild-type (+/+, bottom) fibroblasts, whereas Hmgb1−/− fibroblasts (top) do not express it. Nuclei are revealed by staining with DAPI, and HMGB1 expression by immunohistochemistry. (B) HMGB1 in the supernatant of necrotic wild-type (+/+) or Hmgb1 knockout (−/−) fibroblasts was revealed by immunoblotting. (C) The expression of HLA-DR, CD40, CD83, CD80 and CD86 was assessed by flow cytometry on untreated immature DCs (a) or DCs treated with the supernatants of wild-type necrotic fibroblasts (F/T +/+; b) or their −/− counterparts (c). Only DCs treated with supernatants of necrotic Hmgb1+/+ fibroblasts had significantly higher expression of the markers (P<0.005). (D) The expression of HLA-DR, CD80 and CD86 was assessed on immature DCs, either untreated or treated with the supernatants of necrotic Hmgb1+/+ fibroblasts (F/T +/+ sup), insoluble fractions of necrotic Hmgb1+/+ fibroblasts (F/T +/+ pellet), supernatants of necrotic Hmgb1−/− fibroblasts (F/T −/− sup) and insoluble fractions of necrotic Hmgb1−/− fibroblasts (F/T −/− pellet). Results are expressed as a relative increase (y-axis) in surface expression over untreated DCs. DCs treated with pellets or F/T −/− sup had significantly lower expression of the different markers (*P<0.01). Experiments were repeated at least three times with DCs from different donors. (E) The production of TNF-α by immature DCs treated with F/T −/− sup or F/T −/− sup reconstituted with purified recombinant HMGB1 was assessed in the cell culture supernatants. (F) The development of lymphoma was evaluated in C57BL/6 mice vaccinated with PBS (saline), apoptotic RMA cells or apoptotic RMA cells in the presence of the supernatants of wild-type necrotic fibroblasts (F/T +/+) or from their Hmgb1−/− counterparts (F/T −/−). The results shown depict the fraction of tumour-free mice (y-axis) at different times after injection of living RMA lymphoma cells (x-axis). Protected mice were re-challenged on day 80 (arrow). (G) The results shown depict the mean diameter of the growing tumour (y-axis) at different times after administration of living RMA cells (x-axis). Fisher's exact test results for protection and tumour growth were **P<0.005 and ***P<0.001, respectively.
In living polymorphonuclear neutrophils (PMNs), HMGB1 is contained in cytoplasmic vesicles as well as in the nucleus (Fig 1B). Necrotic PMNs do not release HMGB1 (Fig 1D); the protein is tightly associated with an insoluble fraction and fails to be released by treatment with ionic and nonionic detergents. Necrotic PMN supernatants, which therefore do not contain HMGB1, did not trigger DC maturation (Fig 1A).
We then tested whether HMGB1 was involved in the adjuvant activity of necrotic cells in vivo. We injected C57BL/6 mice with apoptotic RMA lymphoma. After 14 days, we challenged them with a large number of living lymphoma cells and evaluated the development of lymphoma (Fig 2E,F). Apoptotic lymphoma cells syngeneic to C57BL/6 mice are poorly immunogenic (Ronchetti et al, 1999): all mice subsequently challenged with living lymphoma cells developed the tumour within 10 days. We then injected C57BL/6 mice with apoptotic lymphoma cells together with the supernatants of necrotic fibroblasts, either wild type or Hmgb1−/−. The mice that had received wild-type necrotic cell supernatants rejected the tumour; 80 days later, they also rejected a further challenge with living lymphoma cells, indicating that the response was long-lasting. Supernatants of necrotic Hmgb1−/− fibroblasts were less efficient in eliciting a protective immune response: 75% of the mice developed lymphomas and eventually died. The blockade of HMGB1 with antibodies reduced (but did not abolish completely) the protection elicited by necrotic −/− cell supernatants by 50%.
These results indicate that HMGB1 released by necrotic cells is a potent adjuvant in vivo and that other intracellular components contribute to the adjuvant activity of necrotic cell supernatants. Candidates include heat-shock proteins (Basu et al, 2000) and uric acid (Shi et al, 2003); significantly, uric acid can derive from the breakdown of adenosine triphosphate (ATP)—itself a cell component released by necrotic cells—by phosphodiesterases and other enzymes, such as xanthine oxidase that can be found in extracellular fluids (Hare & Johnson, 2003).
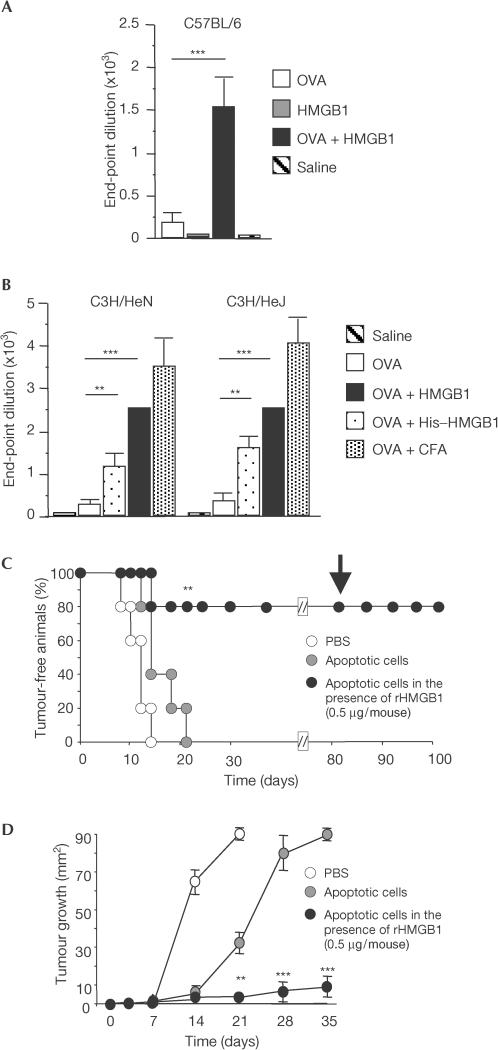
We then verified whether purified recombinant HMGB1 obtained from prokaryotic or eukaryotic cells (Fig 3) was comparable in its adjuvant activity with the natural protein released by necrotic cells. We first verified whether recombinant HMGB1 influenced the primary antibody response against exogenous soluble antigens, a well-characterized feature of endogenous adjuvants (Le Bon et al, 2001). We immunized mice by injecting ovalbumin (OVA) subcutaneously, either in the presence or absence of HMGB1; control mice were injected with phosphate-buffered saline (PBS) or HMGB1 alone. Coinjection with HMGB1 elicited a striking increase in OVAspecific IgG antibody titres (Fig 4A; P<0.001). Similar effects on OVA immunogenicity were obtained in wild-type C57BL/6 mice, wild-type lipopolysaccharide (LPS)-responsive C3H/HeN mice and in C3H/HeJ mice, which are LPS-resistant due to a mutation in TLR4 (Fig 4A,B). This result indicates that contamination with bacterial endotoxin is not involved. Recombinant HMGB1 obtained from prokaryotic or eukaryotic cells exerted similar effects in vivo (compare HMGB1 with His–HMGB1 in Fig 4B). HMGB1 per se did not induce the production of OVAspecific IgG antibodies (Fig 4A,B). At the concentration we used, HMGB1 was consistently less potent than complete Freund's adjuvant, an oil-in-water emulsion containing heat-killed Mycobacterium tuberculosis, considered the gold standard against which all adjuvants are measured.
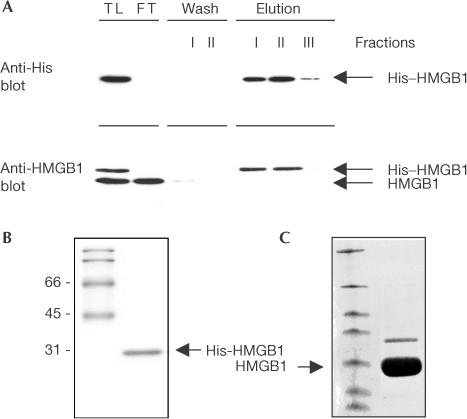
Figure 3.
HMGB1 expression and purification. (A) His–HMGB1 was purified by affinity chromatography from total lysates (TL) of COS7 cells. TL was applied to pre-packed Ni2+ columns and fractions were analysed by SDS–PAGE (FT, flow-through). Immunoblotting with anti-His or anti-HMGB1 antibodies shows that only the His–HMGB1 protein is retained and released by competitive elution with imidazole. His–HMGB1 has a higher molecular weight than endogenous HMGB1 because of the His-tag insertion. (B,C) Recombinant His–HMGB1 and HMGB1 were pure, as shown by Coomassie staining of an SDS–polyacrylamide gel.
Figure 4.
In vivo adjuvant activity of HMGB1 towards soluble and cell-associated antigens. (A) The production of OVAspecific IgG was evaluated in C57BL/6 mice vaccinated with PBS (saline), OVA, OVA plus HMGB1 or HMGB1 alone. The results shown are expressed as mean±s.e.m. of IgG end-point titres (n=5/group). (B) The production of OVA-specific IgG was evaluated in groups of C3H/HeN or C3H/HeJ mice vaccinated with PBS, OVA, OVA plus HMGB1, OVA plus His–HMGB1 or OVA+CFA. Results are expressed as mean±s.e.m. of IgG end-point titres (n=5/group). Differences were statistically significant (*P<0.01 with respect to OVA-injected animals). (C) The development of lymphoma was evaluated in mice vaccinated with PBS, apoptotic RMA cells or apoptotic RMA cells in the presence of rHMGB1 (0.5 μg/mouse). Results depict the fraction of tumour-free mice (y-axis) at different times after injection of living RMA cells (x-axis). Protected mice were re-challenged with living RMA on day 80 (arrow). (D) The subcutaneous growth of RMA cells was evaluated in vaccinated C57BL/6 mice (see above). The results shown depict the mean diameter±s.d. of the growing tumour (y-axis) at different times after injection of living RMA cells (x-axis).
We then verified whether HMGB1 behaved as an adjuvant for cell-associated antigens by injecting apoptotic RMA lymphoma cells in the presence or absence of recombinant HMGB1. Apoptotic lymphoma cells alone were poorly immunogenic: all mice injected with apoptotic lymphoma cells alone developed the tumour (Fig 4C). In contrast, 80% of mice injected with RMA apoptotic cells and HMGB1 rejected the later challenge with living RMA lymphoma cells (Fig 4C). The response was long-lasting, because surviving mice rejected an additional lethal challenge with living lymphoma cells 80 days later (Fig 4C).
Our data suggest that the HMGB1 acts as a bona fide endogenous adjuvant. This is consistent with the general message conveyed by extracellular HMGB1—namely, that tissue damage has occurred (Bianchi & Manfredi, 2004). It also makes good sense from an evolutionary point of view: it is advantageous to activate the immune system immediately after trauma, because infection may follow shortly. HMGB1 is also actively secreted by monocytes and macrophages on activation by LPS, tumour necrosis factor-α (TNF-α) or interleukin-1β (IL-1β) (Wang et al, 1999; Andersson et al, 2000). In myeloid cells, HMGB1 is secreted through specific organelles, the secretory lysosomes (Gardella et al, 2002), and is extensively acetylated in order to reach them from the nucleus (Bonaldi et al, 2003). Thus, HMGB1 secreted actively by inflammatory cells is molecularly different from HMGB1 released passively by necrotic cells. We have no reason to believe that the secreted protein cannot activate immune responses, but this should be verified formally when adequate amounts of acetylated HMGB1 become available.
Whereas the blockade of extracellular HMGB1 might represent a suitable therapeutic target for the treatment of sepsis (Wang et al, 2004) and rheumatoid arthritis (Andersson & Erlandsson-Harris, 2004), the local administration of recombinant HMGB1 may enhance the immune responses against cancer.
Methods
Cells. DCs and PMNs were derived from the blood of healthy donors (Rovere et al, 2000). Embryonic fibroblasts from H-2b C57BL/6 wild-type and Hmgb1−/− mice (Calogero et al, 1999) and H-2b RMA lymphoma cells were grown in RPMI 1640 (Life Technologies, Grand Island, NY, USA) containing 10% FCS (HyClone, Logan, UT, USA). Cells were tested for mycoplasma by PCR. Immature DCs were challenged with necrotic or apoptotic cells (dead cells:DCs ratio=1:2), or their supernatants and insoluble fraction. When indicated, experiments were performed with or without anti-HMGB1 polyclonal antibodies (BD Biosciences PharMingen, San Diego, CA, USA), irrelevant rabbit polyclonal antibodies or the inhibitory HMGB1 fragment box A (Palumbo et al, 2004). Maturation was assessed after 48 h (Rovere et al, 1998).
Apoptosis and necrosis. Cells were killed by necrosis after three cycles of freezing and thawing (Sauter et al, 2000). Apoptosis was induced by ultraviolet irradiation and verified as described (Bellone et al, 1997; Ronchetti et al, 1999).
HMGB1 production and detection. Mouse and human HMGB1 sequences have only two conservative substitutions. Full-length HMGB1 and the inhibitory HMGB1 box A fragment were expressed and purified as described (Palumbo et al, 2004). Endotoxins were removed by passage over Detoxy-Gel (Pierce Biotechnology Inc., Rockford, IL, USA). Indirect immunofluorescence was performed using anti-HMGB1 polyclonal antibodies (BD Biosciences, PharMingen) and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibodies (Boehringer); nuclei were counterstained with 4′-6 diamidino-2-phenylindole (DAPI, Sigma -xAldrich, St Louis, MO, USA; Scaffidi et al, 2002). For expression in eukaryotic cells, full-length HMGB1 was cloned into the pcDNA 4/HisMax vector (Invitrogen), which allowed for the insertion of a poly-histidine (6 × His) tag at the amino-terminus (His–HMGB1). His–HMGB1 was transiently expressed in COS7 cells after transfection with diethylaminoethyl–dextran. After 48 h, cells were lysed and His–HMGB1 purified in a Ni2+-chelating Sepharose column (Amersham) and ultrafiltred on polyethersulphone membranes (Vivascience). Immunoblots were performed using anti-His (Clontech Laboratories Inc.) or anti-HMGB1 antibodies and horse radish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit secondstep reagents (Amersham). Purity was evaluated by Coomassie staining of sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE).
Immunization. C57BL/6 mice were injected subcutaneously (s.c.) twice every other week with PBS or 106 apoptotic RMA cells, in the presence or the absence of HMGB1 (0.5 μg/mouse), or supernatants from necrotic wild-type or Hmgb1−/− fibroblasts. Supernatants of 5 × 106 wild-type fibroblasts contained approximately 500 ng of HMGB1. In selected experiments, the effect of anti-HMGB1 antibodies on the supernatants of wild-type and −/− fibroblasts was evaluated. Endotoxin contamination was assessed by the kinetic-QLC Limulus test (BioWhittaker, Walkersville, MD, USA) and was under the limits of detection (lower than 0.03 U/mouse). After 14 days, mice were challenged s.c. in the opposite flank with 50 × 103 living RMA cells, and tumour appearance and size were evaluated (Ronchetti et al, 1999). Protected mice were rechallenged with living RMA cells after 80 days. C57BL/6 mice, wild-type LPS-responsive C3H/HeN mice and C3H/HeJ mice, which are LPS resistant due to a mutation in TLR4 (Jackson Laboratory), were immunized with purified OVA (10 μg/mouse, Sigma-Aldrich) alone, OVA in CFA (Pierce, Rockford, IL, USA) or with OVA in the presence or the absence of HMGB1 or His–HMGB1 (1 μg/mouse). OVAspecific antibodies were assessed by ELISA: 96-well plates (Nunc) were coated with OVA (10 μg/ml) and blocked with FCS; diluted sera were added and samples developed by sequential incubation with HRP-conjugated IgG-specific monoclonal antibody and substrate (Sigma). Results are expressed as end-point titres, which were the highest serum dilutions that gave an optical density reading five times above that of control samples. The background level was very low at all dilutions and did not vary significantly between experiments.
Acknowledgments
We thank C. Rugarli for discussions, and P. Dellabona, M.P. Protti and R. Pardi for sharing reagents and reading the manuscript. This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (to A.A.M. and M.E.B.), the EC (QLK3-CT-2002-02017-APOCLEAR to P.R.-Q.), and the Fondazione Berlucchi and the Ministero della Sanità (to A.A.M. and M.E.B.). Dr Susanne Müller is the recipient of an EMBO restart grant.
References
- Andersson U, Erlandsson-Harris H (2004) HMGB1 is a potent trigger of arthritis. J Intern Med 255: 344–350 [DOI] [PubMed] [Google Scholar]
- Andersson U et al. (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192: 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ (2002) HMGB1 as a DNA-binding cytokine. J Leukoc Biol 72: 1084–1091 [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol 12: 1539–1546 [DOI] [PubMed] [Google Scholar]
- Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA (1997) Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol 159: 5391–5399 [PubMed] [Google Scholar]
- Bianchi ME, Manfredi A (2004) Chromatin and cell death. Biochim Biophys Acta 1677: 181–186 [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551–5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondanza A, Zimmermann VS, Dell'Antonio G, Dal Cin E, Capobianco A, Sabbadini MG, Manfredi AA, Rovere-Querini P (2003) Dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol 170: 24–27 [DOI] [PubMed] [Google Scholar]
- Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S, Bianchi ME (1999) The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 22: 276–280 [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P (1999) Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5: 1249–1255 [DOI] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3: 995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Johnson RJ (2003) Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 107: 1951–1953 [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Rauvala H (2004) Amphoterin as an extracellular regulator of cell motility: from discovery to disease. J Intern Med 255: 351–366 [DOI] [PubMed] [Google Scholar]
- Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR (1998) Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med 188: 409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF (2001) Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14: 461–470 [DOI] [PubMed] [Google Scholar]
- Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA (2001) An essential role of the NF-κB/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol 166: 7128–7135 [DOI] [PubMed] [Google Scholar]
- Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME (2004) Extracellular HMGB1, a signal of tissue damage, induces mesangioblast migration and proliferation. J Cell Biol 164: 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377 [DOI] [PubMed] [Google Scholar]
- Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti MP, Garancini MP, Manfredi AA, Rugarli C, Bellone M (1999) Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol 163: 130–136 [PubMed] [Google Scholar]
- Rovere P, Vallinoto C, Bondanza A, Crosti MC, Rescigno M, Ricciardi-Castagnoli P, Rugarli C, Manfredi AA (1998) Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol 161: 4467–4471 [PubMed] [Google Scholar]
- Rovere P et al. (2000) The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 96: 4300–4306 [PubMed] [Google Scholar]
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N (2000) Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191: 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2: 965–975 [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195 [DOI] [PubMed] [Google Scholar]
- Shi Y, Rock KL (2002) Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur J Immunol 32: 155–162 [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521 [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL (1998) Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity 8: 657–665 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Nussenzweig MC (2002) Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA 99: 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H et al. (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251 [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Tracey KJ (2004) Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 255: 320–331 [DOI] [PubMed] [Google Scholar]