Summary
Meeting on Cytoskeletal Dynamics: From Cell Biology to Development and Disease
Keywords: actin, cell adhesion, cell motility, cytoskeleton, endocytosis
Introduction
This meeting began with personal retrospective presentations from two of the founding fathers of European cytoskeletal research—V. Small (Vienna, Austria) and U. Lindberg (Stockholm, Sweden)—to commemorate the thirtieth anniversary of the first definitive publication to establish the existence of an actin cytoskeleton in non-muscle cells (Lazarides & Weber, 1974).

The Federation of European Biochemical Sciences (FEBS) Special Meeting on Cytoskeletal Dynamics: From Cell Biology to Development and Disease, which also incorporated the nineteenth meeting of the European Cytoskeletal Forum (ECF), was organized by O. Carpen and P. Lappalainnen, and took place between 12 and 16 June 2004, at the Biomedicum, Helsinki, Finland.
Ancient cytoskeletons
Whereas other meetings often feature an occasional talk on the prokaryotic cytoskeleton, this meeting devoted a whole session to several aspects of our present knowledge of these ancient systems. This was well received and served as a useful reminder that the cytoskeleton is not just the domain of eukaryotic cell biologists.
J. Errington (Oxford, UK) introduced the topic of the bacterial cytoskeleton and reviewed earlier studies that showed the filamentous nature of the ancient bacterial actin proteins MreB and Mbl (MreB-like). As he pointed out, although we have known about actin filaments for 30 years, they have almost certainly existed for more than two billion years. Many early studies on bacterial cell shape indicated that the cell wall alone conveyed the information that was required for morphology. However, the finding that some of the genes that had been identified in mutational cell-shape screens encoded protein products that resided in the cytosol meant that this idea had to be revisited. Errington and colleagues are now investigating the functions of the Mbl protein, which is one of three MreB homologues in Bacillus subtilis. They have used fluorescence recovery after photobleaching (FRAP), which is a technically demanding technique in an organism as small as a bacterium, to show that the helical Mbl cables are dynamic structures in vivo (Fig 1). The kinetics of recovery indicate that this is achieved through turnover from unbleached parts of the cell. These studies also imply that the organization of the cables is not likely to be polar. Errington favours the idea that Mbl and MreB are involved in cylindrical cell extension and act to ensure that cell-wall deposition occurs in the appropriate region of the cell. H. Erickson (Durham, NC, USA) continued the session with a description of studies on bacterial tubulin, filamentation temperaturesensitive protein Z (FtsZ), which assembles into a contractile ring that divides the bacterium during cytokinesis. His group studied FtsZ in vitro and showed that it assembles into thin protofilaments, dozens of which cluster to form the contractile Z ring in vivo. They also used FRAP to determine that the Z ring is extremely dynamic and turns over with a half-life of 9 seconds, which is even faster than the turnover of microtubules in eukaryotic cells. The generation and analysis of many mutant FtsZ proteins has indicated that there is directional end assembly, which raises the possibility of treadmilling. This process is generally considered to be a feature of actin, but not microtubule, assembly in eukaryotes, whereby a filament is continually built at one end and simultaneously falls apart at the other. An exciting recent development by the Erickson group was the generation of a tryptophan FtsZ mutant that provided a real-time assay of assembly using fluorescence spectroscopy. This allowed an analysis of assembly kinetics and nucleation, which yielded two main conclusions: first, that the nucleus for FtsZ filament assembly is a dimer; and second, that assembly is cooperative. The cooperative assembly of FtsZ filaments was also observed by the group of J. Andreu (Madrid, Spain) during their work on FtsZ from Methanococcus.
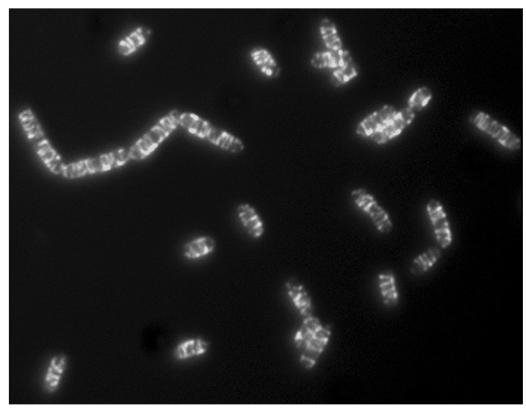
Figure 1.
Helical filaments of the MreB homologue Mbl tagged with green fluorescent protein in growing cells of Bacillus subtilis. Image reproduced courtesy of J. Errington, Oxford, UK.
As well as bacterial actin (MreB) and tubulin (FtsZ), evidence for intermediate filaments in bacteria has been reported. J. Kürner (Martinsreid, Germany) described the use of cryoelectron tomography and reconstruction techniques to study the cytoskeletal elements in the extremely small motile cells of Spiroplasma melliferum. Two types of filament were observed and identified as the intermediate filament-like fibril protein and MreB. Purification of the fibril protein showed that in vitro, similar to in vivo, it assembled as a paired helical filament of 11-nm diameter.
The dynamic actin cytoskeleton
Several sessions during the first two days included talks that were devoted to both structural and functional aspects of actin dynamics. Elegant structural studies from M.-F. Carlier (Gif-sur-Yvette, France), R. Robinson (Stockholm, Sweden) and J. Moseley (Boston, MA, USA) provided mechanistic insight into three important regulators of actin dynamics: thymosin, gelsolin and the formin family of proteins. Using a combination of biochemical and structural approaches, the Carlier group showed how thymosin can be switched from an inhibiting to an activating protein for actin assembly. This is achieved by mutating four important residues in the thymosin molecule to resemble those in domain one (D1) of the ciboulot protein, which regulates actin assembly in Drosophila. Furthermore, by solving the crystal structure of D1 in complex with ATP–actin and comparing this with the nuclear magnetic-resonance spectra of D1 or thymosin bound to actin, they were able to propose a model in which the amino- and carboxy-terminal helices of thymosin lock around the actin monomer and prevent filament assembly. By contrast, in D1 or thymosin-D1-like mutants, the top of the actin is exposed, which allows the barbed-end assembly of filamentous (F)-actin. This model was complemented by the information presented by Robinson on the structure of segment 1 of gelsolin (which severs actin filaments) fused to the C-terminus of thymosin. This showed that the C-terminus can indeed lock the top of actin, as proposed by Carlier (Fig 2A). Robinson also described the structure of segments 1–3 of gelsolin bound to actin. This not only showed how the protein might sever actin, but also is the first atomic resolution structure of an F-actin-binding protein in complex with actin—albeit globular (G)-actin in this case. Using this structure, and previous structures of all six segments of gelsolin alone plus segments 4–6 complexed with actin, Robinson proposed a detailed mechanism for the calcium-dependent activation of gelsolin and the severing of F-actin (Fig 2C).
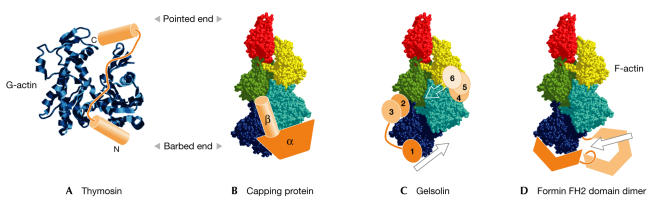
Figure 2.
Schematic representations of the sequestering and capping of actin by thymosin, capping protein, gelsolin and formins. (A) Globular actin (G-actin; blue ribbon) is 'capped' at the top and bottom by the N- and C-terminal helices of thymosin (orange), which are connected by an unstructured loop. (B) The capping protein β-tentacle forms an interface between three actin subunits at the barbed end of a short filament of five actin monomers (shown in space-filling representation). The α-tentacle and the remainder of the β-capping protein are in an equivalent position on the rear side of the filament. (C) The six subunits of gelsolin are coloured in orange and are arranged in their approximate positions on the filament. The planes of severing are indicated by arrows. (D) The formin homology 2 (FH2) domain dimer is represented as dark and light orange, with the darker subunit bound to the dark blue actin monomer at the barbed end of the filament, and the lighter subunit hinged down via the linker and lasso-and-post structures to allow a new actin monomer to be added (arrow).
Actin polymerization is nucleated by formin. Moseley presented mechanistic details of this process, which have been gleaned from the structure of the formin homology 2 (FH2) domain from the Saccharomyces cerevisiae protein Bni1. The FH2 domain dimer is a hinged doughnut-shaped ring that is formed from two largely helical half-doughnut-shaped rings, which are joined by an extended linker with an unusual 'lasso-and-post' ball-and-socket hinge-like structure. Even though each half of the dimer can bind actin, a complete dimer is required for actin nucleation; the linker and lasso-and-post might allow the two halves of the dimer to flex and accommodate the processive addition of actin monomers to the barbed end of a filament (Fig 2D).
The role of actin polymerization at the leading edge of motile cells was the focus of several talks. G. Borisy (Chicago, IL, USA) opened an excellent session with a review of the work of his group on the transition of the branched actin networks that are present in leading-edge lamellipodia to the unbranched parallel filaments that are found in filopodia. Models for leading-edge protrusion indicate that lamellipodia are generated by the dendritic nucleation of F-actin, whereas convergent elongation is responsible for the formation of filopodia (Fig 3). Borisy highlighted the crucial role of capping proteins in shortening filament length and the importance of the Enabled/vasodilatorstimulated phosphoprotein (Ena/VASP) proteins in promoting filament lengthening. Regulating these antagonistic functions is pivotal to the behaviour of actin at the leading edge: if capping dominates, then filament branching occurs and lamellipodia are formed; by contrast, if capping is suppressed, the filaments elongate and can be stabilized by association, which produces filopodia (Fig 3). D. Vignjevic (Chicago, IL, USA) described some elements of filopodia in more detail and presented her own studies on the role of the actin-bundling protein fascin. Unlike many other actin-binding proteins, fascin localizes along the entire length of filopodia. The use of small-interfering RNAs (siRNAs) showed that the depletion of fascin resulted in fewer filopodia and those that were observed contained loosely bundled actin. Inactive fascin (S39E) was found only at the filopodia tips, whereas constitutively active fascin (S39A) was localized along the entire filopodial shafts. Vignjevic proposed that fascin is recruited in an inactive dephosphorylated form by the tip complex of nascent filopodia where it is then dephosphorylated and activated, which allows it to bundle actin filaments as the filopodia extend. M. Barzik (Cambridge, MA, USA) discussed the importance of Ena/VASP proteins at the leading edge. Her studies show that the inhibition of Ena/VASP levels leads to a decreased number of filopodia and that the Ena/VASP-homology domain 2 of these proteins has anti-capping activity. Both the F- and G-actin-binding sites are required in this domain. Furthermore, profilin has been shown to synergize with VASP to enhance actin assembly in the presence of the capping protein.
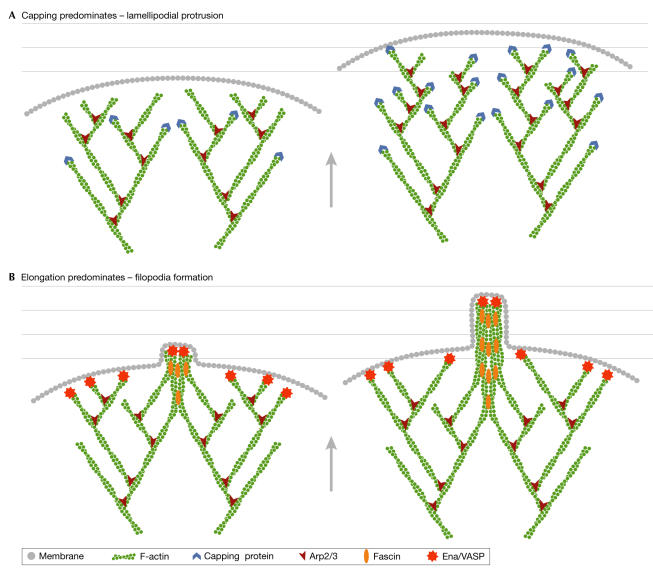
Figure 3.
A schematic representation of the mechanisms of lamellipodia and filopodia formation at the leading edge of cells. (A) If capping activity predominates, then many short branched filaments are formed into a broad dendritic network. (B) If capping activity is reduced and/or elongation is favoured, longer filaments are formed that are then bundled into filopodia by proteins, such as fascin. Arp2/3, actin-related protein 2/3; Ena/VASP, Enabled/vasodilator-stimulated phosphoprotein.
The crystal structure of the capping protein αβ-heterodimer gave rise to the 'tentacle' model, in which the α- and β-subunits are mobile extensions that cap the barbed end of actin filaments. The group of J. Cooper (St Louis, MO, USA) has undertaken a range of biochemical studies that show that the isolated β-tentacle, but not the α-tentacle, has weak capping activity and that this tentacle acts in the heterodimer by binding at the interface of actin subunits (Fig 2B).
T. Pollard (New Haven, CT, USA) described how his group used total internal-reflection fluorescence (TIRF) microscopy to repeat measurements of rate constants for stages of actin dynamics; this work indicates that the crucial concentrations of actin monomers at both ends of the filament are more similar than was previously recognized. His group also performed further research into actin nucleation by formins, which showed that there is insertional barbed-end elongation such that the new actin monomer inserts between formin and the rest of the actin filament. If the pointed end was then tethered in the cell—for example, by binding to myosin—a 1.3-pN buckling force could be measured. Such a mechanism could partly explain how the leading edge of a cell is moved forward.
The importance of a dynamic cytoskeleton was also addressed in talks that concerned other aspects of cell function. D. Drubin (Berkeley, CA, USA) gave a fascinating description of the work of his group using S. cerevisiae to study the role of actin in the endocytic process. From this research, it is now clear that there is a sequential assembly and disassembly of complexes at the plasma membrane that allows different stages of endocytosis to proceed. Interestingly, each of these stages seems to be regulated by a different activator of the actin-related protein 2/3 (Arp2/3) complex, which can initiate new actin polymerization. Additionally, his group has resolved some long-standing questions in the field, such as whether clathrin is involved in endocytosis in budding yeast. Using TIRF microscopy, they have shown that clathrin is present at the plasma membrane and that its lifetime in cortical patches is consistent with it being one of the first proteins to arrive at the endocytic site. Clathrin then remains with the endocytic vesicles until they begin a burst of actin-driven movement into the cell. Drubin and colleagues have also shown that none of the dynamin-like proteins have a role in endocytosis in budding yeast. They are now investigating the importance of the Ark/Prk kinases in the regulation of the endocytic process.
This talk was followed by a presentation by B. Qualmann (Magdeburg, Germany), who discussed the role of actin in endocytosis in mammalian cells. Using evanescent-field microscopy, her group has shown that the actin-polymerization machinery is transiently recruited to sites of endocytosis. Arp2/3 and the actin-regulatory neuronal Wiskott–Aldrich-syndrome protein (N-WASP) can be visualized at these sites, and the depletion of N-WASP leads to a reduction in levels of endocytosis. Syndapins interact with N-WASP, and are potential links between the cortical actin cytoskeleton and endocytosis. Consistent with a role for syndapins in endocytosis, co-overexpression of syndapins rescued the endocytosis block that was caused by N-WASP. Qualmann suggested that the transient modulation of actin polymerization by syndapins, through the activation of the Arp2/3 complex by N-WASP, coordinates dynamin-mediated vesicle fission at the apical plasma membrane of acinar epithelia. Qualmann and colleagues are now investigating the possibility that, as in budding yeast, there might be distinct stages of actin polymerization with the endocytic machinery in mammalian cells, which could be characterized by the sequential action of syndapin, the Arp2/3-activating protein cortactin and the mammalian actin-binding protein 1 (mAbp1).
Finally, K. Ayscough (Sheffield, UK) presented evidence for a newly recognized role for the actin cytoskeleton in the processes of apoptosis and ageing. Using budding yeast, she showed that decreases in actin dynamics trigger the apoptotic pathway, whereas increases in dynamics enhance lifespan. The mechanism for this effect seems to be mediated through the mitochondria, with reduced actin dynamics causing a marked increase in the levels of reactive oxygen species. Conversely, mutations that increase actin dynamics reduce the levels of these damaging molecules. Interestingly, the deletion of a single actin-binding protein gene (scp1) increased the longevity of yeast cells. The mammalian homologue of this protein has previously been isolated in screens for ageing-specific proteins, which points to a conserved role for actin in this process.
Actin-based adhesion, migration and signalling
Many of the advances in our understanding of cytoskeletal dynamics have arisen from developments in imaging technology and analysis, not least through the work of P. Friedl (Wurzburg, Germany) and B. Geiger (Rehovot, Israel). Using time-lapse confocal microscopy of mammalian cells that are embedded in a three-dimensional collagen matrix, Friedl and colleagues have been able to analyse the components of cell invasion and migration through the extracellular matrix, and have determined the requirements for mesenchymal (fibroblast-like) and amoeboid migration. Mesenchymal migration is typified by the formation of integrin-based cell-substrate contacts (that is, focal contacts), local remodelling of the extracellular matrix by metalloproteinase (MMP) activity and the physical deformation of the extracellular matrix that is induced by contractile forces. Once a path has been formed, other cells follow. By contrast, amoeboid movement—as seen in migrating lymphocytes, for example—does not require MMP activity or notable substrate contact, but relies on dynamic cortical contractility to force the cell through small spaces in the matrix. The Geiger group has rigorously analysed the components of different adhesion structures in a two-dimensional context to understand the differential composition of focal adhesions, focal complexes, fibrillar adhesions and podosomes during cell migration on flat surfaces. Using a combination of various constructs of Src-kinase and Src-null cells, they highlighted the importance of tyrosine phosphorylation of tensin for its distribution between focal adhesions and fibrillar adhesions, and showed how Src activity markedly alters the dynamics of podosomes through cortactin phosphorylation.
The mechanisms of lamellipodial-driven cell migration have also been examined in fibroblasts that were isolated from mice deficient in two members of the WASP family: WAVE1 and WAVE2. Using these cells, along with siRNA depletion of either of the Wave proteins, T. Takenawa and colleagues (Tokyo, Japan) showed that WAVE1 and WAVE2 have differential roles in the formation of the dorsal and peripheral ruffles that are involved in fibroblast cell migration. WAVE2 is principally involved in the rapid-spreading phase of migration and actin polymerization at the leading edge, whereas WAVE1 localizes to adhesions and forms more ordered actin structures in an area of consolidation behind the leading edge. WAVE proteins also featured in presentations from P. Hussey (Durham, UK) and M. Huelskamp (Cologne, Germany), whose groups have been studying the actin-signalling machinery in plant cells. Even though plant cells do not migrate or form adhesions in the same way as vertebrate cells, they nonetheless require a dynamic actin cytoskeleton for correct cell shape and cellular organization, and for the rapid growth of many structures, such as root hairs and pollen tubes. It is becoming clear that plants probably have similar components for signalling to actin as those that are found in vertebrate systems, from Rho GTPases through WAVE to Arp2/3, although with a certain degree of added complexity owing, in some cases, to a staggering degree of redundancy. For example, plants seem to have more than 20 genes that encode formins. Some of this redundancy can be accounted for by the presence of specific genes that are encoded by reproductive and vegetative cells, although this is certainly not true for all, and there will be challenging times ahead to unravel this complexity, not least because of the difficulty of identifying plant homologues by sequence alone.
As described above, progress has been made in understanding the mechanisms of formin-induced actin polymerization, and work by A. Albert and co-workers (Grand Rapids, MI, USA) is unravelling some of the complexities of the roles of Diaphanous-related formins (DRF) in mammalian cells. Unlike plant formins, these proteins are regulated directly by Rho family GTPase binding. The three DRFs are differentially regulated by Rho family GTPases and have several overlapping cellular functions. Knockout of one DRF in mice leads to a phenotype that is reminiscent of Wiskott–Aldrich syndrome, which includes multiple lymphomas and leukaemia; this might point to common tissue-specific pathways of formin action in relation to WASP family proteins.
J. Collard (Amsterdam, The Netherlands) has also used knockout mice in conjunction with siRNA to study the role of Rac signalling to actin, as mediated by the Rac guanine nucleotide-exchange factor Tiam1. Depletion of Tiam1 in Madine–Darby canine kidney (MDCK) cells by siRNA resulted in a transition to a mesenchymal phenotype with an increase in apoptosis triggered by loss of contact with the extracellular matrix (anoikis). Interestingly, Ras-transformed MDCK cells have low Rac activity and low levels of Tiam1, which result in an invasive mesenchymal phenotype; the overexpression of Tiam1 restores Rac levels and induces a mesenchymal-to-epithelial transition. These findings were nicely recapitulated in a Tiam1-knockout mouse, in which fewer tumours formed relative to controls owing to an increase in apoptosis, although the tumours that did form were much more aggressive and invasive. In another knockout mouse model, W. Sebastian (Martinsreid, Germany) described the chondrocyte-specific knockout of the actin signalling protein profilin 1. Mutant mice were born the same size as controls but exhibited progressive dwarfism compared with wild-type animals. Examination of the skeleton detected severe chondrodysplasia as a result of delayed or absent ossification. The chondrocytes themselves were differentiated, but they had an apparent cytokinesis defect with many binucleate cells and failed to organize correctly into the columns of cells that are typically seen in cartilage growth plates. Sebastian also showed that profilin-1-knockout cells seem to spread more slowly and have fewer and shorter stress fibres with less numerous adhesions.
FERM domains in dynamics, development and disease
FERM domains, which are the signature motif of the four.1, ezrin, radixin, moesin family of proteins that includes the closely related protein merlin (or schwannomin), are also found in numerous other proteins, including talin, several myosins, Janus kinase and protein tyrosine phosphatases. FERM domains are involved in linking to membrane lipids and to the cytoplasmic tail of transmembrane proteins. V. Niggli and colleagues (Bern, Switzerland) investigated the lipid-binding properties of the myosin V11a and V11b FERM domains and discovered a specific interaction with liposomes that contained phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), and an even stronger interaction with PtdIns(3,4,5)P2, but not with PtdIns(4)P, PtdIns or phosphatidylserine. By contrast, the FERM domain of ezrin binds PtdIns(4,5)P2 and PtdIns(3,4,5)P2 equally well, which provides a basis for the specific signal-dependent regulation of function and membrane location.
Another mode of specifying the membrane location of FERM domains is through binding to a transmembrane protein receptor. S. Winder (Sheffield, UK) provided evidence for the targeting of ezrin to β-dystroglycan, which is part of an adhesion receptor complex that is normally localized to the basal surfaces of cells, through a FERM-domain-mediated interaction with the cytoplasmic tail of β-dystroglycan. Furthermore, the association of β-dystroglycan with ezrin induced numerous dynamic actin-rich filopodial and microvillar protrusions over the apical surface of cells. Mutation of the FERM-binding region in β-dystroglycan abolished the interaction with ezrin and prevented β-dystroglycan-induced cytoskeletal rearrangements.
Ezrin can affect the architecture and dynamics of the cytoskeleton in a single cell, but it also has a role in the organization of multicellular structures, as was elegantly illustrated by the work of A. McClatchey (Charlestown, MA, USA). Redundancy in ezrin, radixin, moesin (ERM) protein function has complicated analyses at the organismal level. However, in an ezrin-knockout mouse, there was a marked phenotype in intestinal epithelium, a tissue in which ezrin is the only ERM protein expressed. Surprisingly, ezrin is not absolutely required for the formation of brush-border microvilli, or for the establishment or maintenance of epithelial polarity. Instead, ezrin organizes the apical terminal web region, which is crucial for the poorly understood process of de novo lumen formation and expansion during villus morphogenesis.
The complexity of ERM family protein function in the nervous system was highlighted in the presentations of M. Giovannini (Paris, France) and M. Gronholm (Helsinki, Finland). Ezrin and merlin are both widely expressed in the brain, but Gronholm and colleagues found that, during development, only ezrin is expressed in the mouse brain until embryonic day 11, at which point merlin expression is robustly switched on. Merlin expression was mainly restricted to neurons, whereas ezrin was found in astrocytes. The role of merlin in the brain and in neurofibromatosis 2 (NF2) in mice was further developed in transgenic studies that were presented by Giovannini. The knockout of merlin is embryonic lethal, but heterozygous animals survive and are prone to increased numbers of tumours, although not the schwannomas or meningiomas that are typified by NF2. Furthermore, the picture is complicated by the presence of two merlin isoforms, and the selective knockout of one or other isoform yielded a different spectrum of tumours. The conditional knockout of merlin in Schwann or arachnoid cells produced the expected schwannomas, which resembled the human NF2 phenotype. Cellular analysis of merlin-deficient cells showed a loss of appropriate growth control, which led to continued high-density growth in low serum conditions, disruption of adherens junctions, and elevated signalling through mitogen-activated protein kinases and cyclin D1. Findings such as these might provide the basis for the identification of therapeutic targets for the treatment of NF2.
With more than 300 delegates and speakers from 20 European countries, as well as Japan and the USA, this meeting was a fitting tribute to the past 30 years of cytoskeletal research. We eagerly await the next update at the twentieth annual meeting of the European Cytoskeletal Forum (ECF), which will be held in Luxembourg from 27 August to 1 September 2005.

Acknowledgments
Thanks go to O. Carpen, P. Lappalainnen and team for putting together an outstanding programme of science, not to mention the hospitality and social events. Work in the authors' laboratories is funded by the Medical Research Council (MRC). We apologize for the fact that, owing to space constraints, it was not possible to discuss of all the excellent presentations in this report.
References
- Carballido-Lopez R, Errington J (2003) A dynamic bacterial cytoskeleton. Trends Cell Biol 13: 577–583 [DOI] [PubMed] [Google Scholar]
- Deeks MJ, Hussey PJ (2003) Arp2/3 and 'the shape of things to come'. Curr Opin Plant Biol 6: 561–567 [DOI] [PubMed] [Google Scholar]
- Friedl P (2004) Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol 16: 14–23 [DOI] [PubMed] [Google Scholar]
- Lazarides E, Weber K (1974) Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci USA 71: 2268–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI (2003) Merlin and ERM proteins: unappreciated roles in cancer development? Nat Rev Cancer 3: 877–883 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465; erratum in 113: 549 [DOI] [PubMed] [Google Scholar]