Abstract
Signalling by the p75 neurotrophin receptor has been implicated in diverse neuronal responses, including increased differentiation or survival, inhibition of regeneration, and initiation of apoptotic cell death. These numerous roles are matched by, but are not yet correlated with, a multiplicity of extracellular ligands and intracellular interactors. Membrane proteins such as sortilin, a member of the Vps10p family of sorting receptors, and the glycosylphosphatidylinositol-linked Nogo receptor (NgR) and the associated adaptor lingo 1 have recently been added to the list of p75-interacting modulators. Other studies have described intramembranal cleavage of p75 and the potential nuclear targeting of cleavage fragments or of the complete receptor after it has been internalized into a putative signalling endosome. These findings suggest that some of the diversity in p75 activities might be due to differential subcellular localization and transport of p75 receptor complexes. We therefore argue that cell-biology-driven approaches are now required to make sense of p75 signalling.
Keywords: p75, sortilin, Vps10p, neurotrophin, Nogo
p75: an overly multifunctional receptor?
The nerve growth factor (NGF) family of neurotrophins has diverse roles in the nervous system, including regulation of progenitor cell numbers, modulation of neurite outgrowth and growth cone guidance, and control of survival or death of neurons and other cell types. These different activities are mediated by two distinct classes of cell-surface receptors, the trk family of receptor tyrosine kinases (Huang & Reichardt, 2003), and the p75 neurotrophin receptor, which belongs to the tumour necrosis factor (TNF)-receptor superfamily (Roux & Barker, 2002). trk receptor signalling and activities have been well characterized over the past 15 years, and it is now widely accepted that a primary role of trk receptors is the control of neuronal survival in response to limiting amounts of neurotrophin ligands (Huang & Reichardt, 2003). trk–p75 cross-talk and interactions have been well documented in the creation of high-affinity binding sites and signalling modulation (Huang & Reichardt, 2003). Beyond this, the independent effects of p75 remain a topic of lively controversy within the field and a bewildering enigma to unfortunate outsiders who delve into the literature. In the past three years alone, different publications have claimed that p75 supports (Bentley & Lee, 2000) or inhibits (Yamashita et al, 2002) axon growth, increases (DeFreitas et al, 2001) or decreases (Troy et al, 2002) neuronal survival, and is crucial (Boyd & Gordon, 2001) or not required (Song et al, 2004) for inhibition of neuronal regeneration. Two independent alleles of p75-null mice have been created, and although both exhibit a pronounced sensory neuron phenotype, additional controversy has been generated by suggestions that both lines express unexpected partial splice variants of the receptor (Paul et al, 2004; von Schack et al, 2001).
Can new accomplices explain many functions?
Recently, the spectrum of p75 ligands has been increased by the addition of unprocessed proneurotrophins (Harrington et al, 2004; Lee et al, 2001), and myelin-associated regeneration inhibiting proteins through a lingo 1-mediated association between p75 and the glycosylphosphatidylinositol (GPI)-linked Nogo receptor (NgR; Mi et al, 2004; Wang et al, 2002a; Wong et al, 2002). Most recently, an intriguing twist in the tale has been added by the implication of sortilin in high-affinity binding of pro-NGF molecules (Nykjaer et al, 2004). Sortilin is a member of the Vps10p family, the proteins of which are thought to act as sorting receptors for molecules in the secretory pathway and on the cell membrane (Mazella, 2001; Nielsen et al, 2001). The recently published crystal structure of NGF complexed with the extracellular domain of p75 reveals an NGF homodimer asymmetrically bound to a p75 monomer, a configuration that might allow the recruitment of another receptor to the complex (He & Garcia, 2004). These findings have led to the suggestion that the outcome of p75 signalling might be determined by the type of co-receptor involved in the complex: if trk is the co-receptor then survival is the outcome, if NgR–lingo 1 then inhibition of regeneration, and if sortilin then death (Nykjaer et al, 2004). However, this model, while appealing in its apparent clarity, has already been called into question by the finding that pro-NGF enhances migration rather than death of A875 melanoma cells, which express high levels of both sortilin and p75 (Shonukan et al, 2003). Furthermore, like TRKs, both sortilin and NgR belong to gene families with several members expressed in the nervous system (Hampe et al, 2001; Lauren et al, 2003; Pignot et al, 2003), raising the possibility that different complexes of these receptors respond to individual neurotrophins and other ligands. Finally, sortilin has been shown to bind to a range of other ligands or cargo proteins at the same site used by pro-NGF (Lefrancois et al, 2003; Mazella, 2001; Munck Petersen et al, 1999), creating diverse options for modulation or interference with proneurotrophin signalling through sortilin in vivo. Therefore, the identification of NgR–lingo 1 and sortilin as p75 co-receptors appears to introduce additional layers of complexity to the field. Conversely, the sortilin–p75 connection directs attention to a relatively neglected facet of p75 research in neurons, namely a cell-biology-driven approach. The field still lacks definitive analyses of p75 biosynthesis, sorting and localization on the one hand, and internalization, transport and the eventual fate of signalling complexes of the receptor on the other hand. Given the polarized morphology of neuronal cells, we argue that such studies would fill the main gaps in our understanding of the modes of action of this multifaceted receptor.
Location, location, location...
The polarization of neurons into somatodendritic versus axonal compartments is a defining aspect of their biology. Although p75 is restricted to the apical domain in epithelial Madine–Darby canine kidney (MDCK) cells (Yeaman et al, 1997), it is expressed in roughly equal amounts in the dendrites and axons of transfected hippo-campal neurons (Jareb & Banker, 1998). Immunohistochemistry and electron microscopy revealed more prominent p75 expression in axons than dendrites in rat dentate gyrus (Dougherty & Milner, 1999). Do NgR or sortilin localize to distinct subcellular compartments in neurons? Interestingly, whereas NgR and its homologues have been observed in lipid rafts on axonal cell surfaces (Pignot et al, 2003; Wang et al, 2002b), sortilin and its homologues are predominantly intracellular and enriched in the somatodendritic domain (Hermey et al, 2001; Sarret et al, 2003), which parallels the distribution of Golgi structures within neurons (Horton & Ehlers, 2003). Thus, it is possible that NgR–lingo 1 modulates the specificity and function of p75 through regulated translocations to lipid rafts in axons, whereas sortilin is a major co-receptor in dendrites. A caveat to such a model is that the vast majority of Vps10p-type receptors in the cell are intracellular and primarily accumulate in the Golgi, where they may be available for the capture and sorting of newly synthesized ligands (Jacobsen et al, 2001; Munck Petersen et al, 1999). Conversely, the lumenal and/or extracellular domains of both sortilin and NgR family members have been observed in cleaved or shed forms in extracellular media (Navarro et al, 2002; Pignot et al, 2003), raising the possibility of soluble ligand–receptor complexes that may activate cellsurface p75. Soluble forms of NgR act as dominant negatives over surface-bound NgR in assays of inhibition of neurite extension (Domeniconi et al, 2002), but this does not preclude the possibility that such ligand-receptor complexes might have distinct activities from that classically ascribed to NgR. It should also be noted that p75 itself undergoes shedding by cellsurface cleavage with metalloproteases in a variety of cell types, including from dorsal-root ganglia (Weskamp et al, 2004). Therefore, p75 signalling might be regulated by extracellular domain cleavage of all three receptor components—p75, NgR and sortilin—and it will be important to determine the spatial and temporal expression and activity of proteases relevant to this process.
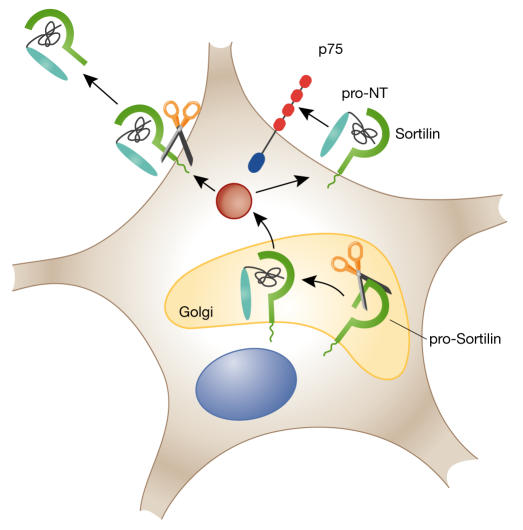
In the case of sortilin, a further intriguing possibility is that proneurotrophin–sortilin interactions could commence intracellularly within the secretory pathway (Fig 1). In such a scenario, sortilin binding to the pro-domain of the neurotrophin in the Golgi apparatus could protect it from proteolytic cleavage, and the molecules could then travel to the cell surface in a pre-formed pro-apoptotic p75-activating complex. Regulating sortilin levels might thereby provide the cell with an efficient mechanism of active suicide. Alternatively, cellsurface cleavage of sortilin could release a soluble sortilin–proneurotrophin complex into extracellular space. Such a complex might provide a circulating reservoir of proneurotrophin that is protected from cleavage to the mature form and is available for interaction with receptors on adjacent or distant cells.
Figure 1.
Different sites of potential interactions between sortilin and pro-neurotrophins. The pro-neurotrophin (pro-NT) binding site in sortilin is exposed after proteolytic cleavage of the sortilin propeptide in the Golgi apparatus, raising the possibility of an intra-Golgi interaction. The molecules would then travel together to the plasma membrane, where they might form an autoactivating complex for p75 on the same cell; alternatively, a second cleavage event might release a soluble complex to the extracellular milieu.
p75 signalling: going the distance?
No catalytic activity has yet been identified for the p75 intracellular domain, so the receptor must rely on recruitment of intracellular interactors to transduce a signal. A wide range of unrelated molecules have been shown to interact with the intracellular domain of p75 (Bai et al, 2003; Roux & Barker, 2002; Tcherpakov et al, 2002; Yamashita & Tohyama, 2003). Most of these also lack catalytic activity, suggesting that p75 signalling requires recruitment of multiple transducers through protein–protein interactions. Once such a signalling complex is formed, how is the signal transported from axonal or dendritic sites of generation to the cell body? This question has been addressed primarily for trk signal transduction, and the hypotheses raised include calcium/ phosphorylation 'waves' progressing along the axon, axonal transport of activated signalling molecules, or retrograde transport of neurotrophin–trk signalling endosomes (Delcroix et al, 2003; Ginty & Segal, 2002). A direct interaction between TRK and the motor protein dynein has been reported, which provides a possible mechanism for microtubule-associated transport of such endosomes (Yano & Chao, 2004; Yano et al, 2001). Although p75 might mediate the transport of a wide variety of neurotrophin and non-neurotrophin ligands (Butowt & Von Bartheld, 2003), the mechanisms of its internalization and movement are poorly understood. Mature NGF induces relatively slow internalization of p75 through clathrin-coated pits to the recycling endosome and to vesicles positive for cholera toxin (a marker of lipid rafts) in the growth cones (Bronfman et al, 2003; Saxena et al, 2004). p75 has been shown to associate with lipid rafts in a protein-kinase-A-regulated manner (Higuchi et al, 2003), and in motor neuron vesicles it colocalizes with tetanus toxin (Lalli & Schiavo, 2002), which is internalized via lipid rafts (Herreros et al, 2001). Therefore, lipid rafts could be regulating the internalization kinetics of p75 and its accessibility to different co-receptor complexes. Strikingly, the presence of sortilin enables more rapid internalization of pro-NGF with p75 (Nykjaer et al, 2004), although the precise kinetics of the process were not determined. Previous studies on sortilin internalization have shown rapid endocytosis of sortilin chimaeras to the trans-Golgi network, with little or no recycling (Nielsen et al, 2001). By contrast, p75 has been implicated in anterograde transport of neurotrophins from the Golgi of retinal ganglion cells (Butowt & von Bartheld, 2001), therefore it will be interesting to determine whether sortilin-mediated rapid internalization is followed by lysosomal degradation or recycling of ligand to anterograde pathways in neurons (Fig 2). Differential kinetics of internalization of liganded p75 complexes might allow the recruitment of different classes of interactors. For example, the association of TNF-receptor associated factor 6 with p75 is rapid and occurs within minutes of exposure to ligand (Khursigara et al, 1999), whereas the interaction of necdin and melanoma-associated antigen H1 (MAGE-H1) requires the presence of ligand for at least one hour (Tcherpakov et al, 2002). Thus, a p75–NgR complex, which is restricted to lipid rafts and either does not internalize (due to an interaction with a membrane-bound component of myelin) or internalizes very slowly, might recruit a completely different complement of interactors than a rapidly internalizing p75–sortilin complex.
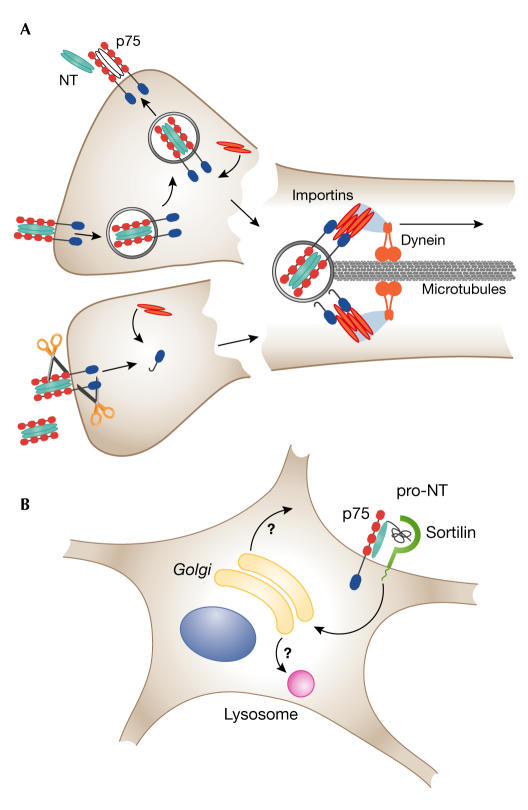
Figure 2.
Plausible scenarios for long-range transduction of p75 signalling complexes. (A) Internalization of p75 with ligand to the recycling endosome (upper) or cleavage of p75 to release the intracellular domain (lower) may be followed in both cases by recruitment of intracellular interactors with nuclear localization signals. These might then serve as adaptors to link the p75 signalling complexes to an importin/dynein retrograde transport ensemble on the microtubules. (B) In the case of a sortilin-containing complex in the somatodendritic compartment, internalization and rapid translocation to the Golgi may be mediated by sorting signals in the cytoplasmic tail of sortilin. The complex might then transfer to lysosomes or, after dissociation, specific components might enter the anterograde pathway for recycling to the cell surface.
Once activated, at least some of the p75 signals must have an impact on the neuronal cell body (Bhakar et al, 2003; Lad & Neet, 2003). Recent studies have provided evidence for two apparently different mechanisms that could underlie this process. On the one hand, formation of a p75 signalling endosome containing ligand, receptor and intracellular MAGE interactors has been demonstrated in the recycling compartment of neuronal PC12 cells (Bronfman et al, 2003); similar endosomes may be shunted into the retrograde transport pathway in nerve axons (Lalli & Schiavo, 2002). On the other hand, two independent studies have now shown that p75 is subject to regulated intramembrane proteolysis (RIP), which produces soluble cleavage products of the intracellular domain that have potential signalling capabilities (Jung et al, 2003; Kanning et al, 2003). Such cleavage could occur at the plasma membrane, but as relevant proteases are also found within vesicles in axons (Kamal et al, 2001), it might also take place in an endosomal compartment after internalization. RIP cleavage products of the p75 homologue neurotrophin receptor homologue 2 (NRH2) have been shown to travel to the nucleus (Kanning et al, 2003). In this context, it is striking that nearly all known p75 intracellular interactors contain nuclear localization signals (NLSs). Moreover, a recent study has shown that nuclear import factors from the importin-α and importin-β families are found in both axons and dendrites, and can mediate retrograde transport of signalling cargoes through an interaction of importin-α with dynein (Hanz et al, 2003). The association of a p75 signalling complex with dynein might be mediated by an NLS-bearing interactor that is able to bind to the high-affinity NLS-binding site on the importins. Such an interaction could provide a mechanism to shunt a p75-containing endosome from the recycling to the retrograde pathway, or to carry a soluble signalling complex formed around the intracellular cleavage products of p75 (Fig 2).
To summarize, recent findings have directed attention to the probable influences of sortilin Vps10p receptors on the formation and endocytosis of p75 signalling complexes, and on possible roles of RIP and importins on the processing and transport of the signals emanating from such complexes. This invasion of cell biology (and hopefully also cell biologists) into the p75 field should help to clarify the mechanisms underlying the many roles of this receptor system.


Acknowledgments
Our work in this field is supported by Fundación Andes, FONDECYT, FONDAP para Biomedicina and DIPUC (F.C.B.); the Israel Science Foundation, BMBF-MOS and IFP Paraplegia (M.F.). M.F. is the incumbent of the Daniel Koshland Sr Career Development Chair at the Weizmann Institute. A special thanks to A. Michaly from the Weizmann Graphics Unit for providing the artwork.
References
- Bai D, Chen H, Huang BR (2003) RanBPM is a novel binding protein for p75NTR. Biochem Biophys Res Commun 309: 552–557 [DOI] [PubMed] [Google Scholar]
- Bentley CA, Lee KF (2000) p75 is important for axon growth and Schwann cell migration during development. J Neurosci 20: 7706–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA (2003) Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J Neurosci 23: 11373–11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JG, Gordon T (2001) The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol 49: 314–325 [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M (2003) Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci 23: 3209–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, von Bartheld CS (2001) Sorting of internalized neurotrophins into an endocytic transcytosis pathway via the Golgi system: ultrastructural analysis in retinal ganglion cells. J Neurosci 21: 8915–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, Von Bartheld CS (2003) Connecting the dots: trafficking of neurotrophins, lectins and diverse pathogens by binding to the neurotrophin receptor p75NTR. Eur J Neurosci 17: 673–680 [DOI] [PubMed] [Google Scholar]
- DeFreitas MF, McQuillen PS, Shatz CJ (2001) A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J Neurosci 21: 5121–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC (2003) NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39: 69–84 [DOI] [PubMed] [Google Scholar]
- Domeniconi M et al. (2002) Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35: 283–290 [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Milner TA (1999) p75NTR immunoreactivity in the rat dentate gyrus is mostly within presynaptic profiles but is also found in some astrocytic and postsynaptic profiles. J Comp Neurol 407: 77–91 [DOI] [PubMed] [Google Scholar]
- Ginty DD, Segal RA (2002) Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol 12: 268–274 [DOI] [PubMed] [Google Scholar]
- Hampe W, Rezgaoui M, Hermans-Borgmeyer I, Schaller HC (2001) The genes for the human VPS10 domain-containing receptors are large and contain many small exons. Hum Genet 108: 529–536 [DOI] [PubMed] [Google Scholar]
- Hanz S et al. (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40: 1095–1104 [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM (2004) Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA 101: 6226–6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Garcia KC (2004) Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 304: 870–875 [DOI] [PubMed] [Google Scholar]
- Hermey G, Riedel IB, Rezgaoui M, Westergaard UB, Schaller C, Hermans-Borgmeyer I (2001) SorCS1, a member of the novel sorting receptor family, is localized in somata and dendrites of neurons throughout the murine brain. Neurosci Lett 313: 83–87 [DOI] [PubMed] [Google Scholar]
- Herreros J, Ng T, Schiavo G (2001) Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol Biol Cell 12: 2947–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Yamashita T, Yoshikawa H, Tohyama M (2003) PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. EMBO J 22: 1790–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD (2003) Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci 23: 6188–6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72: 609–642 [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J, Petersen CM (2001) Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem 276: 22788–22796 [DOI] [PubMed] [Google Scholar]
- Jareb M, Banker G (1998) The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron 20: 855–867 [DOI] [PubMed] [Google Scholar]
- Jung KM et al. (2003) Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem 278: 42161–42169 [DOI] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS (2001) Kinesin-mediated axonal transport of a membrane compartment containing βsecretase and presenilin-1 requires APP. Nature 414: 643–648 [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC (2003) Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci 23: 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursigara G, Orlinick JR, Chao MV (1999) Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem 274: 2597–2600 [DOI] [PubMed] [Google Scholar]
- Lad SP, Neet KE (2003) Activation of the mitogen-activated protein kinase pathway through p75NTR: a common mechanism for the neurotrophin family. J Neurosci Res 73: 614–626 [DOI] [PubMed] [Google Scholar]
- Lalli G, Schiavo G (2002) Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin receptor p75NTR. J Cell Biol 156: 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T (2003) Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci 24: 581–594 [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945–1948 [DOI] [PubMed] [Google Scholar]
- Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR (2003) The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J 22: 6430–6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J (2001) Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal 13: 1–6 [DOI] [PubMed] [Google Scholar]
- Mi S et al. (2004) LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 7: 221–228 [DOI] [PubMed] [Google Scholar]
- Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P (1999) Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 18: 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro V, Vincent JP, Mazella J (2002) Shedding of the luminal domain of the neurotensin receptor-3/sortilin in the HT29 cell line. Biochem Biophys Res Commun 298: 760–764 [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM (2001) The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 20: 2180–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A et al. (2004) Sortilin is essential for proNGF-induced neuronal cell death. Nature 427: 843–848 [DOI] [PubMed] [Google Scholar]
- Paul CE, Vereker E, Dickson KM, Barker PA (2004) A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J Neurosci 24: 1917–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignot V, Hein AE, Barske C, Wiessner C, Walmsley AR, Kaupmann K, Mayeur H, Sommer B, Mir AK, Frentzel S (2003) Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J Neurochem 85: 717–728 [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67: 203–233 [DOI] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A (2003) Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol 461: 483–505 [DOI] [PubMed] [Google Scholar]
- Saxena S, Howe CL, Cosgaya JM, Hu M, Weis J, Kruttgen A (2004) Differences in the surface binding and endocytosis of neurotrophins by p75NTR. Mol Cell Neurosci 26: 292–307 [DOI] [PubMed] [Google Scholar]
- Shonukan O, Bagayogo I, McCrea P, Chao M, Hempstead B (2003) Neurotrophin-induced melanoma cell migration is mediated through the actin-bundling protein fascin. Oncogene 22: 3616–3623 [DOI] [PubMed] [Google Scholar]
- Song XY, Zhong JH, Wang X, Zhou XF (2004) Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci 24: 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherpakov M, Bronfman FC, Conticello SG, Vaskovsky A, Levy Z, Niinobe M, Yoshikawa K, Arenas E, Fainzilber M (2002) The p75 neurotrophin receptor interacts with multiple MAGE proteins. J Biol Chem 277: 49101–49104 [DOI] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ (2002) Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem 277: 34295–34302 [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G (2001) Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci 4: 977–978 [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z (2002a) P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420: 74–78 [DOI] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM (2002b) Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci 22: 5505–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Schlondorff J, Lum L, Becherer JD, Kim TW, Saftig P, Hartmann D, Murphy G, Blobel CP (2004) Evidence for a critical role of the tumor necrosis factor α convertase (TACE) in ectodomain shedding of the p75 neurotrophin receptor (p75NTR). J Biol Chem 279: 4241–4249 [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM (2002) A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci 5: 1302–1308 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M (2003) The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci 6: 461–467 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M (2002) The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol 157: 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Chao MV (2004) Mechanisms of neurotrophin receptor vesicular transport. J Neurobiol 58: 244–257 [DOI] [PubMed] [Google Scholar]
- Yano H, Lee FS, Kong H, Chuang J, Arevalo J, Perez P, Sung C, Chao MV (2001) Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J Neurosci 21: RC125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E (1997) The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol 139: 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]