Abstract
A laser-based ethylene detector was used for on-line monitoring of ethylene released by the phytopathogenic fungus Botrytis cinerea in vitro and in tomato fruit. Ethylene data were combined with the results of a cytological analysis of germination of B. cinerea conidia and hyphal growth. We found that aminoethoxyvinylglycine and aminooxyacetic acid, which are competitive inhibitors of the 1-aminocyclopropane-1-carboxylic acid pathway, did not inhibit the ethylene emission by B. cinerea and that the fungus most likely produces ethylene via the 2-keto-4-methylthiobutyric acid pathway. B. cinerea is able to produce ethylene in vitro, and the emission of ethylene follows the pattern that is associated with hyphal growth rather than the germination of conidia. Ethylene production in vitro depended on the l-methionine concentration added to the plating medium. Higher values and higher emission rates were observed when the concentration of conidia was increased. Compared with the ethylene released by the fungus, the infection-related ethylene produced by two tomato cultivars (cultivars Money Maker and Daniela) followed a similar pattern, but the levels of emission were 100-fold higher. The time evolution of enhanced ethylene production by the infected tomatoes and the cytological observations indicate that ethylene emission by the tomato-fungus system is not triggered by the ethylene produced by B. cinerea, although it is strongly synchronized with the growth rate of the fungus inside the tomato.
The gaseous plant hormone ethylene regulates various physiological processes ranging from seed germination to organ senescence and is involved in the reactions to abiotic and biotic stresses (1, 27, 29). An increase in ethylene production is frequently observed during the interaction between a host and a pathogen (1). It has been suggested that ethylene released during infection represents an early response of plants to the perception of a pathogen attack and can be associated with induction of a defense reaction (6). On the other hand, ethylene is considered to be very important in the development of disease symptoms (27). However, its role in pathogenesis and resistance is far from clear (6, 9, 16).
Some microorganisms, including phytopathogenic fungi and bacteria, can synthesize ethylene themselves; therefore, analysis of ethylene emission from a host-pathogen system is a complex problem. Plants can produce ethylene via the 1-aminocyclopropane-1-carboxylic acid (ACC) pathway (36). In microorganisms there are two known pathways for ethylene biosynthesis (19). Ethylene can be produced either via 2-keto-4-methylbutyric acid (KMBA), as it is in Escherichia coli (22) and Cryptococcus albidus (18), or via 2-oxoglutarate, as it is, for example, in Penicillium digitatum (17) and Pseudomonas syringae (28).
The fungus Botrytis cinerea is a plant necrotrophic pathogen that colonizes senescent or dead plant tissues and softening fruits, causing grey mold. Fungal hyphae can penetrate through wounds or natural openings of the plant tissue and spread from previously colonized dead tissues into healthy tissues. This fungus is a major cause of postharvest rot of perishable plant products, including tomatoes at harvest and in storage. Since it is also able to infect at low temperatures, it can result in important economic losses in either pre- or postharvest crops (26).
The ability of B. cinerea to adapt to various environmental conditions has been well investigated, and different mechanisms for its action in attacked host tissue have been proposed (3, 12, 33, 34, 37). These mechanisms include hydrolytic enzymes secreted during germination (12), other cell wall-degrading enzymes which have increased activity in infected ripening fruit (3), and active oxygen species that induce cell death caused by B. cinerea (34).
Usually, grey mold development is associated with an increase in ethylene production from the infected tissues, which is most often attributed to the host plant (13). Previously, it was reported that B. cinerea grown on various nutrient sources did not produce ethylene (11). This result contradicts the later finding (31) that ethylene is released by B. cinerea when it is grown on a defined medium. The mechanism used by the fungus to synthesize ethylene is not known. There is limited information available about ethylene production by B. cinerea; the possible role of fungus-produced ethylene in B. cinerea has been debated over the years (4, 14, 23, 24, 31), and controversial conclusions concerning the role of ethylene in spore germination and mycelium growth have been described. Recently, it has been reported that ethylene is a primary marker for fruit pathogenesis, and several other infection-related plant products have also been studied as early markers of pathogenesis (30).
In order to improve our knowledge about grey mold development in plant tissues and the role of ethylene during the host-pathogen interaction, we studied ethylene production by B. cinerea in vitro with regard to the effects of culture media and ethylene production by infected tomato fruits. The ability of B. cinerea to synthesize ethylene from l-methionine either via the ACC pathway or via the KMBA pathway was examined. The aim of this study was to determine the relationship between ethylene released by the fungus in vitro and the enhanced ethylene production in B. cinerea-infected tomatoes with respect to disease development.
To do this, we used a laser-based ethylene detector which has high sensitivity and relatively high time resolution. This instrument allows on-line monitoring of ethylene emission in a flowthrough system with a detection limit of 10 parts per trillion (5). To our knowledge, this is the first report of real-time monitoring of ethylene production by B. cinerea and infected tissue. Use of the laser-based ethylene detector in combination with a broad range of well-characterized biochemicals related to ethylene biosynthesis allowed us to produce a comprehensive description of the mode of ethylene formation and action by B. cinerea both in vitro and in vivo.
MATERIALS AND METHODS
Growth of fungal strains and isolation of conidia.
An isolate of B. cinerea (strain VTF1, kindly provided by E. Fallik, ARO-Volcani Centre, Bet-Dagan, Israel) was maintained on potato dextrose agar (PDA) plates for 14 days at 22°C before conidia were isolated. To prepare a conidial suspension, the fungus was removed from the cultured plates by gentle brushing of the plate surfaces with a sterile platinum loop and was suspended in distilled water. The fungal suspension was filtered through two layers of gauze to separate the conidia. The concentration of conidia was determined by using a Neubauer counter (0.0025 mm2) and a light microscope. Three concentrations, 1.5 × 108, 2 × 107, and 2 × 105 conidia ml−1, were prepared in sterilized water supplemented with 0.03% Tween 20 to ensure uniform distribution of the conidia.
In vitro fungal growth for ethylene measurement.
To investigate the ability of B. cinerea to produce ethylene in vitro, strain VTF1 was grown in both PDA and PDA supplemented with different concentrations of l-methionine, an ethylene precursor (15 concentrations between 0.05 and 70 mM were used). The precursor was added to PDA, and the pH of each medium was then adjusted to 3.8 before autoclaving. Portions (160 μl) of a suspension containing a specific concentration of conidia were uniformly plated by placing 40 4-μl droplets on 9-cm-diameter petri dishes containing 25 ml of solidified PDA. The plates were then immediately placed inside a closed glass cuvette for ethylene measurement with the laser-based ethylene detector and flushed with humidified air at a continuous flow rate of 2 to 3 liters h−1 and at atmospheric pressure.
Chemical treatment of the fungus.
To study inhibition of ethylene biosynthesis, we used two inhibitors of ethylene biosynthesis via the ACC pathway, aminoethoxyvinylglycine (AVG) at concentrations of 0.25, 2.5, and 5 mM and aminooxyacetic acid (AOA) at concentrations of 1 and 10 mM. The chemicals were added to suspensions containing 2 × 107 conidia ml−1 before they were placed on PDA supplemented with 25 to 35 mM l-methionine. Stimulation of ethylene production was examined by adding ACC or KMBA to a conidial suspension before it was placed on PDA without l-methionine. All the chemicals were provided by Sigma Aldrich.
Along with ethylene monitoring with the laser-based ethylene detector, fungal radial growth was measured. A single drop of conidia (40 μl of a suspension containing 2 × 107 conidia ml−1) was spotted onto a petri dish, and the radial growth of the fungal colony was measured.
Plant material and tissue inoculation.
Tomato (Lycopersicon esculentum) cultivars Money Maker and Daniela were grown in a greenhouse under natural light supplemented with artificial illumination provided by high-pressure metal halide lamps (Philips type HPI-T 400 W) at 70% relative humidity with 18-h photoperiods by using 25°C in the light period and 18°C in the dark period. Tomato fruits were harvested at the pink-light red stage of development (according to the U.S. Department of Agriculture, Agricultural Marketing Service, standard grading system for fresh tomatoes), and each fruit surface was gently cleaned with a wet pad before inoculation. Four injections, each consisting of 40 μl of a conidial suspension, were performed at an equatorial site with a needle head (diameter, 0.8 mm) at constant depth of 2 mm below the fruit skin. Fruits injected similarly with 40 μl of sterile distilled water per injection served as controls (mock-infected fruits). Immediately after inoculation the fruits were placed into a glass cuvette (0.7 liter) connected to the laser-based ethylene detector and flushed with humidified air at a continuous flow rate of 3 to 4 liters h−1 and at a total pressure of 1 atm. The development of decay was monitored, and the extent of decay was expressed as the lesion diameter (in millimeters).
Experiments were also performed with single slices of tomato pericarp (cultivar Daniela; fresh weight, 22 g; thickness, 5 mm); two inoculations, each consisting of 40 μl of a suspension containing 2 × 107 conidia ml−1, were used. The slices were then placed on petri dishes and imbibed in 20 ml of distilled water or a solution of AOA (2 mM).
Real-time monitoring of ethylene production.
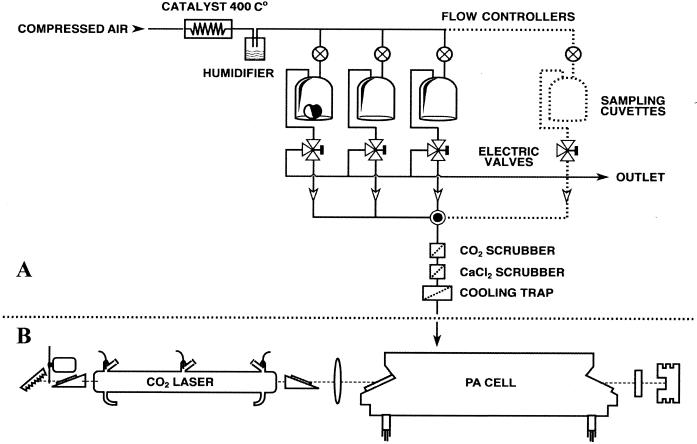
Ethylene production was monitored in real time by using a sensitive laser-based ethylene detector in combination with a gas flowthrough system (5). A schematic diagram of the setup is shown in Fig. 1. A detailed description of the system has been given elsewhere (20, 32). Briefly, the detector consists of a line-tunable CO2 laser emitting radiation in the 9- to 11-μm infrared wavelength region and a photoacoustic cell, in which the gas is detected. The laser-based ethylene detector is able to distinguish between different gases by making use of their wavelength-dependent fingerprint absorption characteristics. Trace gases released by the biological samples (plated fungi or infected fruits) were transported to the photoacoustic cell through a flow system by using air as the carrier gas. Ethylene gas mixtures are sensitively measured by the laser-based ethylene detector due to the distinct fingerprint-like spectrum of ethylene in the CO2 laser wavelength range (7). Inside the photoacoustic cell traces of ethylene can absorb the laser radiation; the absorbed energy is released into heat, which creates an increase in pressure inside a closed volume. By modulating the laser beam with a chopper, pressure waves (i.e., sound) are generated and detected with a sensitive miniature microphone. The amplitude of the acoustic waves is directly proportional to the concentration of ethylene in the photoacoustic cell.
FIG. 1.
Ethylene detection setup. (A) Gas flow system. (B) Laser-based ethylene detector consisting of a photoacoustic (PA) cell placed inside a CO2 laser.
The gas flow through the measuring system can be controlled by using electrical three-way valves that route a particular gas stream to the photoacoustic cell (on position) or into the laboratory (off position). In this way the gases emitted from a number of cuvettes (up to six cuvettes per experiment) containing the biological samples were transported to the photoacoustic cell alternately and at controlled flow rates, which prevented accumulation-induced effects. Usually, in the off position the gas stream was vented to avoid interruption of the flow through the cuvettes. Switching between different cuvettes with a flow rate of 3 liters h−1 caused a delay of 4 min before the photoacoustic cell was completely refilled. The flow was adjusted by a flow controller and was continuously monitored with a mass flow sensor (Brooks Instruments type 5850 S).
The laser-based ethylene detector and the electric three-way valves were operated fully automatically by a computer program and could be used to obtain measurements continuously for periods of up to several weeks.
To eliminate other interfering gases which may have influenced the results due to the overlap between their spectral absorption characteristics and the CO2 laser wavelengths, a number of filters and scrubbers were introduced into the measuring system. A platinum-based catalyzer (platinum on Al2O3), which operated at a minimum temperature of 400°C and was placed before the entrance of the cuvettes, provided airflow free of any traces of external ethylene (or other hydrocarbons).
To measure low ethylene concentrations, it was necessary to reduce the CO2 and water concentrations before the samples entered the photoacoustic cell. These compounds could introduce undesired supplementary absorption profiles. Even if these gases were not present in the original gas flow, they could be produced in the cuvettes. Consequently, a scrubber with KOH (moist pellets) was used to reduce the CO2 concentration to less than 1 ppm, and a tube with CaCl2 (granules) was placed directly after this scrubber in order to decrease the water content in the gas. To remove ethanol and some other heavier hydrocarbons, a cooling trap (−150°C) was inserted into the gas flow system just before the photoacoustic cell. The gas was filtered by passing it through 0.2-μm-pore-size Millipore filters placed at the inlet and outlet of the sampling cuvettes.
The ethylene levels for an empty cuvette were subtracted from the emission rates obtained. All experiments were conducted under normally illuminated laboratory conditions at a constant temperature of 22°C. The ethylene production by the fungi was related to the emission rate by multiplying the measured value by the flow rate; the results were expressed in nanoliters per hour. For the tomato data the rate of ethylene production was expressed in nanoliters per hour per gram (fresh weight).
To obtain a better overview of the ethylene emission rates, we displayed the results of the experiments as averages of the sampling rate every 3 h (the errors due to averaging were smaller than the symbol sizes). Higher sampling rates were used for the ethylene released by fungus grown on PDA without l-methionine. Each experiment was repeated at least six times, and representative data are shown below.
Microscopy.
In separate experiments, samples of plated fungi or infected fruits kept under the conditions that were used during ethylene measurements were collected at specific times and analyzed by light microscopy to determine fungal morphology.
Mycelium of a plated B. cinerea culture was gently scraped from the growth medium (PDA) with a sterile platinum loop. The sample was suspended in water on a glass microscope slide and covered with a glass coverslip. Mycelium growing on an infected fruit surface was also scraped from the fruit and analyzed on a microscope slide as described above. Tissue from the inoculation site was briefly softened in a 0.2 M NaOH solution at 60°C and then gently squashed on a glass microscope slide with a glass coverslip. The microscope slides were examined with a Zeiss AxioPlan 2 Imaging microscope and photographed with a digital camera (AxioCamera), and the images were processed with specialized software (AxioVision).
RESULTS
Ethylene production by B. cinerea.
We measured ethylene emission from B. cinerea grown on PDA and PDA supplemented with l-methionine and analyzed the morphology of fungal development in vitro.
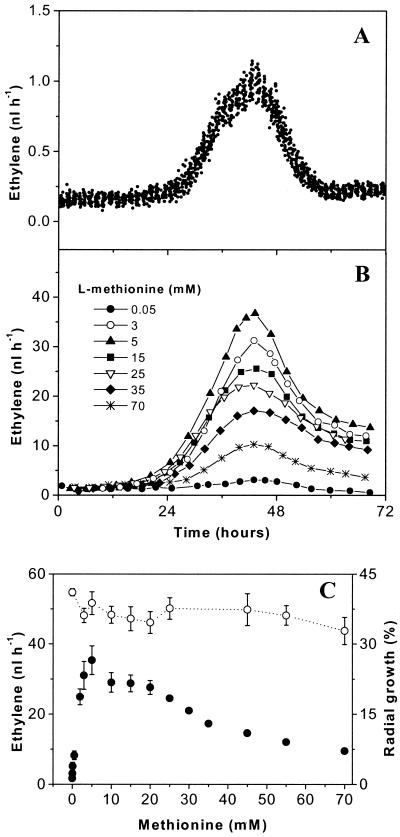
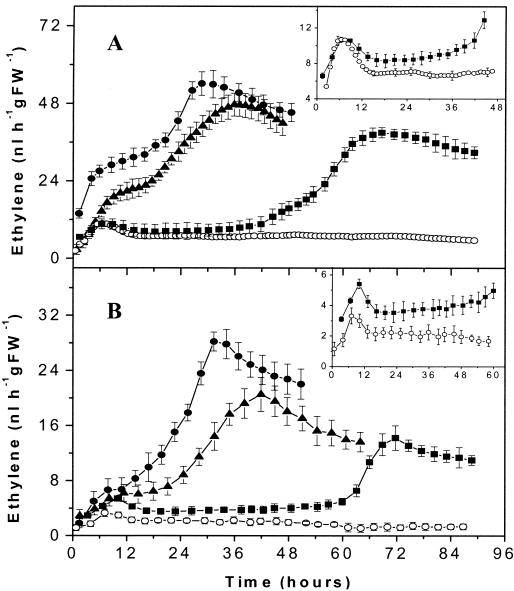
Previously, it was estimated that very little ethylene was produced by B. cinerea in the absence of l-methionine in basal media, and there was no quantification or description of ethylene evolution over time (31). Our measurements proved that B. cinerea produced small amounts of C2H4 when it was grown in vitro on PDA without l-methionine. By using the laser-based ethylene detector, the C2H4 released by the plated conidia could be quantitatively monitored every 72 s. A typical pattern is shown in Fig. 2A. Constant emission at a rate of of 0.17 ± 0.04 nl h−1 was detected during the first 24 h for 160 μl of a suspension containing 2 × 107 conidia ml−1. The rate of ethylene production increased to a peak of about 1 ± 0.05 nl h−1 43 h after the conidia were plated on PDA, after which it decreased to 0.2 ± 0.04 nl h−1. As controls we used 160 μl of an autoclaved conidial suspension and 160 μl of autoclaved hyphae plated on PDA (data not shown). In these cases, no increase in ethylene emission was observed over a 3-day period. The equivalent rates of ethylene production by the controls, representing the nonenzymatically produced ethylene background, were constant and were 0.18 ± 0.05 nl h−1 for the autoclaved conidial suspension and 0.18 ± 0.04 nl h−1 for the autoclaved hyphae. When only PDA alone was monitored (with no conidia or autoclaved sample plated on it), the ethylene detector measured a constant background value of 0.17 ± 0.03 nl h−1.
FIG. 2.
Ethylene released by B. cinerea in vitro. Fungus (160 μl of a conidial suspension containing 2 × 107 conidia/ml) was plated at zero time on PDA (only 75% of the data are displayed) (A) and PDA supplemented with l-methionine (B). For a better overview the results for only 7 of the 15 l-methionine concentrations used are displayed (0.05, 3, 5, 15, 25, 35, and 70 mM). (C) Maximum ethylene production (•) and radial growth (○) for different l-methionine concentrations. The values are means of the maximum amount of ethylene released at 43 h and the radial growth measured at 43 h; the error bars indicate standard deviations (for l-methionine concentrations greater than 25 mM the standard deviations of the ethylene values are smaller than the symbols).
Ethylene emission from plated conidia increased substantially if l-methionine was added to the growth medium. We monitored on-line ethylene release from 2 × 107 B. cinerea conidia ml−1 (160 μl of conidial suspension) plated on PDA containing different concentrations of l-methionine (between 0.05 and 70 mM). The pattern of ethylene production (Fig. 2B) was similar to the pattern for the fungus grown on PDA without l-methionine. The rate of production started at low values, increased to a peak about 43 h after the conidia were plated, and then declined to the background level. Long-term experiments over more than 2 weeks showed that the background level also stayed constant during sporulation (data not shown).
Ethylene production increased as the l-methionine concentration increased up to 10 mM, reached a plateau at 10 to 15 mM l-methionine, and decreased when more L-methionine was added to the growth medium (Fig. 2C). However, some variability in ethylene production at l-methionine concentrations less than 20 mM was observed. To eliminate such variables in our study, all of the experiments described below were performed with l-methionine concentrations of 25 to 35 mM, at which low variability was found. Radial growth was slightly less for media containing l-methionine than for medium without l-methionine, although no significant variations were observed for media containing different l-methionine concentrations (Fig. 2C).
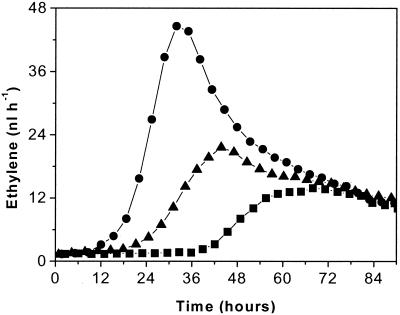
Ethylene emission by B. cinerea was also found to be dependent on the concentration of conidia plated on the growth medium. Figure 3 shows the amounts of ethylene produced after 160-μl suspensions containing 1.5 × 108, 2 × 107, and 2 × 105 conidia ml−1 were plated on PDA containing 25 mM l-methionine. The amount of ethylene released and the rate of production increased as the concentration of conidia increased.
FIG. 3.
Ethylene production by the fungus (160 μl of a conidial suspension) when different concentrations of conidia, including 1.5 × 108 conidia ml−1 (•), 2 × 107 conidia ml−1 (▴), and 2 × 105 conidia ml−1 (▪), were plated on PDA containing 25 mM l-methionine.
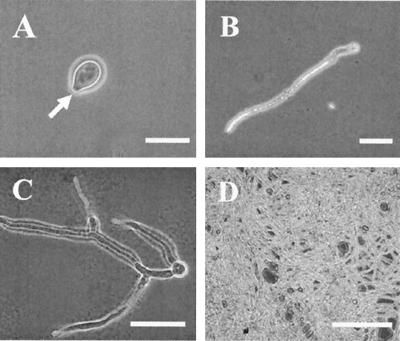
Light microscope analysis showed that under the conditions used in our experiments, germination of conidia occurred within the first 3 h after harvesting and plating (Fig. 4A). At 18 h after plating, elongating hyphae were clearly visible (Fig. 4B), and at 24 h after plating, before the concentration of ethylene reached the maximum value, the fungal hyphae extensively grew and branched (Fig. 4C). There was no further differentiation of the fungus, only continuous hyphal growth, up to 72 h after plating (Fig. 4D).
FIG. 4.
Cytological observation of germination of B. cinerea conidia and hyphal growth in vitro. Conidia of B. cinerea at a concentration of 2 × 107conidia ml−1 were plated on PDA supplemented with 25 mM l-methionine. (A) Conidium at 3 h after plating. The conidia are oval, and germination occurs at one end (arrow). Bar = 10 μm. (B) Conidium at 18 h after plating. Hyphal elongation is clearly visible. Bar = 40 μm. (C) Conidium at 24 h after plating. The fungal hyphae are branching and starting to create a dense skein. Bar = 25 μm. (D) Hyphae at 72 h after plating. Fungal growth is proceeding randomly in all directions, and there is no further visible differentiation. Bar = 500 μm.
Ethylene biosynthesis by B. cinerea.
The high rate of emission of ethylene by the fungus grown on PDA with l-methionine led to the hypothesis that ethylene is biosynthesized via a pathway in which l-methionine is used as a precursor. Furthermore, addition of another compound, 2-oxoglutarate, to PDA did not increase the amount of ethylene released (data not shown).
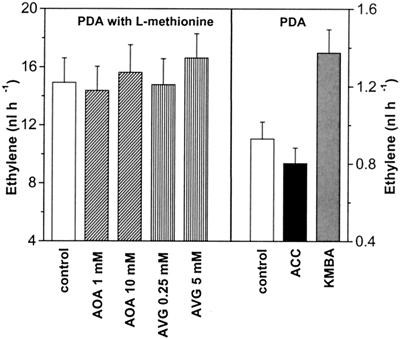
To investigate the ethylene biosynthesis pathway, AOA (1 and 10 mM) and AVG (0.25 and 5 mM), both of which are competitive inhibitors of ACC synthase, were applied along with the conidial suspension. The results show that although l-methionine stimulated the release of ethylene, no inhibitors of the ACC metabolic pathway were effective in reducing the rate of ethylene production by B. cinerea (Fig. 5, left side).
FIG. 5.
Ethylene production by B. cinerea (concentration, 2 × 107 conidia ml−1). At zero time suspensions of conidia containing AOA (1 or 10 mM) or AVG (0.25 or 5 mM) were plated on PDA supplemented with 35 mM l-methionine (left side). Suspensions of conidia containing ACC (10 mM) or KMBA (10 mM) were also plated at zero time on PDA without l-methionine (right side). The control samples contained no additional chemicals.
The ability of B. cinerea to convert externally applied ACC or KMBA to ethylene was tested by directly adding ACC (10 mM) or KMBA (10 mM) to a conidial suspension placed on PDA without l-methionine (Fig. 5, right side). The effect of adding 10 mM ACC to PDA was the same as the effect of adding 1 mM ACC (data not shown). The presence of ACC did not stimulate ethylene production, while addition of KMBA resulted in a ∼47% increase in ethylene emission compared to the emission of the control.
The same results were obtained when these chemicals were added to unsolidified PDA after autoclaving (data not shown).
Ethylene release by B. cinerea-infected tomato fruit.
In order to evaluate the relationship between the ethylene produced by B. cinerea in vitro and the ethylene released by the fungus-host system, we considered the ethylene responses of artificially inoculated fruits of two tomato cultivars, one which is known to be fast ripening (cultivar Money Maker) and one which is known to be slow ripening (cultivar Daniela).
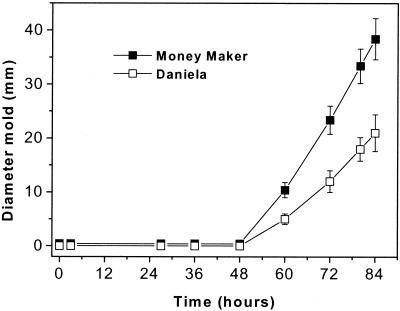
Significant differences between the amounts of ethylene released by infected tomatoes and the amounts of ethylene released by mock-infected fruits of both cultivars were observed (Fig. 6). When 160-μl portions of conidial suspensions (the same amount that was for in vitro ethylene measurements) containing 107 to 108 conidia ml−1 were inoculated inside tomatoes, no visible disease was noted during the first day after inoculation, while our measurements showed that elevated levels of ethylene were released after 6 h. When the signs of infection became visible, there were rapid increases in production, up to four- to sixfold increases; the maximum levels occurred after 36 to 43 h, depending on the concentration of the inoculum. Higher and faster ethylene emission was observed for the fast-ripening cultivar, cultivar Money Maker (Fig. 6A), than for the slow-ripening cultivar, cultivar Daniela (Fig. 6B). When a lower inoculum concentration (160 μl of a suspension containing 2 × 105 conidia ml−1) was used, ethylene emission started to increase slowly 24 h after inoculation (Fig. 6, insets) and reached a maximum level after 70 h. Decay (Fig. 7) became visible after 48 h, when the rate of ethylene emission started to increase more than it increased during the first 24 h. Faster disease development was observed for cultivar Money Maker infected tomatoes than for cultivar Daniela infected tomatoes.
FIG. 6.
Ethylene released by tomato cultivars Money Maker (A) and Daniela (B) that were mock infected (○) or were inoculated with B. cinerea at a concentration of 1.5 × 108 conidia ml−1 (•), 2 × 107 conidia ml−1 (▴), or 2 × 105 conidia ml−1 (▪). At zero time tomatoes were inoculated and immediately placed into cuvettes with a continuous airflow at a rate of 3 to 4 liters h−1. The insets show the ethylene emission from mock-infected tomatoes (○) and tomatoes infected with 2 × 105 conidia ml−1 (▪) for the first 2 to 3 days. Measurement was stopped when the fruits were completely deteriorated.
FIG. 7.
Development of decay in cultivar Money Maker (▪) and Daniela (□) tomatoes infected with 2 × 105 B. cinerea conidia ml−1.
Compared with the ethylene measurements obtained with the fungus in vitro, there are two aspects which have to be considered. First, the levels of ethylene produced from B. cinerea-infected tomatoes were much higher (more than 100-fold higher for typical 80- to 100-g [fresh weight] tomatoes) than the levels produced by the fungus in vitro. Second, the rate of infection-related ethylene production was higher than the corresponding rate for the fungus. For example, the ethylene emission in vitro from 160 μl of a suspension containing 2 × 105 conidia ml−1 started to increase after 36 h (Fig. 3), while the ethylene emission from infected tomatoes (both cultivars) inoculated with the same amount and concentration of conidia started to increase in less than 24 h (Fig. 6, insets).
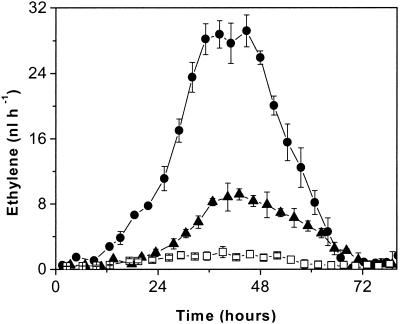
To discriminate between the ethylene released by the host and the ethylene released by the fungus inside the host, slices of tomato pericarp were imbibed in a solution of AOA (2 mM) to block ethylene biosynthesis and then inoculated with 80 μl of solution containing 2 × 107 conidia ml−1 (Fig. 8). The pattern of ethylene release from an infected slice was similar to the pattern of ethylene release from an infected fruit and showed a maximum about 43 h after inoculation. When the tomato slice was imbibed in AOA, the infection-related ethylene level was three times less than the level of ethylene released by the slice imbibed in distilled water. Very little ethylene was produced by the uninfected slice imbibed in AOA. At 12 h after inoculation, ethylene emission from the uninhibited sample started to increase at high rate, reaching about 9 nl h−1 after 24 h. The ethylene released by the AOA-treated and infected slice exhibited the same pattern as the ethylene released by the AOA-treated and uninfected slice, and for both samples a constant level of only 1.5 nl h−1 was observed during the first 24 h. This observation confirmed the inhibitory action of AOA on ethylene biosynthesis from an infected slice.
FIG. 8.
Slices of tomato pericarp (22 g) imbibed in 20 ml of water (•) or 2 mM AOA (▴) and directly infected with 2 × 107 B. cinerea conidia ml−1 (two inoculations, 40 μl each). The effect of inhibition of ethylene production by AOA on tomato slices that were not infected was also determined (□).
Light microscope analysis of a Botrytis-infected tomato (data not shown) indicated that fungal growth inside the infected fruit tissue proceeds essentially as it does under in vitro conditions; germination of conidia occurred within the first 3 h after inoculation, and after 18 h elongating fungal hyphae were clearly visible. Growth continued with no further differentiation of the fungus up to 72 h after infection.
DISCUSSION
Ethylene production has been thoroughly studied in higher plants (1), and it has been shown that ethylene is also released by algae, lichens, fungi, and even humans as a result of lipid peroxidation (15, 19, 21, 25, 37). In this work, we studied ethylene production and action related to B. cinerea and the associated development of grey mold. This fungus is well known for its extremely broad host spectrum; however, the factors which are important for the infection process are still not well understood. The study of fungal infection-related plant hormones is therefore of great interest.
Our results showed that the fungus B. cinerea is able to produce ethylene in vitro; emission can be correlated with fungus growth. When B. cinerea grew on media without l-methionine, ethylene release was almost undetectable with standard procedures; use of the laser-based ethylene detector enabled us to detect it. Addition of l-methionine greatly enhanced ethylene production by B. cinerea. A small amount of l-methionine (0.05 mM l-methionine) in PDA resulted in up to a threefold increase in the amount of ethylene released compared with the amount of ethylene released by the fungus growing on PDA without l-methionine. The highest rate of ethylene production occurred when 3 to 15 mM l-methionine was added to PDA; ethylene production decreased when higher concentrations of l-methionine were added. Our results obtained by investigating 15 concentrations of l-methionine differ from those reported by Qadir et al. (31), who examined the effects of adding 1, 5, 10, 35, and 50 mM l-methionine to PDA and found that the maximum amount of ethylene was produced by fungus grown on PDA supplemented with 35 mM l-methionine. This may have been due to the fact that large errors were introduced by the integration method over 7 days of ethylene production (31). Additionally, Qadir et al. used only a few concentrations of l-methionine, and no measurements were obtained for concentrations between 10 and 35 mM or less than 1 mM.
Microorganisms and higher plants can biosynthesize ethylene by different pathways, and in two of these pathways l-methionine is a precursor (19). The most-studied pathway for ethylene biosynthesis is via ACC and occurs mainly in higher plants (36), but there are two known pathways for ethylene formation by microorganisms, namely, the 2-oxoglutarate and KMBA pathways (19). The 2-oxoglutarate pathway does not occur in B. cinerea according to our results (data not shown), which confirms the findings of Qadir et al. (31). By using a pharmacological approach, we found that B. cinerea most likely uses the KMBA pathway; inhibitors of the ACC ethylene formation pathway had no effect on the emission of ethylene from this fungus, whereas addition of KMBA to the fungus resulted in an increase in ethylene emission compared to the emission in the control grown on PDA without l-methionine. The amount of ethylene released after KMBA is added to a conidial suspension or PDA is less than the amount released in the presence of l-methionine. This can be explained by taking into account the possibility that KMBA is not completely taken up by the fungus or the possibility that B. cinerea may use an additional pathway for ethylene formation.
Using light microscopy, we observed that ethylene production by the fungus in vitro is associated primarily with the period when the most active growth of the fungus occurs.
Cytological analyses indicated that germination of conidia occurred before the amount of ethylene released became substantial. Thus, the ethylene released by B. cinerea must be associated with hyphal growth rather than with germination of conidia. The production of ethylene in vitro depends on the l-methionine concentration added to the plating medium, and higher levels are observed when the concentration of conidia is higher. Note that at higher concentrations of conidia the ethylene emission rate is higher. This finding can be related to fungal neighborhood sensing.
The level of ethylene production by B. cinerea-infected tomatoes was significantly higher than the level in mock-infected tomatoes and started to increase before there was visible decay. Our results demonstrate that ethylene can be considered a sensitive marker for early infection of harvested fresh products, in accordance with a recent study (30). In an earlier work it was shown that higher inoculum concentrations of B. cinerea increased infection both in flowers and in leaf removal wounds (10). We found that in the case of infected tomato fruit the development of decay depends on the inoculum concentration and that decay occurs more rapidly with higher concentrations of conidia. In particular, at higher inoculum concentrations (1.5 × 108 and 2 × 107 conidia ml−1) we observed faster development of decay for cultivar Money Maker infected tomatoes, which was accompanied by high levels of ethylene release, than for cultivar Daniela infected tomatoes. The difference can be explained by taking into account the fact that cultivar Money Maker is a fast-ripening cultivar and that its ripening rate can be increased due to ethylene induced by conidia inoculated into the fruit.
The amount of infection-related ethylene produced by both tomato cultivars was much higher (>100-fold higher) than the amount of ethylene released by the fungus in vitro, although the evolution patterns were similar. Therefore, ethylene generation by infected tomatoes can be considered a likely response of the host to the stress caused by B. cinerea infection.
For the fungus in vitro, we found that exogenous application of ethylene by fumigation with 10 or 20 ppb or 1 ppm of C2H4 in air did not affect conidial germination or hyphal growth (data not shown). In this case, the ethylene produced by the fungus and its temporal evolution were similar to the results obtained for the fungus in vitro that was not fumigated (data not shown). Thus, the presence of ethylene may provide an advantage to the fungus indirectly, because it stimulates the softening of the plant tissue and therefore facilitates tissue penetration and fungal spread (8).
However, in our conditions, the ethylene levels produced by the fungus in vitro were too low even compared with the levels of ethylene released by mock-infected fruits to be considered substantial in inducing fruit spoilage. Moreover, enhanced formation of ethylene by the infected tomatoes was observed before the amount of ethylene released by the fungus in vitro started to increase. Cytological analysis indicated that germination of conidia and fungal growth inside the fruit are similar to germination of conidia and fungal growth under vitro conditions and that equivalent dynamics of ethylene release correspond to similar fungal growth.
These results led us to the conclusion that ethylene production by B. cinerea does not trigger the emission of ethylene by the tomato-fungus system. Rather, the emission is synchronized with the growth rate of the fungus inside the tomato.
Experiments performed with slices of tomato pericarp showed that it is possible to use the laser-based ethylene detector to determine the contribution of ethylene by the fungus and, separately, the contribution of the infected fruit tissue. Inhibition of ethylene biosynthesis in Botrytis-infected tomato pericarp with AOA prior to inoculation significantly decreased the ethylene emission. In this case, the ethylene released from the infected tissue could be produced by the fungus itself, as we demonstrated in the in vitro experiments, and could also be partially released by the tissue due to possible stimulation of ethylene production by the fungus. Our results demonstrate that the activity of the inhibitor was strong enough to inhibit the release of ethylene from the tomato after inoculation. Moreover, the ethylene evolution from an AOA-treated and infected slice showed a pattern similar to that of ethylene production by the fungus in vitro (Fig. 2B). Additionally, the level of ethylene emission from a tomato slice imbibed with AOA is very low, and consequently, the ethylene produced from an AOA-inhibited and B. cinerea-infected slice should be attributed to the fungus more than to residual tissue activity. From these observations we concluded that the ethylene released by Botrytis-infected tomato pericarp treated with AOA was primarily due to emission by fungal hyphae.
The decrease in ethylene emission from an infected tomato after the peak was reached corresponded with an advanced stage of the fungal infection. It has been suggested that during the infection process the infected tissue gradually loses the capacity to convert ACC to ethylene and that in the last stages of decay the host ethylene-producing mechanism is suppressed (2).
In summary, our observations indicate that B. cinerea is able to produce ethylene autonomously in vitro and in tomato fruit and to induce ethylene synthesis by an infected tomato due to its own growth. We have shown that a laser-based ethylene detector is a suitable instrument for on-line measurement of ethylene released by a fungus in vitro and in vivo. Moreover, this instrument is a powerful tool for early detection of traces of ethylene released in vegetal tissue infected by microorganisms (fungi or bacteria) after long periods of incubation (weeks). Its high sensitivity and fast response should allow workers to investigate new aspects of the temporal and functional relationships between fungal and plant ethylene biosynthesis. This may lead to future applications in postharvest technologies based on alternative strategies for fresh plant produce protection, mainly focused on the activity of the fungus rather that on inhibition of plant-produced ethylene, which usually associate enhanced shelf life with decreased flavor and quality.
The role played by ethylene in the infection process remains to be clarified. The new molecular tools for studying B. cinerea molecular biology described in the late 1990s (33, 35) should enable characterization of the role of fungus-produced ethylene in pathogenesis. Isolation of the genes involved in ethylene biosynthesis by the fungus and deletion of these genes should reveal whether ethylene production by the fungus plays a role in the fungus-plant interaction. In addition, ethylene perception by the fungus should also be investigated.
Acknowledgments
We thank K. T. van de Pas-Schoonen (Microbiology Department, University of Nijmegen) and Huub Geurts (Botany Department, University of Nijmegen) for their help with light microscopy and C. Kuhlemeier (Institute of Plant Science, Berne, Switzerland) for critically reading the manuscript and helpful comments.
This study was supported by EU/FAIR grant CT98-4211.
REFERENCES
- 1.Abeles, F. B., P. W. Morgan, and M. E. Saltveit, Jr. 1992. Ethylene in plant biology, 2nd ed. Academic Press, San Diego, Calif.
- 2.Achilea, O., Y. Fuchs, E. Chalutz, and I. Rot. 1985. The contribution of host and pathogen to ethylene biosynthesis in Penicillium digitatum. Physiol. Plant Pathol. 27:55-63. [Google Scholar]
- 3.Barkai-Golan, R., G. Lavy-Meir, and E. Kopeliovitch. 1988. Pectolytic and cellulolytic activity of Botrytis cinerea Pers. related to infection of non-ripening tomato mutants. J. Phytopathol. 123:174-183. [Google Scholar]
- 4.Barkai-Golan, R., G. Lavy-Meir, and E. Kopeliovitch. 1989. Effects of ethylene on the susceptibility to Botrytis cinerea infection of different tomato genotypes. Ann. Appl. Biol. 114:391-396. [Google Scholar]
- 5.Bijnen, F. G. C., J. Reuss, and F. J. M. Harren. 1996. Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection. Rev. Sci. Instrum. 67:2914-2923. [Google Scholar]
- 6.Boller, T. 1991. Ethylene in pathogenesis and disease resistance, p. 293-324. In A. K. Mattoo and J. C. Suttle (ed.), The plant hormone ethylene. CRC Press, Boca Raton, Fla.
- 7.Brewer, R. J., C. W. Bruce, and J. L. Mater. 1982. Optoacoustic spectroscopy of C2H4. Appl. Optics 21:4092-4100. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, J., A. ten Have, and J. A. L. van Kan. 2002. The role of ethylene and wounding signalling in resistance of tomato to Botrytis cinerea. Plant Physiol. 129:1341-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, X. 1998. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1:316-323. [DOI] [PubMed] [Google Scholar]
- 10.Eden, M. A., R. A. Hill, R. Beresford, and A. Stewart. 1996. The influence of inoculum concentration, relative humidity, and temperature of infection of greenhouse tomatoes by Botrytis cinerea. Plant Pathol. 45:795-806. [Google Scholar]
- 11.Elad, Y. 1990. Production of ethylene by tissue of tomato, pepper, French-bean and cucumber in response to infection by Botrytis cinerea. Physiol. Mol. Plant Pathol. 36:277-287. [Google Scholar]
- 12.Elad, Y. 1997. Responses of plants to infection by Botrytis cinerea and novel means involved in reducing their susceptibility to infection. Biol. Rev. 72:381-422. [Google Scholar]
- 13.Elad, Y., and K. Eversen. 1995. Physiological aspects of resistance to Botrytis cinerea. Phytopathology 85:637-643. [Google Scholar]
- 14.El-Kazzaz, M. K., N. F. Sommer, and A. A. Kader. 1983. Ethylene effects on in vitro and in vivo growth of certain postharvest fruit-infecting fungi. Phytopatology 73:998-1001. [Google Scholar]
- 15.Epstein, E., O. Sagee, J. D. Cohen, and J. Garty. 1986. Endogenous auxin and ethylene in the lichen Ramalina duriaei. Plant Physiol. 82:1122-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feys, B. J., and J. E Parker. 2000. Interplay of signalling pathways in plant disease resistance. Trends Genet. 16:449-455. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda, H., H. Kitajima, T. Fujii, M. Tazaki, and T. Ogawa. 1989. Purification and some properties of novel ethylene-forming enzyme produced by Penicillium digitatum. FEMS Microbiol. Lett. 59:1-6. [Google Scholar]
- 18.Fukuda, H., M. Takahashi, T. Fujii, M. Tazaki, and T. Ogawa. 1989. An NADH:Fe(III) EDTA oxidoreductase from Cryptococcus albidus: an enzyme involved in ethylene production in vivo? FEMS Microbiol. Lett. 60:107-112. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda, H., T. Ogawa, and S. Tanase. 1993. Ethylene production by micro-organisms. Adv. Microb. Physiol. 35:275-306. [DOI] [PubMed] [Google Scholar]
- 20.Harren, F. J. M., and J. Reuss. 1997. Photoacoustic spectroscopy, p. 413-435. In G. L. Trigg (ed.), Encyclopedia of applied physics, vol. 19. VCH Publishers, Inc., Weinheim, Germany.
- 21.Harren, F. J. M., R. Berkelmans, K. Kuiper, S. te Lintel Hekkert, P. Scheepers, R. Denhuijzen, P. Hollander, and D. H. Parker. 1999. On-line laser photoacoustic detection of ethene in exhaled air as biomarker of ultraviolet radiation damage of the human skin. Appl. Phys. Lett. 74:1761-1763. [Google Scholar]
- 22.Ince, J. E., and C. J. Knowles. 1986. Ethylene formation by cell-free extracts of Escherichia coli. Arch. Microbiol. 146:151-158. [DOI] [PubMed] [Google Scholar]
- 23.Kepczynska, E. 1989. Ethylene requirement during germination of Botrytis cinerea spores. Physiol. Plant. 77:369-372. [Google Scholar]
- 24.Kepczynska, E. 1993. Involvement of ethylene in the regulation of growth and development of the fungus Botrytis cinerea Pers. ex. Fr. Plant Growth Regul. 13:65-69. [Google Scholar]
- 25.Lurie, S., and J. Garty. 1991. Ethylene production by the lichen Ramalina duriaei. Ann. Bot. 68:317-319. [Google Scholar]
- 26.Mansfield, J. W. 1980. Mechanism of resistance to Botrytis, p. 181-218. In J. R. Coley-Smith, K. Verhoeff, and W. R. Jarvis (ed.), The biology of Botrytis. Academic Press, New York, N.Y.
- 27.Mattoo, A. K., and J. C. Suttle. 1991. The plant hormone ethylene. CRC Press, Boca Raton, Fla.
- 28.Nagahama, K., T. Ogawa, T. Fujii, M. Tazaki, S. Tanase, Y. Morino, and H. Fukuda. 1991. Purification and properties of an ethylene-forming enzyme from Pseudomonas syringae pv. phaseolicola PK2. J. Gen. Microbiol. 137:2281-2286. [DOI] [PubMed] [Google Scholar]
- 29.Pazout, J., S. Pazoutova, and V. Vancura. 1982. Effects of light and oxygen on ethylene formation and conidiation in surface cultures of Penicillium cyclopium. Curr. Microbiol. 7:133-136. [Google Scholar]
- 30.Polevaya, Y., S. Alkalai-Tuvia, A. Copel, and E. Fallik. 2002. Early detection of grey mould development in tomato after harvest. Postharvest Biol. Technol. 25:221-225. [Google Scholar]
- 31.Qadir, A., E. W. Hewett, and P. G. Long. 1997. Ethylene production by Botrytis cinerea. Postharvest Biol. Technol. 11:85-91. [Google Scholar]
- 32.te Lintel Hekkert, S., M. J. Staal, R. H. M. Nabben, H. Zuckermann, S. Persijn, L. J. Stal, L. A. C. J. Voesenek, F. J. M. Harren, J. Reuss, and D. H. Parker. 1998. Laser photoacoustic trace gas detection, an extremely sensitive technique applied in biological research. Instrum. Sci. Technol. 26:157-175. [Google Scholar]
- 33.ten Have, A., W. O. Breuil, J. P. Wubben, J. Visser, and J. A. L. van Kan. 2001. Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet. Biol. 33:97-105. [DOI] [PubMed] [Google Scholar]
- 34.von Tiedemann, A. 1997. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol. Mol. Plant Pathol. 50:151-166. [Google Scholar]
- 35.Wubben, J. P., A. ten Have, J. A. L. van Kan, and J. Visser. 2000. Regulation of endopolygalacturonase genes expression in Botrytis cinerea by galacturonic acid, ambient pH and carbon catabolite repression. Curr. Genet. 37:152-157. [DOI] [PubMed] [Google Scholar]
- 36.Yang, S. F., and N. E. Hoffman. 1984. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35:155-189. [Google Scholar]
- 37.Zuckermann, H., M. Staal, L. J. Stal, J. Reuss, S. te Lintel Hekkert, F. J. M. Harren, and D. H. Parker. 1997. On-line monitoring of nitrogenase activity in cyanobacteria by sensitive laser photoacoustic detection of ethylene. Appl. Environ. Microbiol. 63:4243-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]