Summary
Workshop on Molecular Mechanisms of Vesicle Selectivity
Keywords: clathrin, endocytosis, adaptors
Introduction
Maintenance of the structural and functional organization of eukaryotic cells requires the correct targeting of proteins and lipids to their final destinations. This workshop focused on the mechanisms that mediate the formation of transport intermediaries, which carry cargo between intracellular compartments and the plasma membrane. The formation of transport vesicles is a highly complex process requiring the coordinated and selective recruitment of lipids, transmembrane proteins and their lumenal cargo, and the sorting machinery components that are required for vesicle transport and fusion with acceptor compartments.

The Workshop on Molecular Mechanisms of Vesicle Selectivity was held in Madrid, Spain, between 29 and 31 March 2004. It was organized by I. Sandoval and B. Pearse and was sponsored by the Juan March Foundation.
Although different types of transport vesicles have been described, clathrin-coated vesicles (CCVs), which mediate endocytosis from the plasma membrane, are the best characterized and are therefore often considered a model for transport-intermediary formation. Recently, the resolution of the structure of several coat-component domains through X-ray crystallography and the identification of new regulatory proteins has greatly advanced our understanding of CCV formation and function.
Selected highlights from these and other recent studies that were presented at the meeting are the subject of this report.
Formation and function of the clathrin scaffold
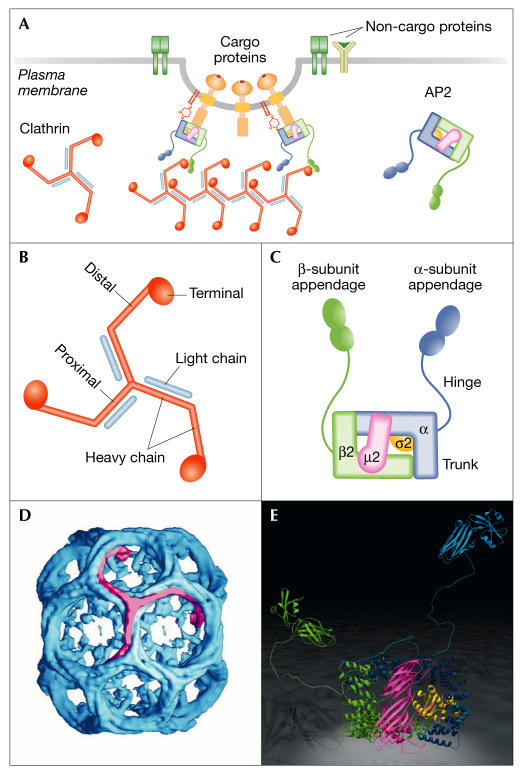
In the mid-1970s, the isolation of CCVs by density centrifugation allowed B. Pearse (Cambridge, UK) to purify clathrin, one of the main components of these structures (Pearse, 1976). The assembly unit of clathrin, commonly referred to as the 'triskelion', consists of three heavy chains (∼190 kDa) and three light chains (∼30 kDa) that oligomerize to form three-dimensional (3D) polygonal lattices, or cage-like structures (Fig 1). Clathrin heavy chains can be sub-divided into five structurally and functionally distinct regions: an aminoterminal globular domain; a highly flexible linker region composed of short α-helices; a distal segment; a proximal segment and a carboxy-terminal domain, which mediates the trimerization of the clathrin chains (reviewed in Brodsky, 2001). The heavy-chain proximal domain has a superhelical structure composed of clathrin heavy-chain repeats of ten short α-helices and contains binding sites for clathrin light chains (Ybe et al, 1999). It has been proposed that light chains might prevent the spontaneous self-assembly of heavy chains.
Figure 1.
Clathrin and adaptor protein 2 are the main components of clathrin-coated vesicles. (A) Clathrin-coated vesicles (CCVs) consist of three concentric layers: an inner membrane layer in which different cargo proteins are embedded; adaptors, such as adaptor protein 2 (AP2), that provide a cargo selection function; and an outer clathrin coat with the ability to polymerize and bind adaptors and other regulatory factors, which allows the concentration of associated proteins into regular protein networks. Schematic representation of clathrin (B) and AP2 (C). (D) A 21 Å resolution map of clathrin assembled into cages obtained by cryo-electron microscopy. The image has been coloured to show the disposition of a single triskelion. (E) Crystal structure of the AP2 adaptor. Note that while the core and appendage domains have been solved by X-ray crystallography, the linkers between these two domains are represented according to their predicted extended conformation. D and E were reproduced from Pearse et al (2000) and Owen (2004) with permission of the authors and copyright permission from Elsevier and the Biochemical Society, respectively.
Crystallographic studies have revealed that the clathrin heavy-chain N-terminal domain consists of a seven-blade β-propeller structure (ter Haar et al, 1998). A binding site for the LφXφ[DE ] (where φ is a hydrophobic residue) motif, which is present in different endocytic proteins, has been identified between blades one and two of the β-propeller. Recently, it has been revealed that amphiphysin contains a clathrin-binding sequence, PWXXW, designated the W-box. This sequence also binds to the clathrin N-terminal domain but does not compete with the canonical LφXφ[DE ] motif, which indicates the presence of two independent binding regions. In support of this, D. Owen (Cambridge, UK) presented the crystal structure of the bovine clathrin N-terminal domain in complex with a TLPWDLWTT peptide derived from amphiphysin 1. As expected, the W-box binds to a complementaryshaped pocket in the centre of the top surface of the β-propeller at a location distant from the binding site for the LφXφ[DE ] motif (Miele et al, 2004).
F. Brodsky (San Francisco, CA, USA) presented studies on the role of clathrin heavy-chain distal and proximal segments in the formation of clathrin baskets. Measurements of the binding kinetics between isolated clathrin segments or chimaeras indicated that all proximal–proximal, proximal–distal and distal–proximal interactions collectively contribute to clathrin polymerization, although individually these interactions are very weak (Wakeham et al, 2003). These findings imply that clathrin cage assembly depends on the additive effect of multiple weak interactions during the polymerization process, which allow the clathrin lattice to 'breathe'. The membrane curvature that results from this facilitates CCV formation and provides several opportunities for cellular regulation. For example, adaptors and regulatory proteins could overcome the negative regulation of the clathrin light chains through promoting the correct alignment of triskelion leg segments, thus facilitating clathrin polymerization. In this regard, Brodsky presented evidence that the monomeric adaptor protein GGA1 contains two different clathrin-binding sites located in its hinge and appendage domains that interact with the N-terminal domain and the first linear repeat (CHCR1) of the proximal domain, respectively. The fact that the motif located at the GGA1 appendage domain is not required for clathrin recruitment suggests that this interaction might be specific for the regulation of clathrin assembly.
Clathrin and AP2 cooperation in cargo recruitment
After clathrin, the adaptor protein 2 (AP2) is the second most abundant protein at clathrin-coated pits (CCPs), which are the sites of CCV formation. AP2 is a large heterotetrameric complex composed of two large subunits (α and β2), a medium subunit (μ2) and a small subunit (σ2). The large subunits can be subdivided into an N-terminal domain, or trunk, and a globular C-terminal region, or appendage, which are connected by an extended flexible linker (Fig 1). AP2 binds to clathrin through specific motifs located at the β2-hinge and appendage domains, whereas the α- and β2-appendage domains recruit alternative adaptors and regulatory proteins (Fig 2). The AP2 μ2subunit functions mainly in the recognition of the YXXφ sorting motifs that are present in the cytosolic tail of different cargo proteins. In addition, binding sites for phosphatidylinositol (4,5)-bisphosphate (PIP2) have been identified in both the α- and the μ2-subunits and seem to be involved in the recruitment of AP2 to the plasma membrane.
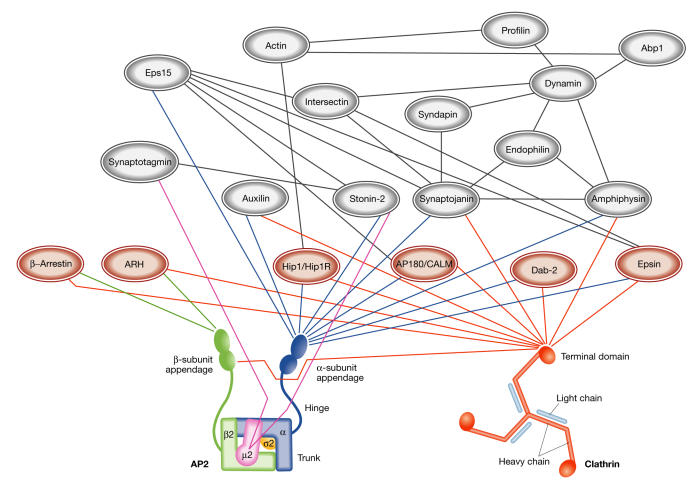
Figure 2.
The endocytic network. Schematic representation of the complex protein network involved in the formation of clathrin-coated vesicles at the plasma membrane. Interactions mediated by the clathrin N-terminal domain (red), the α-appendage (blue), β-appendage (green), μ-subunit (pink) of adaptor protein 2 (AP2) are shown. Interactions among accessory proteins are indicated in grey. Cargo-specific adaptors are represented in brown.
Owen presented the crystal structure of a complex comprising the N-terminal two-thirds of the α- and β2-trunk domains and the full-length μ2- and σ2-subunits (Collins et al, 2002). Surprisingly, the site in μ2 that interacts with the YXXφ motif was found to be buried within this complex. This new insight suggests that a conformational change might occur when AP2 is recruited onto the plasma membrane to make this binding site accessible for interactions with cargo proteins. E. Smythe (Sheffield, UK) proposed that this conformational change could be mediated by the phosphorylation of the μ2subunit by the adaptor-associated kinase 1 (AAK1). It has been shown that clathrin can act as a specific activator of AAK1 in vitro. In support of this, Smythe showed that AP2 containing phosphorylated μ2 mainly associates with assembled clathrin in vivo, whereas the level of phosphorylated μ2 is dramatically reduced in cells that are depleted of clathrin (Jackson et al, 2003). These data fit with the proposed model in which AP2 is recruited to the plasma membrane via interactions with PIP2, followed by clathrin binding. Clathrin might then in turn activate a μ2-kinase, thus driving AP2 phosphorylation and allowing efficient interactions with the binding motifs on cargo proteins.
Cargo-specific adaptors: novel recruitment machinery
In addition to clathrin and AP2, more than 30 proteins have been isolated from precursor CCPs and mature CCVs. These numerous proteins have traditionally been considered accessory factors in the regulation of CCV formation. However, it has recently been proposed that some of these so-called accessory proteins might have a more direct role as cargo-specific adaptors that serve to recruit specific receptors to the same population of coated vesicles and promote coat assembly. All the cargo-specific adaptors described so far have clathrin box and AP2 interaction sequences that allow them to colocalize with clathrin and AP2 at the plasma membrane, as well as N-terminal lipid-binding domains that mediate interactions with PIP2. However, it is important to note that, according to the data presented by M. Robinson (Cambridge, UK), depletion of AP2 by small interfering RNAs (siRNAs) causes a dramatic reduction in the number of coated pits. This indicates that, despite the ability of alternative adaptors to regulate the uptake of certain receptors, AP2 is still required for efficient coat assembly and transportation in most cell types (Motley et al, 2003).
L. Traub (Pittsburgh, PA, USA) presented data on two alternative adaptors, the autosomal recessive hypercholesterolaemia (ARH) protein and Disabled 2 (Dab2). These adaptors contain phosphotyrosine binding domains that also bind to non-tyrosine-phosphorylated FXNPXY sorting motifs present in the cytosolic tails of members of the low-density lipoprotein (LDL) receptor superfamily, such as the LDL receptor, the scavenger receptor megalin and ApoER2. Recently, mutations in the ARH gene were shown to be linked to a rare autosomal recessive form of hypercholesterolaemia, which suggests a possible role for ARH in the internalization of the LDL receptor (Soutar et al, 2003).
Another class of alternative adaptor, the β-arrestins, mediates the internalization of most G-protein-coupled receptors (GPCRs) through physically linking agonist-activated, phosphorylated receptors, AP2 and clathrin. Other proposed cargo-specific adaptors include AP180/CALM, HIP1/Hip1R and epsin, which have recently been shown to mediate the internalization of synaptobrevin, glutamate receptors and ubiquitinated proteins, respectively. Some of these proposed adaptors have additional roles in the regulation of CCV formation. One example is epsin, a protein known to induce membrane curvature through binding to PIP2 in combination with clathrin polymerization. Another example is Hip1R, which is a protein that interacts with both clathrin and F-actin and seems to be crucial for the proper association between the endocytic machinery and the actin cytoskeleton. Hip1R binds to actin through a talin homology domain located at the C-terminus of the protein. Through the use of a limited proteolysis approach, T. Brett (St Louis, MO, USA) has identified a 200-amino-acid domain in Hip1R, termed THATCH (talin–HIP1/HIP1R–actin-tethering C-terminal homology), which binds to F-actin. The crystal structure of the THATCH domain revealed a five-helix bundle with a unique topology that contains the F-actin-binding core. In addition, Brodsky presented evidence that Hip1R interacts with clathrin light chains and that this interaction is mediated by a 22-residue sequence, which is conserved in all light chains and which contains several negative charges known to regulate clathrin polymerization. Moreover, the preference of Hip1R to bind to light chains that are associated with clathrin heavy chains versus free light chains suggests that Hip1R could help to overcome the inhibitory effect of light chains, thus inducing clathrin assembly.
New roles for accessory proteins in CCV formation
In addition to the components described above, a large complement of regulatory proteins has been implicated in the temporal and spatial coordination of endocytosis from the cell surface.
V. Haucke (Göttingen, Germany) presented evidence that stonin 2, a protein that contains two Eps 15-binding NPF motifs and a μ-homology domain, is recruited to axonal vesicle clusters and that this localization is mediated by an interaction between the μ-homology domain and synaptotagmin 1. In addition, WVXF motifs in stonin 2 are capable of associating with the AP2 α-adaptin ear domain and the tyrosine-sorting motif-binding pocket of μ2. These data might suggest a regulatory role for stonin 2 in AP2 function (Walther et al, 2004).
Members of the dynamin family are high-molecular-mass GTPases that associate with the 'necks' of coated pits and promote vesicle scission. All dynamins contain an N-terminal GTPase domain, a pleckstrin-homology domain implicated in binding to PIP2 and a GTPase effector domain that is involved in oligomerization and self-assembly, and increases the GTPase activity of dynamin by 50- to 100-fold. The C-terminal region of dynamin contains a proline-rich region that interacts with the Src homology 3 domains of several proteins, such as amphiphysin, endophilin and actin-binding proteins. J. Hinshaw (Bethesda, MD, USA) reported on the conformational changes she could observe in dynamin following the addition of GTP using cryo-electron microscopy and 3D-image processing techniques. The transition from a non-constricted to a constricted state was accompanied by a change from a straight to a zigzag pattern for the GTPase effector domain/middle region of dynamin. Hinshaw suggested that this conformational change reveals a strong interaction between the GTPase effector domain and the GTPase domain from a neighbouring dimer and indicates that dynamin can act as a force-generating 'constrictase' (Chen et al, 2004).
Amphiphysins are regulatory proteins known to interact with clathrin, AP2, dynamin and synaptojanin. The most conserved feature between different amphiphysins is the BAR domain (Bin/Amphiphysin/Rvs) located at the N-terminus. H. McMahon (Cambridge, UK) presented the crystal structure of the amphiphysin BAR domain from Drosophila melanogaster (Peter et al, 2004). This module dimerizes to form an elongated 'bananashaped' structure. Each BAR monomer is a coiled-coil comprised of three long, kinked α-helices that form a six-helix bundle around the dimer interface. The intersection of the monomers and the kinks in helices 2 and 3 induce a strong curvature in the BAR dimer. In addition, the dimer's concave surface has several positively charged patches that might mediate the interaction with lipids. This suggests that the BAR domain binds to highly curved, negatively charged membranes.
There are also two forms of the regulatory protein auxilin, one specific to neurons and a second, termed auxilin 2 or cyclin-G-associated kinase, that is ubiquitously distributed. The N-termini of both auxilins contain a phosphatase and tensin homology (PTEN) domain followed by a clathrin-binding domain, several AP2-interacting motifs, and a J-domain that binds to and stimulates the ATPase activity of heat-shock cognate 70 (Hsc70), the chaperone implicated in the uncoating of CCVs. C. Smith (Warwick, UK) showed data describing how auxilin interacts with clathrin cages. The use of cryo-electron microscopy and single-particle analysis allowed Smith to detect auxilin on the inside of the clathrin cage. Moreover, a proposed clathrin-binding motif in auxilin has been shown, in peptide competition assays, to displace the LLNLD clathrin box motif of the AP2 β2-subunit from the clathrin β-propeller domain (Smith et al, 2004). Auxilin might, therefore, exert a double function in directing Hsc70 to the correct position on clathrin cages and in displacing clathrin from AP2, both of which would potentially facilitate the uncoating process.
The long list of identified regulatory proteins also includes Eps 15, synaptotagmin, intersectin and endophilin, among others. Interestingly, most of the proteins described above have long flexible regions that interact with the appendage domains of AP2, as well as with multiple components of the endocytic machinery, to form an interconnected network (Fig 2). The ability of many of these factors to bind simultaneously to clathrin, lipids and the actin cytoskeleton might be crucial in regulating CCV formation.
Roles for phosphatidylinositols and the cytoskeleton
Phosphatidylinositols (PtdIns) have recently been revealed as major players in the regulation of clathrin-mediated internalization. Many of the proteins implicated in endocytosis have domains that specifically interact with PIP2. This is the case for the α- and μ-subunits of AP2, the ENTH domain of AP180/CALM and epsin and the plekstrin-homology domain of dynamin. Although the structural determinants implicated in the binding of PIP2 are different for each of these proteins, in each case a patch of positive residues is present that interacts with the negatively charged phosphorylated head groups of the lipid.
Haucke proposed a role for the GTPase ADP-ribosylation factor 6 (ARF6) in the generation of PIP2 in synaptic vesicles through the direct stimulation of a 4-phosphate 5-kinase type Iγ (PIPKIγ; a kinase involved in PIP2 formation), which would lead to the nucleation of AP2 and clathrin at the membrane (Krauss et al, 2003). Interestingly, synaptojanin, a protein previously implicated in the uncoating of endocytosed CCVs, is known to contain a 5'-phosphatase catalytic region that can hydrolyse PIP2. It has been proposed that the conversion of PIP2 to PtdIns could be involved in the removal of AP2 and other regulatory proteins from membranes.
There is also growing evidence for inter-regulation between clathrin-mediated endocytosis and the actin cytoskeleton. In this regard, profilin, a PIP2-binding protein and Abp1 interact with both dynamin and actin; synapsin interacts with amphiphysin and actin; whereas syndapin binds to synaptojanin, dynamin, and the neural Wiskott–Aldrich syndrome protein (N-WASP). In the nerve terminal, endocytic regions contain a dynamic actin matrix (Shupliakov et al, 2002). L. Brodin (Stockholm, Sweden) presented microinjection and immunogold electron microscopy studies of the giant synapse in lamprey in different states of synaptic activity. These data showed that, on activation and vesicle fusion, syndapin accumulates in the endocytic region where it promotes actin polymerization and supports proper recycling of synaptic vesicles.
CCV-mediated sorting at intracellular compartments
Clathrin not only mediates vesicle formation at the plasma membrane but also participates in CCV-mediated protein sorting at the trans-Golgi network (TGN) and endosomal compartments.
At the TGN, CCV-mediated transport has been implicated in the transport of numerous lysosomal enzymes. The adaptors that participate in this process are the heterotetrameric AP1 complex and the GGA family of monomeric adaptors. Similar to AP2, AP1 and the GGAs coordinate interactions with membrane protein cargo, clathrin and accessory proteins. The small GTPase ARF1 regulates the recruitment of adaptors to the TGN, although recent evidence suggests that PtdIns, such as PtdIns(4)P, might also have a role in this process. Presumably, the formation of CCVs at the TGN must also require the coordinated activity of different regulatory proteins; however, only a few of these factors have been identified so far. Robinson presented new data on epsinR, also known as enthoprotin, and CLINT, a protein that interacts with the appendage domains of AP1 and the GGAs. Through purifying CCVs from control and siRNA-treated HeLa cells, Robinson demonstrated that epsinR is required for the incorporation of the SNARE Vti1b into CCVs, which suggests that epsinR could be acting as a cargo-specific adaptor at the TGN.
At endosomes, clathrin forms flat lattices and interacts with Hrs, a key component of the machinery that is implicated in the recognition of ubiquitinated proteins and their delivery into multivesicular bodies for degradation. In this case, clathrin does not seem to be important for vesicle formation, but rather in the segregation of cargo and the generation of different domains on the endosomal membranes. R. Puertollano (Bethesda, MD, USA) showed that a subpopulation of GGAs localize to endosomal compartments. In addition, depletion of GGA3 by siRNA techniques resulted in defects in the transport and degradation kinetics of internalized epidermal growth factor. This, together with the ability of GGA3 to bind both to ubiquitin and to the endosomal sorting complex required for transport I (ESCRTI) component Tsg101, led Puertollano to suggest that GGAs might have a role in cargo sorting at endosomes (Puertollano & Bonifacino, 2004).
Future perspectives
The emerging picture of transport vesicle dynamics reveals these processes to be extraordinarily elaborate and to require the coordinated formation of complex protein networks. In the coming years, we will undoubtedly expand our understanding of the mechanisms that regulate intracellular transport. This will come largely from the evolution of modern cell-biology technologies, such as microscopy techniques involving fluorescence-tagged reporter proteins and live cell imaging, which continually provide new and exciting insights into the dynamics of coat structure and function in vivo. In addition, the use of siRNA-based techniques has proven a powerful tool for addressing the specific function of different proteins. Finally, it remains essential to identify new components of the sorting machinery. In this regard, it is important to point out the novel genetic approach presented by E. Conibear (Vancouver, Canada). On the basis of the idea that mutations in components of the same protein complexes will have similar phenotypic effects, Conibear carried out multiple quantitative genome-wide screens on several yeast genome deletion sets using a robotic system. Following different functional assays, genes were grouped according to their phenotypic effect by using clustering algorithms. Several uncharacterized open reading frames were found to cluster with different protein complexes that regulate specific sorting events. Subsequent functional assays proved that those genes were indeed part of the complexes, thus validating the experimental approach.
Certainly, the combination of biochemical, cellular and genetic approaches will allow us to continue unravelling the molecular mechanisms of vesicle assembly and selectivity.
Acknowledgments
My thanks to I. Sandoval and B. Pearse for organizing this interesting meeting and to the Juan March Foundation for their excellent organization and assistance. I am also grateful to C. Mullins for his valuable comments and suggestions. My apologies to those speakers whose talks I have been unable to include due to space limitations.
References
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE (2001) Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol 17: 517–568 [DOI] [PubMed] [Google Scholar]
- Chen YJ, Zhang P, Egelman EH, Hinshaw JE (2004) The stalk region of dynamin drives the constriction of dynamin tubes. Nat Struct Mol Biol 11: 574–575 [DOI] [PubMed] [Google Scholar]
- Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ (2002) Molecular architecture and functional model of the endocytic AP2 complex. Cell 109: 523–535 [DOI] [PubMed] [Google Scholar]
- Jackson AP, Flett A, Smythe C, Hufton L, Wettey FR, Smythe E (2003) Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase. J Cell Biol 163: 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V (2003) ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J Cell Biol 162: 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele AE, Watson PJ, Evans R, Traub LM, Owen DJ (2004) Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain β-propeller. Nat Struct Mol Biol 11: 242–248 [DOI] [PubMed] [Google Scholar]
- Motley A, Bright NA, Seaman MN, Robinson MS (2003) Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol 162: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ (2004) Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation. Biochem Soc Trans 32: 1–14 [DOI] [PubMed] [Google Scholar]
- Pearse BM (1976) Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci USA 73: 1255–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM, Smith CJ, Owen DJ (2000) Clathrin coat construction in endocytosis. Curr Opin Struct Biol 10: 220–228 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Puertollano R, Bonifacino JS (2004) Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol 6: 244–251 [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustaffson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, Brodin L (2002) Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci USA 99: 14476–14481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Dafforn TR, Kent H, Sims CA, Khubchandari-Aswani K, Zhang L, Saibil HR, Pearse BM (2004) Location of auxilin within a clathrin cage. J Mol Biol 336: 461–471 [DOI] [PubMed] [Google Scholar]
- Soutar AK, Naoumova RP, Traub LM (2003) Genetics, clinical phenotype, and molecular cell biology of autosomal recessive hypercholesterolemia. Arterioscler Thromb Vasc Biol 23: 1963–1970 [DOI] [PubMed] [Google Scholar]
- ter Haar E, Musacchio A, Harrison SC, Kirchhausen T (1998) Atomic structure of clathrin: a β propeller terminal domain joins an α zigzag linker. Cell 95: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeham DE, Chen CY, Greene B, Hwang PK, Brodsky FM (2003) Clathrin self-assembly involves coordinated weak interactions favorable for cellular regulation. EMBO J 22: 4980–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Diril MK, Jung N, Haucke V (2004) Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc Natl Acad Sci USA 101: 964–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybe JA, Brodsky FM, Hofmann K, Lin K, Liu SH, Chen L, Earnest TN, Fletterick RJ, Hwang PK (1999) Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature 399: 371–375 [DOI] [PubMed] [Google Scholar]