Summary
The use of friendly microbes to thwart pathogens has a long history. Will the latest wave of discoveries bring us any closer to new biological medicines?
Lin Tao, a researcher at the College of Dentistry at the University of Illinois (Chicago, IL, USA), may have found a way to stop countless infants contracting HIV. This May, at the American Society for Microbiology general meeting in New Orleans (LO, USA), he presented his discovery of a specific strain of Lactobacillus, a commensal bacterium in human saliva, which could reduce the likelihood of HIV transmission from mother to breast-fed child. Giving this particular bacterium in the form of lyophilized powder to infants to populate their digestive tract may effectively block HIV from entering its target cells. Around 800,000 infants worldwide contract HIV in this way every year, most of them in Africa. But if Tao's research receives the necessary funding to go into development, a prophylactic against this particularly distressing route of transmission could be in sight. Although the discovery is still some way from application, it reinforces the wisdom that enlisting your enemy's enemy can be as good as attacking him yourself.
It is simply a case of working out who eats whom
Since the first half of the twentieth century, scientists and medics have realized that the eternal struggles of the microbial world could be exploited for human benefit. Isolating the weapons of these wars provided physicians with a powerful arsenal against disease and infection. No sooner had penicillin been discovered, than pharmaceutical companies brought us even more antibiotics, such as other β-lactams and later polyketides and many other naturally occurring compounds. However, the declining effectiveness of many antibiotics—caused by the misuse of nature's gifts—combined with the convergence of many biological disciplines, is creating a renaissance in the field of biological control of pathogens. The new tools provided by genomics and proteomics are producing a mountain of information on how formerly intractable organisms interact with their surroundings, both biotic and abiotic, and how host and prey interact at the genetic level. Many a microbe that preys on us is in turn preyed on by something else. It is simply a case of working out who eats whom.
Among our best microbial friends are the lactobacilli, some of which have been used by humans ever since milk turned sour and inadvertently made cheese. Others have happily inhabited various parts of our anatomy for much longer than that. The growing list of probiotic foods is evidence of their important role in maintaining a healthy intestinal flora. And clearly their beneficial properties do not stop there. The lactobacilli identified by Tao have an unexpected affinity for the sugar mannose, which is present in the polysaccharides of the HIV viral envelope. By blocking the mannose residues, this specific strain of Lactobacillus hinders the spread of the virus, as it is no longer able to bind the cell surface receptors that trigger its entry into human cells.
In 1.5 m of large intestine, the average human being gives table space to around 1014 bacteria, ten times the number of cells in the human body
And lactobacillus could probably do more. “It could be useful not only for HIV, but also possibly all viruses that have an envelope,” Tao remarked, cautioning “but we don't have these data yet.” But for fighting HIV, not just any lactobacillus will do. Representatives of the same species identified in saliva samples taken in Africa apparently do not have the mannose-binding ability. Tao suspects that what he is seeing is a new strain, which has obtained a plasmid encoding the mannose-binding protein.
Understandably, he is very careful not to give too much away, but at the same time he needs to attract investment to develop his National Institutes of Health (NIH)-funded research further. “I would like to get more publicity,” he said, because “for me to develop the product I need venture capitalists to fund me.” Unfortunately, venture capital is more likely to flow towards discoveries that address the ailments of westerners rather than African mothers who cannot afford to buy medicines. “Venture capitalists still don't think the discovery has monetary value, but I strongly believe this technology can be used for other applications,” Tao remarked. For women across the globe, it could, for instance, be administered as a vaginal suppository to erect a partial barrier to sexually transmitted HIV. And Tao's mannose-binding lactobacilli are not the only microbial enemy that HIV may have to contend with in the future. Other NIH-funded researchers have been working on cyanovirin-N, a mannose-binding protein from cyanobacteria (Han Z et al. (2004) Peptides 5: 551–561; Tsai CC et al. (2004) AIDS Res Hum Retroviruses 20: 11–18).
Bdellovibrio's voracious appetite for bacteria has been known for more than 40 years, and it is puzzling to Schuster that it was not more vigorously pursued by researchers
Elsewhere, lactobacilli are busy fighting caries. Carina Krüger's research group at the Institute for Laboratory Medicine at the Karolinska Institute in Stockholm, Sweden, recently reported a possible cost-effective passive immunization strategy to protect teeth (Krüger C (2004) PhD thesis, Karolinska Institute, Karolinska University Press, Stockholm, Sweden). The trick is to engineer the lactobacilli to produce a single-chain antibody that binds to the cell surface adhesion molecules of the major caries-causing bacterium Streptococcus mutans. Even normal Lactobacillus rhamnosus GG, when consumed in milk, has already been shown to drastically reduce the incidence of cavities in children.
Further down the alimentary canal, lactobacilli jostle with a host of other bacteria, some friendly, some not. The lactic-acid-producing bacteria—Acidophilis, Lactobacillus and Bifidobacillus—are increasingly in the limelight. Termed 'probiotics', they turn up in a growing number of food products, and are even creating a new market in functional foods. Although the mechanism is not understood, they even seem to have an immuno-modulating or adjuvant activity on the immune system through the intestinal mucosa, which can help to protect us against pathogens.
Some observers might be tempted to believe that we are at the brink of another explosion in biotechnology known as 'probiotic biotech'. But they would do well to talk to Koen Venema, Product Manager for Intestinal Microbiology at TNO Nutrition and Food Research in Zeist, and Project Leader at the Wageningen Centre for Food Sciences, both in the Netherlands. Although there is certainly a great deal of potential in this field, the sheer complexity of the flora in the human gastrointestinal tract needs to be understood better before technologies can be developed, he thinks. In 1.5 m of large intestine, the average human being gives table space to around 1014 bacteria, ten times the number of cells in the human body. As Venema remarked, “We are probably more microbe than anything else.” And given that this number represents between 400 and 1,000 different species, it is not surprising that analysing their interrelationships is a formidable task. Venema cautioned that we are only at the very beginning of understanding, and have not yet reached a stage at which we can substantially exploit existing species, let alone genetically modify them for improved performance. So far, a few general mechanisms by which lactic-acid bacteria control other bacterial populations are known: the lowering of pH by lactic-acid production, the production of bacteriocin (a pore-forming protein that punctures bacterial membranes), competition for food and competition for binding sites to epithelial cells.
...the curative powers of phages had first been observed by researchers at the Pasteur Institute in Paris in 1917, where they were administered to sufferers of dysentery and several other bacterial infections, with remarkable results
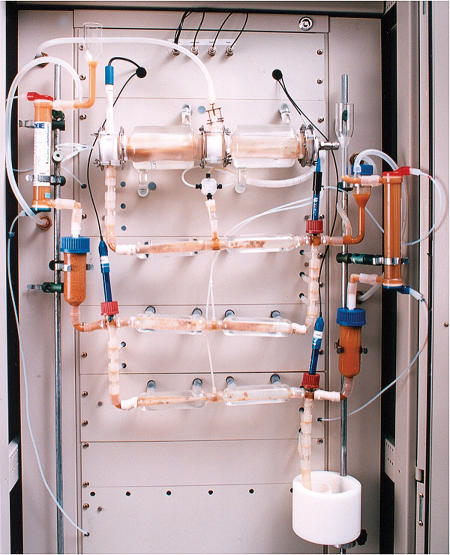
In vitro experiments using simulated gastrointestinal epithelium and donated faecal samples are the current method of investigating gut flora and bacterial metabolites at TNO Nutrition and Food Research (Fig 1). However, studies on individual species are fraught with difficulties; perhaps only half of them can be cultured in vitro. “The microbes live in symbiosis, and that is probably why we can't grow them [individually]; they kill each other, but at the same time they live together,” Venema said. Some even produce butyrate, which provides 70% of the energy source for colonocytes (cells lining the colon), and without which we too would die. With the estimate of the number of gut commensal rising almost daily, research in this field appears to have a rosy future—Venema thinks the number in a given human population could eventually be as high as 5,000.
Figure 1.
TIM-1 (TNO gastrointestinal model) used to simulate human intestines. Courtesy of Koen Venema, TNO Nutrition and Food Research, Zeist, the Netherlands.
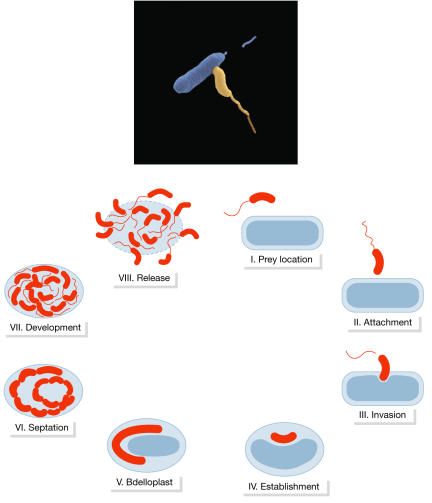
One of these is a vicious little predator called Bdellovibrio bacteriovorus—literally 'bacteria eating'. This January, the research group of Stephan Schuster at the Max Planck Institute for Developmental Biology in Tübingen, Germany, announced its complete genome sequence, and suggested that it could become a living antibiotic (Rendulic S et al. (2004) Science 303: 689–692). The aptly named microbe literally eats its victim alive from inside, residing in the periplasmic space between the two host bacterial membranes until it is big enough to divide and burst its host (Fig 2). The powerful techniques of genomics and proteomics enabled the researchers not only to identify the enzymes involved in this murderous act, but also to show that B. bacteriovorus does not have the molecular machinery necessary to infect human cells. Furthermore, its surface proteins are less allergenic than those of other bacteria, thus making its use as an antibiotic even more plausible.
Figure 2.
Life cycle of a killer. Top: Bdellovibrio bacteriovorus infecting a host bacterium. Courtesy of the Max Planck Institute for Developmental Biology and Snjezana Rendulic, Jürgen Berger and Stephan Schuster, Tübingen, Germany. Bottom: Life cycle of Bdellovibrio.
“I would say it's an excellent starting point: many of the features of the system make it plausible that it would work, but its development would be similar to that of pharmaceuticals, of which less than 5% finally make it to market,” Schuster explained. Unlike blockbuster antibiotics, different Bdellovibri species have very specific tastes in different host bacteria. Schuster's species has a penchant for Pseudomonas aeruginosa, a growing scourge of hospitals on account of its rapidly emerging resistance to most antibiotics, including vancomycin. An estimated 1,400 deaths per year in the USA alone are due to hospital-acquired pneumonia caused by Pseudomonas.
Bdellovibrio's voracious appetite for bacteria has been known for more than 40 years, and it is puzzling to Schuster that the subject was not more vigorously pursued by researchers. He has already started cooperating with a biotechnology company to develop a treatment for external wounds infected by Pseudomonas. “So far we're concentrating on external applications,” he said, but he is disappointed in the low level of interest from industry, which considers the discovery to be too far from application for investment. Meanwhile, the sequencing of another Bdellovibrio strain is allowing genomic comparisons, further increasing the understanding of the genes involved in predation. These number over 240. Not surprisingly, “genetic modification [of Bdellovibrio] is still a thing of the future; we need to understand the genetic traits first,” Schuster commented. But the basic research is already a perfect example of the confluence of many fields: microbiology, cell biology, molecular biology and immunology.
Schuster already thinks that his potential living antibiotic stands a better chance of success than a much older field of research involving bacteriophages. These bacterium-infecting viruses, he says, have a much higher mutation rate and would therefore be less stable in applications. Phages have evolved specifically to attack only bacteria, and are very selective about their prey species. But given that as long ago as the 1930s, a US pharmaceutical company included phages in its list of biological therapies, one cannot help but wonder why they have not yet made it into widespread use. And it was not as if the company was pushing some kind of alchemist's panacea: the curative powers of phages had first been observed by researchers at the Pasteur Institute in Paris in 1917, where they were administered to sufferers of dysentery and several other bacterial infections, with remarkable results. The broad-spectrum efficacy of penicillin, first used in the 1940s, however, eclipsed these minute killing machines.
The specificity of phages, as with many organism-based strategies, precludes their use as a broad-spectrum antibiotic; the disease-causing pathogen must first be accurately identified before treatment. But this is also their advantage: unlike broad-spectrum antibiotics, which not only simultaneously breed resistance in many bacteria, but also wipe out friendly commensals, a particular phage delicately picks off only the bacterial species that it naturally infects. That may make phages the doctor's friend, but does not necessarily interest large pharmaceutical companies. Biotech companies, on the other hand, see plenty of fresh pickings, as Glenn Morris, co-founder of Intralytix Inc. (Baltimore, MD, USA) and Chairman of the Department of Epidemiology and Preventive Medicine at the University of Maryland School of Medicine (also in Baltimore) agrees. He thinks the time is ripe for phages to make it to market. Commenting on the abrupt decline in phage preparations in the 1940s, he said, “there were some problems with initial phage preparations because they weren't pure, and frequently [the medics] did not know what they were treating.” That has all changed: “[today] you're talking about the sophistication that comes with a further 60 years of science,” he added.
Intralytix Inc. has already performed trials of phages against vancomycin-resistant enterococci in animal models, showing the phages to be “very safe, and very efficacious” in the words of Morris. Multidrug-resistant Pseudomonas is also on the hit list of the phage therapy developers, and Morris is confident, despite Schuster's scepticism, that here too a treatment for humans is not far off. Given that a phage is a much simpler biological entity than a bacterium, he is sure of who the winner will be: “It's going to be tough enough to get approval for phage use in medications; I'm not sure I'd like to take a Bdellovibrio through an FDA approval process.” Although Morris concedes that phages will not be a cure-all, this is precisely their strength: in combination with traditional antibiotics, they will probably contribute to new antibiotic strategies that keep pathogens on their toes more than antibiotics alone.
Developing the antibiotics of the future is not really about whether bacteria, phages or chemical antibiotics will win. The combination of increasing pressure to attack infectious disease with new and diverse strategies, and a market sector that takes risks and is content with smaller profits than the pharmaceutical giants, will probably generate a range of novel biological products. Nature has, after all, done half the work for us.