Summary
The evolution of PtdIns(3,4,5)P3 synthesis
Keywords: PtdInsP3 synthesis, phosphoinositides, membrane transport, fission yeast
Since the identification of the lipid second messenger phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3; Traynor-Kaplan et al, 1988; Auger et al, 1989), this molecule has become a paradigm for how phosphoinositides (PIs) mediate complex signalling events in cells. PtdIns(3,4,5)P3 can regulate chemotaxis, cell survival, translation and cytoskeletal rearrangement (Cantley, 2002), the deregulation of which contributes to the pathology of diseases such as diabetes, and breast and prostate cancer.
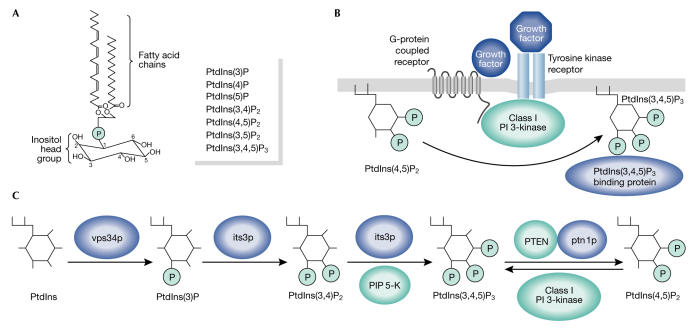
PtdIns(3,4,5)P3 is a phosphorylated form of the PI phosphatidylinositol (PtdIns). All PIs have two fatty-acid chains that anchor them to membranes and a hydrophilic inositol head group that is exposed to the aqueous cytosol (Fig 1A). This head group can be phosphorylated at the 3-, 4- and 5-positions and so far, a total of seven phosphorylated derivatives of PtdIns have been reported (Fig 1A).
Figure 1.
Pathways of PtdIns(3,4,5)P3 synthesis. (A) Phosphatidylinositol (PtdIns) is the basic building block of phosphorylated phosphoinositides (PIs). The inositol head group can be phosphorylated in the 3-, 4- and 5-positions to generate derivatives of PtdIns, which are listed on the right. (B) Schematic for receptor-driven PtdIns (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) production. Agonist stimulation (tyrosine kinase or G-protein-coupled receptors) activates class I PI 3-kinase to induce phosphorylation of PtdIns(4,5)P2 on the 3-position of the inositol group. (C) The proposed new pathway for PtdIns(3,4,5)P3 production in Schizosaccharomyces pombe. Fission yeast enzymes are shown in blue. Their mammalian counterparts are depicted in turquoise. PIP 5-K, phosphatidylinositol-4-phosphate 5-kinase; PTEN, phosphatase and tensin homologue.
After the treatment of cells with certain agonists, the transient synthesis of PtdIns(3,4,5)P3 is initiated by class I PI 3-kinases. These lipid kinases are normally inactive; however, agonist stimulation increases their ability to phosphorylate the 3-position of PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3 (Hawkins et al, 1992). The receptor-driven activation of class I PI 3-kinases can give rise to greater than a tenfold increase in the cellular levels of PtdIns(3,4,5)P3 (which rarely approach 0.1% of total PI levels, making its detection difficult—even for the more hardened lipid biochemists among us). In turn, subsets of proteins that specifically recognize and bind to PtdIns(3,4,5)P3 (mediated by specialist lipid-binding modules such as pleckstrin homology (PH) domains) are then recruited to the membrane, which often leads to their activation and further transduction of the agonist signal (Fig 1B; Lemmon, 2003).
Recently, PtdIns(3,4,5)P3 has stepped into the scientific limelight once again, this time not because of its biological function but intriguingly because of the way it is synthesized in fission yeast. A recent article in the Journal of Cell Biology (Mitra et al, 2004) has revealed for the first time that Schizosaccharomyces pombe can generate the 3-phosphorylated lipid PtdIns(3,4,5)P3. This is exciting because researchers have assumed that yeast are incapable of producing PtdIns(3,4,5)P3. This dogma has arisen as PtdIns(3,4,5)P3 has never been found in yeast, and yeast do not have a class I PI 3-kinase, which suggests that they lack one of the essential reaction steps necessary to generate PtdIns(3,4,5)P3.
The paper by Mitra and co-workers begins with the identification of ptn1, a homologue of the mammalian phosphatase and tensin homologue (PTEN) gene. PTEN is a lipid phosphatase (Maehama et al, 1998), which dephosphorylates the 3-position of PtdIns(3,4,5)P3 to generate PtdIns(4,5)P2. When the authors tested the lipid phosphatase activity of Ptn1 in vitro, they found that it also dephosphorylated PtdIns(3,4,5)P3. This led Mitra and colleagues to question why S. pombe would have maintained a functional PtdIns(3,4,5)P3 phosphatase in its genome if not to regulate PtdIns(3,4,5)P3 levels.
In mammalian cells, disruption of PTEN activity leads to elevated PtdIns(3,4,5)P3 levels and surprisingly, examination of the phosphoinositide levels in an S. pombe strain lacking ptn1 (ptn1Δ) revealed a dramatic increase in PtdIns(3,4)P2 and PtdIns(3,4,5)P3. This finding is noteworthy in itself; however, the authors went on to postulate that S. pombe synthesizes PtdIns(3,4,5)P3 through a pathway that is different to the one used by mammalian cells after growth factor stimulation.
Yeast have only one PI 3-kinase homologue, Vps34, which regulates vesicle transport to the vacuole (Takegawa et al, 1995). When a strain lacking vps34 was crossed with ptn1Δ, the authors found that levels of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 decreased. Unlike class I PI 3-kinases, Vps34 can only phosphorylate PtdIns to generate PtdIns(3)P in vitro, indicating that it is not the 3-phosphorylation of PtdIns(4,5)P2, but the synthesis of PtdIns(3)P that is the important step in S. pombe PtdIns(3,4,5)P3 synthesis. Mitra and colleagues then turned their attention to the kinases that might phosphorylate the 4- and 5-positions of the inositol head group. The lipid kinase Fab1 can phosphorylate PtdIns(3)P at the 5-position; however, its deletion did not affect the PtdIns(3,4)P2 or PtdIns(3,4,5)P3 production in ptn1Δ cells, indicating that there was no role for Fab1 in PtdIns(3,4,5)P3 synthesis. Next, they examined Its3, the S. pombe homologue of the mammalian phosphatidylinositol-4-phosphate 5-kinase (PIP 5-K) family, which are key enzymes in the regulation of PtdIns(4,5)P2 levels. When a strain retaining only 10% of wild-type PIP 5-K activity was crossed with ptn1Δ, levels of PtdIns(4,5)P2 were lowered as expected. Surprisingly, PtdIns(3,4)P2 and PtdIns(3,4,5)P3 levels were also dramatically attenuated. As PtdIns(4,5)P2 is not a substrate for Vps34, this suggests a novel role for Its3 in PtdIns(3,4,5)P3 synthesis. (It is possible that yeast have a PtdIns(4,5)P2 3-kinase that is yet to be identified or that PtdIns(4,5)P2 can allosterically regulate PtdIns(3,4,5)P3 synthesis.) In vitro, mammalian PIP 5-Ks can sequentially phosphorylate PtdIns(3)P at the 4- and 5-positions to generate PtdIns(3,4,5)P3 (Zhang et al, 1997), and this led Mitra and colleagues to propose that PtdIns(3)P generated by Vps34 is converted to PtdIns(3,4,5)P3 by Its3 (Fig 1C).
What is the function of PtdIns(3,4,5)P3 in yeast? Although the ptn1Δ strain displayed vacuolar defects and was more susceptible to osmotic stress than wild-type yeast, neither of these observations were directly linked to elevated PtdIns(3,4)P2 and PtdIns(3,4,5)P3 levels. Importantly, the authors demonstrated that at least two S. pombe proteins can bind PtdIns(3,4,5)P3 in vitro, with the intracellular localization of one of them showing some dependency on the presence of Ptn1. This raises the possibility that PtdIns(3,4,5)P3-dependent signalling networks exist in yeast, analogous to those described in metazoan systems.
So, what are the consequences of these findings? The fact that the PIP 5-K-driven PtdIns(3,4,5)P3 synthesis pathway is present in yeast indicates that, historically, this was the original pathway that organisms evolved to generate PtdIns(3,4,5)P3. Class I PI-3-kinases evolved much later, which suggests that the growth-factor-driven pathway found in Caenorhabditis elegans and mammalian cells is a 'new kid on the block'. If so, then what became of the PIP 5-K-driven PtdIns(3,4,5)P3 pathway in mammalian cells? Intriguingly, whereas growth-factor-stimulated PtdIns(3,4,5)P3 synthesis is dependent on class I PI 3-kinases in mammalian cells, we recently showed that certain stress agonists, such as H2O2, increase PtdIns(3,4,5)P3 levels in vivo by the PIP 5-K-mediated 5-phosphorylation of PtdIns(3,4)P2 (Halstead et al, 2001).
Finally, on a clinical note, PTEN (a tumour suppressor) deletion in human cancers leads to the activation of cell survival pathways through the upregulation of PtdIns(3,4,5)P3 levels. In light of these new findings that connect PTEN with PIP 5-K-driven PtdIns(3,4,5)P3 synthesis, it would be interesting to dissect how PtdIns(3,4,5)P3 is generated in these cancer cells.
At the moment, this new, but ancient, biochemical pathway for PtdIns(3,4,5)P3 synthesis seems to be an 'outsider' in the field of cell biology. Hopefully, with further study, it too can find a place of its own.

References
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC (1989) PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57: 167–175 [DOI] [PubMed] [Google Scholar]
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657 [DOI] [PubMed] [Google Scholar]
- Halstead JR, Roefs M, Ellson CD, D'Andrea S, Chen C, D'Santos CS, Divecha N (2001) A novel pathway of cellular phosphatidylinositol(3,4,5)-trisphosphate synthesis is regulated by oxidative stress. Curr Biol 11: 386–395 [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Jackson TR, Stephens LR (1992) Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature 358: 157–159 [DOI] [PubMed] [Google Scholar]
- Lemmon MA (2003) Phosphoinositide recognition domains. Traffic 4: 201–213 [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378 [DOI] [PubMed] [Google Scholar]
- Mitra P et al. (2004) A novel phosphatidylinositol(3,4,5)P3 pathway in fission yeast. J Cell Biol 166: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegawa K, DeWald DB, Emr SD (1995) Schizosaccharomyces pombe Vps34p, a phosphatidylinositolspecific PI 3-kinase essential for normal cell growth and vacuole morphology. J Cell Sci 108: 3745–3756 [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan AE, Harris AL, Thompson BL, Taylor P, Sklar LA (1988) An inositol tetrakisphosphate-containing phospholipid in activated neutrophils. Nature 334: 353–356 [DOI] [PubMed] [Google Scholar]
- Zhang X, Loijens JC, Boronenkov IV, Parker GJ, Norris FA, Chan J, Thum O, Prestwich GD, Majerus PW, Anderson RA (1997) Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J Biol Chem 272: 17756–17761 [DOI] [PubMed] [Google Scholar]