Abstract
Resealing after wounding, the process of repair following plasma membrane damage, requires exocytosis. Vacuolins are molecules that induce rapid formation of large, swollen structures derived from endosomes and lysosomes by homotypic fusion combined with uncontrolled fusion of the inner and limiting membranes of these organelles. Vacuolin-1, the most potent compound, blocks the Ca2+-dependent exocytosis of lysosomes induced by ionomycin or plasma membrane wounding, without affecting the process of resealing. In contrast, other cell structures and membrane trafficking functions including exocytosis of enlargeosomes are unaffected. Because cells heal normally in the presence of vacuolin-1, we suggest that lysosomes are dispensable for resealing.
Keywords: chemical genetics, membrane repair, exocytosis
Introduction
In a widespread form of regulated exocytosis, internal membranes are deposited at the plasma membrane in response to surface injury. This response, known as ‘resealing', is essential to cell survival in many mammalian cell tissues (McNeil & Steinhardt, 2003).
Exocytic fusion of lysosomes triggered by plasma membrane damage is proposed to be the major source of the membrane required for resealing (Rodriguez et al, 1997; Andrews, 2002; Jaiswal et al, 2002; McNeil, 2002). Lysosomal markers appear at the cell surface or are released into the medium on transient elevation of cytosolic Ca2+, including that induced by plasma membrane disruption (Reddy et al, 2001). This lysosomal exocytosis can be inhibited in cells deficient in synaptotagmin VIIa by expression of a dominant-negative form of synaptotagmin VIIa or by microinjection of antibodies against synaptotagmin VIIa or of the cytoplasmic domain of the lysosomal membrane protein Lamp-1 (Martinez et al, 2000; Andrews, 2002; Chakrabarti et al, 2003). These perturbations in synaptotagmin VII function are associated with partial inhibition of resealing. It has been suggested that lysosomes are required for the process, as synaptotagmin VIIa is predominantly localized to lysosomes (Martinez et al, 2000; Andrews, 2002; Chakrabarti et al, 2003). Numerous observations, however, suggest that membranes other than lysosomes also participate in wound healing (McNeil & Steinhardt, 2003).
Definitive reagents for assessing the role of lysosomes in resealing, relative to other organelles, have hitherto been unavailable. We have found a set of such reagents, the ‘vacuolins', discovered in an image-based phenotypic screen for inhibitors of the secretory pathway (Feng et al, 2003). Although vacuolins do not affect transport along the endocytic or secretory pathways, they induce the formation of large, swollen structures derived from endosomes and lysosomes. The vacuoles seem to arise from homotypic fusion of these organelles, with further contributions, in the case of multivesicular bodies, from fusion between inner and limiting membranes. We find that vacuolin-1 blocks both ionophore- and wound-induced, Ca2+-dependent exocytosis of lysosomes, but does not block Ca2+-dependent fusion of enlargeosomes with the plasma membrane. Because cells reseal normally in the presence of vacuolin-1, we suggest that lysosomes are dispensable for this important cell response to physiologically generated injury.
Results
Identification of vacuolins using an image-based screen
In the course of an image-based phenotypic screen to find small molecules affecting transport of membranes from the endoplasmic reticulum (ER) to the plasma membrane (Feng et al, 2003), 16 compounds that induce formation of vacuoles in the cytoplasm were discovered (supplementary Fig 1 online). We call the compounds vacuolins and have selected the most potent, vacuolin-1, for analysis (supplementary Fig 2 online).
Cellular origin of the vacuoles
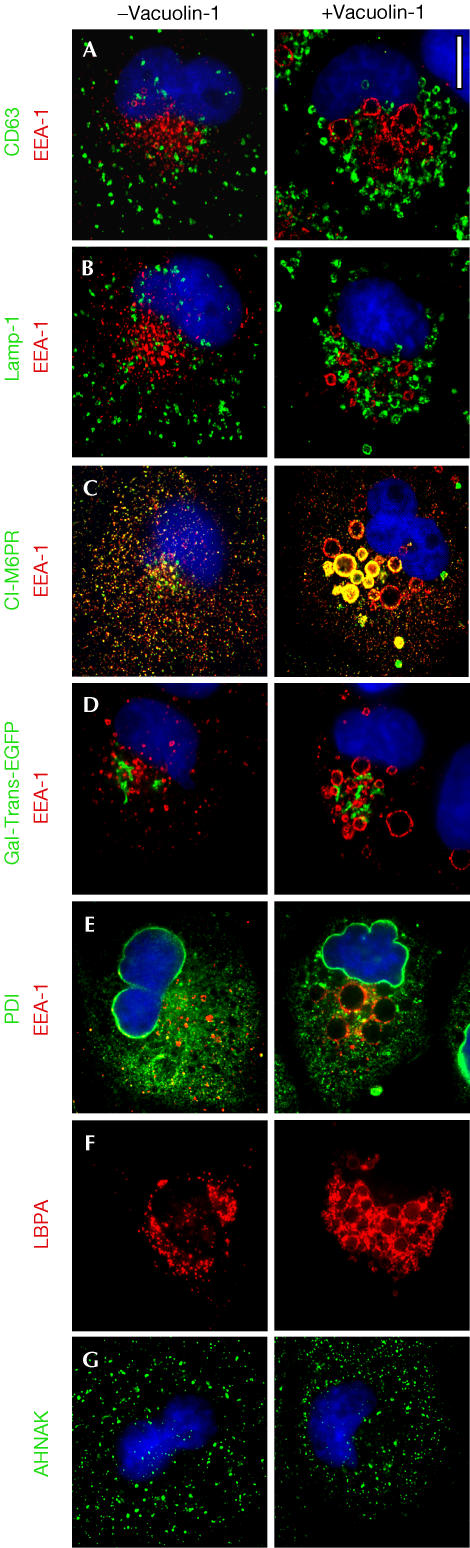
Immunofluorescence images demonstrate the endosomal and lysosomal origin of the swollen structures. One set of vacuoles was labelled by markers of late endosomes (Fig 1A) and lysosomes (Fig 1B); these were relatively small (∼1 μm diameter), showed almost complete conversion from the originating organelles and appeared throughout the cell. In contrast, markers for early endosomes (Fig 1A–E) and the fraction of CI-M6PR colocalizing with EEA1 (Fig 1C) were in larger vacuoles (up to 3 μm in diameter) found mostly in perinuclear regions. No vacuoles contained markers specific for both early and late endosomes (Fig 1A) or for both early endosomes and lysosomes (Fig 1B). Thus, vacuolin-1 seems not to disrupt the homotypic fusion characteristic of unperturbed organelles. Conversion of early and recycling endosomes to vacuoles was not complete. Live-cell imaging of BSC-1 cells expressing Rab7-EGFP revealed conversion of small vacuoles into larger ones as well as fusion events between vacuoles (supplementary Movie 1 online). There was no evidence for vacuoles containing markers for the Golgi apparatus (Fig 1D), ER (Fig 1E) or enlargeosomes (Fig 1F). The vacuolins did not affect the organization of the nucleus (Figs 1,4 and supplementary Fig 5 online), mitochondria (not shown) or the actin or tubulin cytoskeletal networks (supplementary Fig 3 online), and did not induce apoptosis or affect cell viability. The vacuolation effect was reversible, as no swollen structures were apparent 13 h after removal of vacuolin-1 from the medium (supplementary Fig 4 online).
Figure 1.
Origin of the vacuoles induced by vacuolin-1. BSC-1 cells were labelled with the indicated markers in the absence (left) or following 1 h incubation with 1 μM vacuolin-1 (right). Vacuolin-1 exhibits a vacuolating effect specific for endosomal and lysosomal compartments, manifested by generation of relatively large vacuoles (early endosomes) and small vacuoles (late endosomes and lysosomes). The identity of the resulting vacuoles is maintained, as the markers for each compartment do not intermix. In contrast, the enlargeosomes, Golgi apparatus, ER and nucleus (4,6-diamidino-2-phenylindole staining, in blue) are not affected, and the vacuoles do not contain markers from these organelles. Antibody markers are specific for early endosomes (EEA1), early and late endosomes (CI-M6PR), late endosomes (CD63 and LBPA), lysosomes (Lamp-1), the Golgi apparatus (Gal-Trans-EGFP), ER (PDI) and enlargeosomes (desmoyokin/AHNAK). Scale bar, 10 μM.
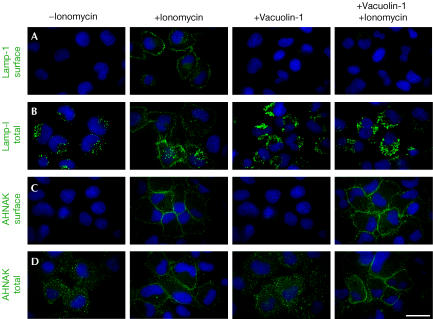
Figure 4.
Vacuolin-1 blocks ionomycin-induced exocytosis of lysosomes but not of enlargeosomes. The fluorescent images show the effect of vacuolin-1 on the intracellular distribution in BSC-1 cells of the markers Lamp-1, specific for lysosomes (A), (B), and desmoyokin/AHNAK, specific for enlargeosomes (C), (D). The cells were immunostained before or after permeabilization with 0.05% saponin to identify the fusion of lysosomes and enlargeosomes with the plasma membrane and their intracellular location, respectively. In resting cells, lysosomes stain as cytosolic dots slightly larger than those corresponding to enlargeosomes. After treatment with ionomycin (5 μM, 5 min), both markers shift towards the cell periphery and appear at the surface, reflecting the Ca2+-regulated exocytosis of lysosomes (A), (B) and enlargeosomes (C), (D). Pretreatment with 1 μM vacuolin-1 results in vacuolation of lysosomes (A) and complete inhibition of the surface expression of Lamp-1 (D). In contrast, the appearance (C) and the exocytosis of the enlargeosome marker (D) remain normal. Vacuolin-1 alone does not induce surface expression of any marker. Scale bars, 20 μM.
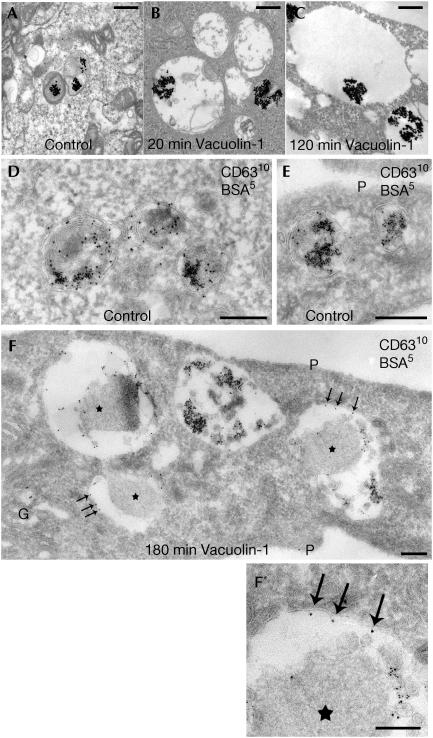
Vacuolin-1 disrupts the segregation of inner and limiting membranes characteristic of endosomes and lysosomes (Gruenberg & Stenmark, 2004). Two examples, shown in Fig 1A and F, illustrate that the membranes surrounding the vacuoles became labelled with antibodies against CD63 and lysobisphosphatidic acid, markers normally restricted to the endosomal inner membranes. Ultrastructural analysis also shows that vacuolin-1 favours fusion of inner and limiting membranes (Fig 2).
Figure 2.
Enlargement of endosomes and lysosomes and loss of their inner membranes by treatment with vacuolin-1. HeLa cells were incubated for 10 min with bovine serum albumin (BSA) conjugated to 5 nm gold particles to mark endocytic compartments, followed by an overnight (A–C) or 3 h (D–F) chase. Control samples (A,D,E) were not incubated with vacuolin-1. The remaining samples were treated before fixation with 1 μM vacuolin-1 as follows: (B) 20 min, (C) 120 min and (F) 180 min. (A,D,E) Representative examples of late endosomal compartments in control cells showing BSA–gold within internal vesicles and membrane sheets of late endosomes/lysosomes primarily labelled with CD63. (B), (C) and (F) show a marked loss of inner membranes on treatment with vacuolin-1 and the frequent redistribution of the CD63 marker to the limiting membranes (arrows). The CD63-positive endosomes are hugely swollen (note the differences in bar sizes). Some endosomes contain few or no internal vesicles; instead, many have an amorphous content (asterisks) that is never observed in control cells. G, Golgi complex; P, plasma membrane. (F′) An enlarged portion of (F). Sections were stained with osmium (A–C) or ultrathin cryosections were prepared and labelled for CD63 (10 nm gold particles; D–F). Scale bars: (A–C, F′) 500 nm; (D–F) 250 nm.
The swollen structures generated by vacuolin-1 are competent to receive cargo internalized by receptor-mediated endocytosis, such as transferrin, EGF and LDL, and maintain the ability to sort internalized receptor–ligand complexes (supplementary Fig 3 online).
Vacuolin-1 blocks some fusion events
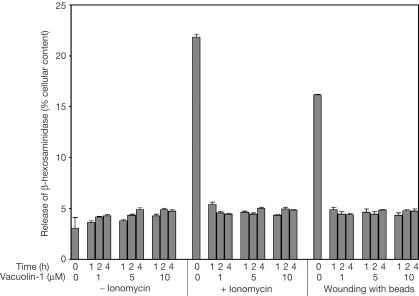
In the absence of vacuolin-1, treatment of HeLa cells for 10 min with 5 μM ionomycin resulted in the expected (Veldman et al, 2001) release of 18–20% of lysosomal β-hexosaminidase (Fig 3). In contrast, cells that were pretreated with 5 or 10 μM vacuolin-1 for 2 h released no more β-hexosaminidase compared with cells that were not exposed to ionomycin (∼4%). Membrane damage can also trigger Ca2+ influx (McNeil & Warder, 1987), leading to partial release of β-hexosaminidase. Vacuolin-1 inhibited this release (Fig 3). It also blocked the ionomycin-induced, Ca2+-dependent cell surface appearance of the luminal epitope from the lysosomal membrane protein, Lamp-1, a marker for fusion between the limiting membranes of lysosomes and the cell surface (Fig 4A,B). Thus, vacuolin-1 blocks the Ca2+-dependent fusion of lysosomes with the plasma membrane and the release of lysosomal contents. Like lysosomes, enlargeosomes also undergo a Ca2+-dependent exocytic fusion with the plasma membrane (Borgonovo et al, 2002; see also Fig 4C,D). Pretreatment of HeLa (Fig 4C) or BSC-1 cells (not shown) with 1 μM vacuolin-1 had no effect on the cell surface appearance of the enlargeosome marker in response to ionomycin. Thus, the inhibitory effect of vacuolin-1 on Ca2+-dependent exocytosis is not a general phenomenon. Rather, it seems to be a specific effect for endosomes and lysosomes but not for enlargeosomes.
Figure 3.
Vacuolin-1 blocks the Ca2+-dependent release of β-hexosaminidase from lysosomes. HeLa cells were incubated for the indicated times in the presence or absence of vacuolin-1 and then treated or not with 5 μM ionomycin for 10 min. Alternatively, the cells were wounded by gently rolling glass beads on top of the cell monolayer. The media were then exchanged, and the amount of β-hexosaminidase released during 10 min was determined. Data, obtained in quadruplicate, are expressed as average ± standard error normalized to the total cellular content of β-hexosaminidase. The same results were obtained with BSC-1 cells (not shown).
Vacuolin-1 does not block resealing
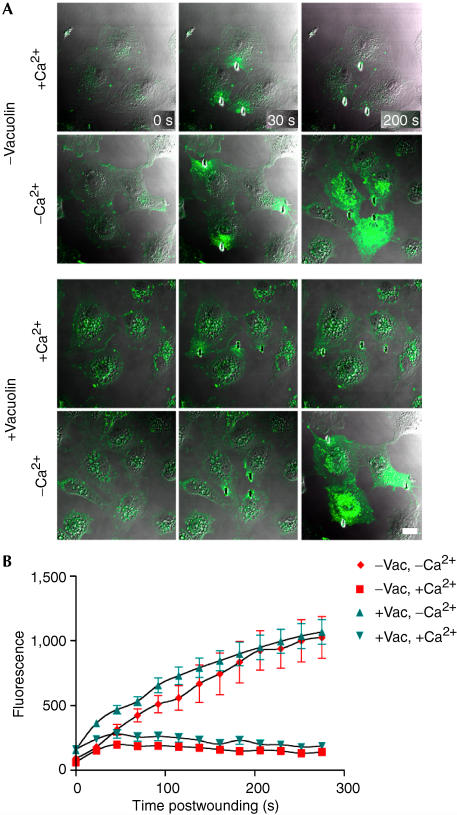
Two established methods were used to assess the effect of vacuolin on resealing. First, the extent of cell labelling produced by addition of FM 1–43 dye 5 min after shear-induced wounding was measured by flow cytofluorimetry (McNeil et al, 2003) as a function of vacuolin treatment (supplementary Fig 7 online). Vacuolin-1 had no effect on resealing. Second, the kinetics of FM 1–43 entry through a laser-induced disruption was observed microscopically (Fig 5A) as an increase in whole-cell fluorescence (Bansal et al, 2003; McNeil & Steinhardt, 2003). The kinetics and magnitude of fluorescence increase, indicative of resealing, were identical in the presence and absence of vacuolin-1 (Fig 5B). Thus, two independent assays failed to reveal any effect of vacuolin-1 on resealing efficiency.
Figure 5.
Vacuolin does not inhibit laser-induced resealing. (A) BSC-1 cells exposed to DMSO (−vacuolin) or 10 μM vacuolin-1 for 1 h (+vacuolin) are shown before (0 s) and after (30 and 200 s) laser-mediated wounding. Burn marks in the plastic indicate the wound sites. Entry of the FM 1–43 dye present in the medium is restricted in cells wounded in the presence of 1.5 mM Ca2+ (+Ca2+), regardless of whether the cells were treated or not with vacuolin-1. In contrast, the FM 1–43 dye freely enters and stains intracellular membranes in cells wounded in the absence of Ca2+ (−Ca2+). (B) Quantitative evaluation of dye uptake in the absence (−Vac) or presence (+Vac) of vacuolin-1 before (t=0 s) and after laser wounding (for each data point, n=6 cells, scale bars=1 s.d.).
Discussion
We describe the discovery of vacuolins, a group of molecules that induce vacuole formation in a variety of cell types, and their use to dissect the relative contribution of lysosomes to resealing in response to plasma membrane disruption. The effects of vacuolin-1 are rapid and specific. Vacuolin-1 converts endosomes and lysosomes into vacuoles, whereas enlargeosomes and other membrane-bound organelles are unaffected. Moreover, the vacuolins do not affect the organization of the actin or tubulin cytoskeletal networks, or interfere with membrane traffic along the constitutive secretory pathway from the ER to the plasma membrane. Treatment with vacuolin-1 did not prevent receptor-mediated endocytosis and did not seem to interfere with the sorting of different receptor-ligand complexes to endosomes. Extensive exposure to vacuolin-1 did not affect cell viability.
The size of the vacuoles and their final cellular distribution is easy to discern; vacuoles derived from early and recycling endosomes are larger and are found in the perinuclear region, whereas those derived from late endosomes and lysosomes are smaller and more peripheral. It is likely that the generation of vacuoles reflects a combination of two processes: fusion of internal with limiting membranes to generate enlarged versions of the original organelle and homotypic fusion of early endosomes, late endosomes and lysosomes.
Vacuolin-1 strongly inhibits the Ca2+-dependent exocytic fusion of lysosomes with the plasma membrane. In cells treated with ionomycin, which normally elicits a strong exocytic effect (Rodriguez et al, 1997; Andrews, 2002; McNeil, 2002), vacuolin-1 completely blocks the surface appearance of Lamp-1 and completely inhibits β-hexosaminidase release. In contrast, vacuolin-1 does not inhibit the ionomycin-induced, Ca2+-dependent exocytosis of enlargeosomes.
Does Ca2+-dependent exocytic fusion of lysosomes have an essential role in wound healing, as generally believed (Andrews, 2002; McNeil, 2002)? Numerous observations, as well as those reported here, argue against this view. First, although the Ca2+-dependent exocytic fusion of lysosomes is completely blocked by loading the cells with antibodies against synaptotagmin VII or by overexpression of the recombinant C2A domain of synaptotagmin VII (Martinez et al, 2000), inhibition of resealing is only partial (Reddy et al, 2001; Andrews, 2002). This discrepancy could be explained if synaptotagmin VII is, in fact, not specific for lyososomes, and/or if the reagents used for inhibiting it are not specific for synaptotagmin VII. Second, using total internal reflection to monitor fusion events with the plasma membrane, it has been shown that lysosomes predocked to the plasma membrane fuse to the plasma membrane in response to Ca2+, but do so with a time course (minutes) incompatible with that required for resealing (seconds; Jaiswal et al, 2002). Third, using this same imaging approach, synaptotagmin VII has very recently been shown to restrict lysosome fusion: cells from synaptotagmin knockout mice, described as resealing-deficient in a previous publication (Chakrabarti et al, 2003), actually exhibited an accelerated rate and extent of lysosome-based exocytosis (Jaiswal et al, 2004). Fourth, in Chinese hamster ovary cells, the size of the average lysosome is larger than that of most vesicles fusing to the plasma membrane in response to an increase in cytosolic Ca2+ (Ninomiya et al, 1996). This result suggests that in nonsecretory cells, an organelle other than the lysosome can undergo Ca2+-dependent exocytic fusion. Indeed, PC12-27 cells manage to reseal in a process that involves fusion of exocytic vesicles that are smaller than lysosomes (Kasai et al, 1999), despite the finding that lysosomal markers do not appear at the cell surface in response to a transient increase in cytosolic Ca2+ (Borgonovo et al, 2002). Finally, dyspherlin, a membrane protein required for resealing in skeletal muscle cells (Bansal et al, 2003), does not localize to the Lamp-1-positive compartment of the myocyte (P.L. McNeil and K. Miyake, unpublished observation).
We have shown that resealing takes place, with kinetics indistinguishable from untreated cells, when lysosomal exocytosis has been ablated chemically. As an exocytic increase of membrane surface is necessary for repair (Bi et al, 1995; Miyake & McNeil, 1995), we conclude that an exocytic organelle other than lysosomes is involved, at least in cells treated with vacuolin-1. Discovery of a specific way to prevent enlargeosome fusion is now required to test whether enlargeosomes, or some other organelles, are indeed the source of membrane required to sustain resealing.
Methods
Cells and antibodies. Human HeLa and A431 and monkey BSC-1 cells were grown on glass coverslips in Dulbecco's modified Eagle's medium to 50–70% confluency. Confirmatory experiments were carried out with monkey CV1 and COS cells, hamster CHO cells, human macrophages isolated from blood and dendritic cells, both differentiated in vitro, and mouse peritoneal macrophages. The mouse monoclonal antibodies MEM259, H4A3 and LBPA, against CD63, lysosomal membrane protein 1 (Lamp-1) and lysobisphosphatidic acid (LPBA) were provided by V. Horejsi, D. King and J. Gruenberg, respectively; the rabbit polyclonal antibodies against early endosomal antigen 1 (EEA-1), mannose-6-phosphate receptor (CI-M6R) and protein disulphide isomerase (PDI) were provided by M.J. Clague, S. Kornfeld and T. Rapoport, respectively; the rabbit polyclonal antibody against Lamp-1 was provided by S. Carlsson and M. Fukuda. The monoclonal for desmoyokin-AHNAK was the same used by Borgonovo et al (2002).
Immunofluorescence imaging. For surface staining, the cells were incubated for 30 min at 4°C with Lamp-1 or desmoyokin-AHNAK antibodies dissolved in Eagle's minimal essential medium supplemented with HEPES (N-2-hydroxyethylpiperazine-N′-2 ethanesulfonic acid; HMEM) with 1% BSA, followed by fixation with 3.7% paraformaldehyde and staining with fluorescently labelled secondary antibodies. For total staining, the cells were fixed, permeabilized with 0.05% saponin and stained with the appropriate antibodies. Epifluorescence and spinning disk confocal microscopy were used for imaging under control of SlideBook (Intelligent Imaging Innovations Inc.).
Electron microscopy. HeLa cells grown on 25 mm diameter coverslips in six-well plates were incubated for 10 min with BSA conjugated with 5 nm gold particles, followed by a 3–12 h incubation in medium without BSA–gold. During the last 20–180 min of the chase, the cells were incubated in 0.2% DMSO with or without 1 μM vacuolin-1. After treatments, cells were washed with PBS, fixed at room temperature for 30 min using 2% paraformaldehyde and 0.2% glutaraldehyde and processed for ultrathin cryosectioning and immunolabelling (Slot et al, 1991; Liou et al, 1996).
Ionomycin treatment, β-hexosaminidase release and glass bead wounding. Confluent monolayers of HeLa cells in six-well plates were transferred to HMEM at 37°C and stimulated at 37°C for 10 min with 5 μM ionomycin (in HMEM) followed by three washes with ice-cold phosphate-buffered saline and transfer to ice. For cell wounding (McNeil & Warder, 1987), we used 100 mg of 425–600 μm in diameter, acid-washed glass beads (Sigma Co.), mixed with 0.1 ml media and evenly sprinkled from an Eppendorf tube held 2 cm above the cells (∼400 beads/coverslip). The 100-mm-diameter dishes containing the cell monolayers were gently rocked five times at 21–22°C to allow rolling of the beads on top of the cells. After wounding, 0.9 ml of prewarmed HMEM media was added for 5 min at 37°C. The media from these cells or from those treated with ionomycin were then removed and centrifuged (10 min, 12,000 rpm), followed by measurement of released β-hexosaminidase activity into the supernatant (Veldman et al, 2001). Total cellular β-hexosaminidase was determined in parallel samples following permeabilization of the cells with 1 ml HMEM supplemented with 1% BSA and 1% NP-40.
Laser-mediated cell wounding and analysis of resealing. Cell wounding was performed with the infrared laser of a two-photon microscope (LSM 510, Zeiss Inc.); analysis of resealing was on the basis of kinetics and the extent of FM1-43 dye entry through open disruptions at 37°C (Bansal et al, 2003; McNeil et al, 2003).
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v5/n9/extref/7400243s1.pdf).
Supplementary Material
Supplementary Fig 1
Supplementary Fig 2
Supplementary Fig 3
Supplementary Fig 4
Supplementary Fig 5
Supplementary Fig 6
Supplementary Fig 7
Supplementary Movie 1
Supplement for embo 48044
Supplement for embo 48044
Acknowledgments
We thank members of the ICCB for use of their facilities and for ongoing advice and cooperation, various colleagues who generously supplied antibody reagents, and G. Racchetti, E. Cocucci and V. Oorschot for antibody production and immunoelectron microscopy. We acknowledge NIH grant GM62566 (to T.K.), grant LN 00A026 from the Project Center of Molecular and Cellular Immunology, Ministry of Education, Youth and Sports of the Czech Republic (to J.C.), a grant from NASA (to P.L.M.) and grants from the European Community (Growbeta) and the Italian Prin system (2001-053389-033) in the framework of the Center of Excellence on Physiopathology of Cell Differentiation (to J.M.).
References
- Andrews NW (2002) Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol 158: 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP (2003) Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172 [DOI] [PubMed] [Google Scholar]
- Bi GQ, Alderton JM, Steinhardt RA (1995) Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol 131: 1747–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J (2002) Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol 4: 955–962 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW (2003) Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol 162: 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Yu S, Lasell TK, Jadhav AP, Macia E, Chardin P, Melancon P, Roth M, Mitchison T, Kirchhausen T (2003) Exo1: a new chemical inhibitor of the exocytic pathway. Proc Natl Acad Sci USA 100: 6469–6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H (2004) The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 5: 317–323 [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Andrews NW, Simon SM (2002) Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol 159: 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM (2004) Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol 2: E233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Kishimoto T, Liu TT, Miyashita Y, Podini P, Grohovaz F, Meldolesi J (1999) Multiple and diverse forms of regulated exocytosis in wild-type and defective PC12 cells. Proc Natl Acad Sci USA 96: 945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Slot JW (1996) Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol 106: 41–58 [DOI] [PubMed] [Google Scholar]
- Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW (2000) Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol 148: 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL (2002) Repairing a torn cell surface: make way, lysosomes to the rescue. J Cell Sci 115: 873–879 [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA (2003) Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19: 697–731 [DOI] [PubMed] [Google Scholar]
- McNeil PL, Warder E (1987) Glass beads load macromolecules into living cells. J Cell Sci 88(Part 5): 669–678 [DOI] [PubMed] [Google Scholar]
- McNeil PL, Miyake K, Vogel SS (2003) The endomembrane requirement for cell surface repair. Proc Natl Acad Sci USA 100: 4592–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, McNeil PL (1995) Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol 131: 1737–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H (1996) Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J Biol Chem 271: 17751–17754 [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW (2001) Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106: 157–169 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Webster P, Ortego J, Andrews NW (1997) Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol 137: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE (1991) Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol 113: 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman RJ, Maestre N, Aduib OM, Medin JA, Salvayre R, Levade T (2001) A neutral sphingomyelinase resides in sphingolipid-enriched microdomains and is inhibited by the caveolinscaffolding domain: potential implications in tumour necrosis factor signalling. Biochem J 355: 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1
Supplementary Fig 2
Supplementary Fig 3
Supplementary Fig 4
Supplementary Fig 5
Supplementary Fig 6
Supplementary Fig 7
Supplementary Movie 1
Supplement for embo 48044
Supplement for embo 48044