Abstract
We have addressed the mechanism of insertion of both transmembrane segments (TMs) of leader peptidase, a double-spanning protein, into the Escherichia coli inner membrane. Using photo-crosslinking, the first TM (H1) was shown to insert at a Sec-translocon/YidC interface in a fixed orientation. H1 lost its contacts with the Sec-translocon and gained access to lipids near YidC soon after complete exposure outside the ribosome. Following lipid integration, it moved away from the Sec/YidC insertion site. The second TM (H2) inserted and interacted with SecY and YidC in a similar transient fashion. The data are consistent with a linear integration model in which the TMs of polytopic inner membrane proteins move one by one from a Sec/YidC insertion site into the lipid bilayer. We propose that YidC assists the lipid partitioning of single TMs.
Keywords: membrane protein, YidC, Sec-translocon, leader peptidase, membrane integration
Introduction
The Escherichia coli Sec-translocon has originally been identified as an integral membrane complex that transports proteins across the inner membrane (reviewed in Mori & Ito, 2001). The core of the Sec-translocon consists of SecY, SecE and SecG, which form a narrow pore (van den Berg et al, 2004). The same core-translocon is also used for the membrane integration of most inner membrane proteins (IMPs; reviewed in de Gier & Luirink, 2001). Transmembrane segments (TMs) in nascent IMPs must be recognized in the Sec-translocon and must partition laterally into the lipid bilayer. This ultimately leads to the stable integration of the TMs and their folding into the native conformation. The molecular details of these latter processes are poorly understood. YidC has recently been identified as a novel translocon associated IMP that specifically interacts with TMs during synthesis and membrane integration (Scotti et al, 2000). YidC is also required for the integration of IMPs that do not use the Sec-translocon (Samuelson et al, 2000).
To investigate membrane integration of polytopic E. coli IMPs and the role of YidC in this process, we have used the relatively simple polytopic IMP Leader peptidase (Lep), which spans the membrane twice. Sequential interactions of both TMs (H1 and H2) were examined by site-specific photo-crosslinking using nascent chains of different length, which were produced by in vitro translation in the presence of membrane vesicles.
Results
Membrane-targeted nascent Lep: early encounters
Lep, the principal signal peptidase in E. coli, was used as a model protein to investigate the molecular details of the insertion of polytopic IMPs into the lipid bilayer. Lep has two TMs, H1 and H2, and both the short amino-terminus and the large catalytic carboxy-terminal domain face the periplasmic site of the inner membrane (Fig 1A). Previously, the initial insertion steps of Lep have been investigated by site-directed photo-crosslinking of in vitro translated nascent chains (Houben et al, 2002). Surprisingly, both YidC and SecY contacted nascent Lep very early during biogenesis, at only 50 amino acids nascent chain length.
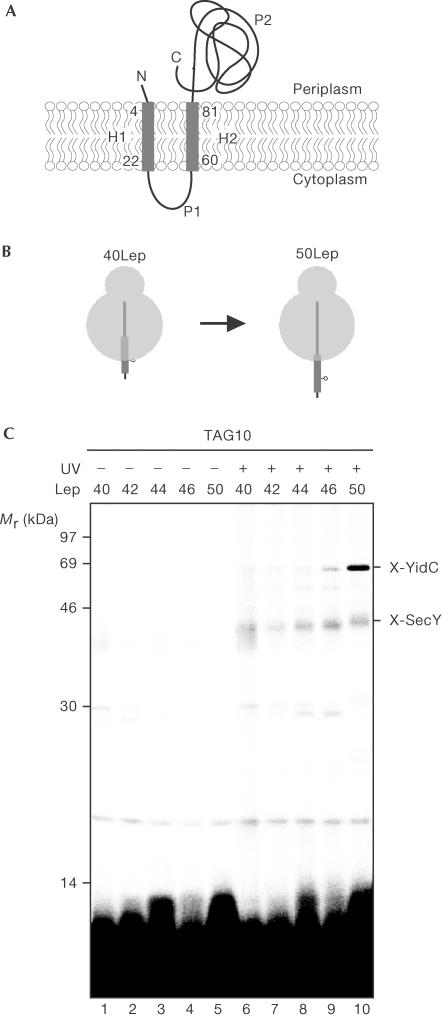
Figure 1.
Initial contacts of Lep H1 during membrane insertion. (A) Topology of Lep in the inner membrane. (B) Schematic representation of the 40 and 50Lep constructs with a crosslinking probe at position 10. The transmembrane regions are presented as thick lines. (C) In vitro translation of nascent Lep 40–50-mer was carried out in the presence of inner membrane vesicles and the (Tmd)Phe-tRNAsup. After translation, samples were UV irradiated to induce crosslinking and extracted with sodium carbonate.
To further define the first contacts of H1 with YidC, Lep nascent chains of 40, 42, 44, 46 and 50 amino acids were prepared with the photo-crosslinker at position 10 (Fig 1B). The Lep 48-mer failed to be synthesized and was not included in this experiment. The nascent chains were prepared by in vitro translation in the presence of [35S]methionine using truncated mRNAs that encode N-terminal Lep nascent chains with a C-terminal 4 × methionine tag to increase the labelling efficiency. A stop codon was introduced at position 10 in H1, which was suppressed during translation by including a suppressor tRNA, which carried a phenylalanine with a photoreactive crosslinking probe Tmd (see Methods). The nascent chains were synthesized in the presence of inner membrane vesicles to generate insertion intermediates. Interactions were fixed by irradiation with UV light to induce crosslinking. Subsequently, the membranes were treated with sodium carbonate and sedimented to enrich for membrane-integrated material.
50LepTAG10 showed clear crosslinking to YidC and very inefficient crosslinking to SecY, as observed before (Houben et al, 2002; Fig 1C, lane 10). At shorter nascent chain lengths, the crosslink efficiency to YidC rapidly reduced and became undetectable at a nascent chain length of 44 amino acids (Fig 1C, lane 8). SecY was found to be crosslinked with similar low efficiencies independent of the length of nascent Lep (quantifications not shown). Conceivably, 40–44LepTAG10 inserts into the aqueous Sec-translocon but is not specifically bound. This results in quenching of the photoprobe by water, which explains the inefficient crosslinking to SecY. Importantly, position 10 contacts YidC directly when nascent Lep has reached a length of 46 amino acids without an earlier intimate contact with SecY or SecE.
Transient interactions of H1 with Sec and YidC
To probe the integration of H1 into the lipid bilayer, we monitored the interactions of H1 in longer nascent Lep chains. Previous experiments of this kind have indicated that position 10 stays near YidC at least until a nascent chain length of 100 amino acids (Houben et al, 2002). However, closer examination of the longer (>70 amino acids) nascent chains on high-resolution gels showed that in addition to polypeptides of the anticipated length, shorter products were also present (Fig 2B). This heterogeneity in nascent chain length is probably due to premature pausing or blocking of translation, events that commonly occur in cell-free E. coli extracts (Hardesty & Kramer, 2001). To address this technical problem, the C-terminal eight amino acids of nascent Lep constructs (72–162 amino acids) were replaced by a Myc epitope of the same length (Fig 2A). This allows the selective recognition and immunopurification of nascent chains that have reached the desired length and their crosslinked partners.
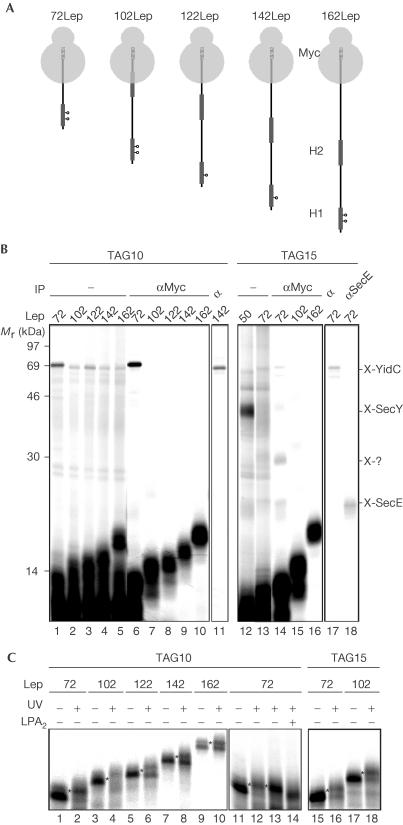
Figure 2.
Consecutive interactions of Lep H1 during membrane insertion. (A) Nascent Lep species used to study insertion of H1. The photo-crosslinking probes are indicated (positions 10 and 15). The C-terminal Myc tags are indicated as hatched bars. (B) In vitro translation, crosslinking and carbonate extraction of nascent Lep 50–162-mer were carried out as described in Fig 1. Carbonate-insoluble material was immunoprecipitated as indicated. (C) Nascent Lep 50–162-mer was produced, crosslinked or kept in the dark, immunoprecipitated using anti-Myc serum and analysed by 15% SDS–PAGE. To identify lipid crosslinking adducts (indicated by asterisks), membranes of photo-crosslinked 72LepTAG10 samples were not carbonate extracted but spun through a highsalt sucrose cushion and incubated with bee venom phospholipase A2 (PLA2) or mock treated with incubation buffer (lanes 13 and 14).
72–162Myc-LepTAG10 showed crosslinking to YidC (Fig 2B, lanes 1–5, verified by immunoprecipitation of the 142-mer, lane 11). However, crosslinking products of the 102–162-mer were weakly present and migrated at the same position for all nascent chain lengths, indicating crosslinking of a dominant premature translation product. Indeed, immunoprecipitation with anti-Myc antibodies, which selectively precipitated nascent Lep of the expected length, showed that only the 72-mer was crosslinked to YidC (Fig 2B, cf. lanes 1–5 and 6–10). The anti-Myc purified samples were also analysed for crosslinking to lipids using 15% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) for a better resolution in the low-molecular-weight area (Fig 2C, lanes 1–10). Notably, 50LepTAG10 does not contact lipids (Houben et al, 2002). At every nascent chain length studied here, crosslinking adducts were detected that migrated slightly slower than the noncrosslinked nascent chains. The adducts were not present after phospholipase treatment of the crosslinked samples, confirming that they represent crosslinking to lipids (shown for the 72-mer in Fig 2C, lanes 11–14). These results demonstrate the necessity and efficacy of the Myc-tag approach. Importantly, the results suggest that position 10 in H1 has moved away from YidC and other translocon components already at a nascent chain length of 102 amino acids. This position in H1 is, furthermore, close to lipids at all tested stages in the insertion process starting from the 72-mer.
Previous scanning photo-crosslinking using 50Lep has shown that, opposite to the results with the TAG10 construct, TAG15 is strongly crosslinked to SecY and only weakly to YidC. Furthermore, weak crosslinking to SecE and no crosslinking to lipids could be observed at this position (Houben et al, 2002; Fig 2B, lane 12; data not shown). To investigate the molecular environment of position 15 in H1 at later stages in the insertion process, Myc-tagged 72, 102 and 162LepTAG15 constructs were made (Fig 2A) and analysed (Fig 2B, lanes 12–18 and Fig 2B, lanes 15–18). For comparison, the crosslink pattern of the 50LepTAG15 is shown as well (Fig 2B, lane 12). In contrast to 50LepTAG15, 72Myc-LepTAG15 did not detectably crosslink to SecY nor did any of the longer constructs, suggesting that position 15 moves very rapidly from SecY. The 72-mer only yielded weak adducts of ∼68, ∼30 and ∼25 kDa that represent crosslinking to YidC, an unknown factor and SecE, respectively (Fig 2B, lanes 17 and 18). The adducts were also visible following anti-Myc precipitation confirming crosslinking to the 72-mer (Fig 2B, lane 14). Anti-Myc precipitation of the crosslinked 102- and 162-mer showed no crosslinking adducts, indicating that position 15 has entirely left the Sec/YidC site at this stage. Both the anti-Myc-precipitated 72- and 102-mer demonstrated efficient crosslinking to lipids (Fig 2C, lanes 15–18). Together, the data suggest that position 15 in H1 inserts near SecY but swiftly moves into a lipid environment without appreciable halting at YidC.
H1 and H2 interact sequentially with Sec and YidC
At a nascent chain length of 102 amino acids, H1 has completely moved away from the Sec/YidC insertion site into the lipid bilayer (see previous section). At this stage in translation, H2 has only partly emerged from the ribosomal exit site (Fig 2A). It therefore is already evident that the two TMs of Lep insert one by one into the inner membrane. To study the consecutive interactions of H2 and to compare these with the interactions observed for H1 during insertion, 102–162Lep constructs were made that include a TAG codon at position 71 or 73 in the core of H2 and a C-terminal Myc tag (Fig 3A). When H2 adopts a helical conformation, positions 71 and 73 would face different sides of the helix. The distance of H2 to the peptidyl transferase centre in 102-, 122/142- and 162Lep was comparable to the distance of H1 to the peptidyl transferase centre at a nascent chain length of 50, 72 and 102 amino acids, respectively (Figs 2A, 3A).
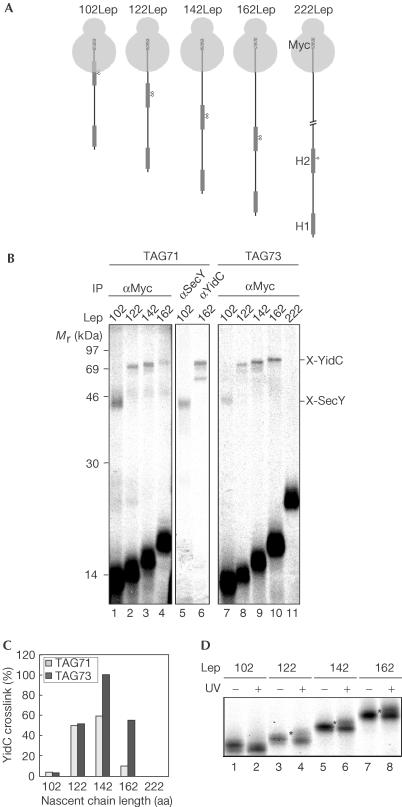
Figure 3.
Consecutive interactions of Lep H2 during membrane insertion. (A) Schematic representation of the 72–222Lep constructs with a C-terminal Myc tag and the crosslinking probe at position 71 or 73. (B) 102–222LepTAG71 and 73 were produced, crosslinked, carbonate extracted and immunoprecipitated as described in Fig 2. (C) Quantification of nascent Lep photo-crosslinked to YidC relative to the amount of immunoprecipitated nascent chains using the anti-Myc serum. The highest value for crosslinking efficiency was taken as 100%. (D) To identify lipid crosslinking, 102–162LepTAG73 constructs were produced, crosslinked or kept in the dark, immunoprecipitated using anti-Myc serum and analysed by 16% tricine SDS–PAGE (indicated by asterisks).
Following crosslinking and anti-Myc precipitation, both 102Myc-LepTAG71 and 73 showed one crosslinking product of ∼45 kDa, which could be immunoprecipitated using antiserum raised against SecY (shown for TAG71; Fig 3B, lane 5). As position 73 is 29 residues from the peptidyl transferase centre, crosslinking to SecY at this position suggests an intimate contact of the ribosome with the Sec-translocon. The 122-mer did not appreciably crosslink to SecY (the weak signal just above the SecY crosslink is caused by the heavy chains of the Myc antibody), suggesting a very transient interaction of H2 with the Sec-translocon. A component ∼58 kDa was crosslinked from both positions in the 122-, 142- and 162-mer, which could be identified as YidC (shown for 162LepTAG71; Fig 3B, lane 6). Quantification of the crosslink efficiencies to YidC demonstrated that YidC was optimally crosslinked to both positions at a nascent chain length of 142 amino acids (Fig 3C). YidC crosslinking at position 71 was almost diminished in the 162-mer. In addition, no crosslinks were detected at position 73 after extending the nascent chain length to 222 amino acids (Fig 3B, lane 12). Crosslinking to lipids from position 73 was investigated by analysing the crosslinked samples using 16% tricine SDS–PAGE (Fig 3D). UV irradiation of the 122-mer but more clearly of the 142- and 162-mer showed the small shift in the migration of a portion of the nascent chains that is diagnostic for lipid crosslinking (Fig 3D, lane 4). These results indicate that H2 inserts near SecY, at least when probed from the positions specified, before being handed over to YidC in proximity to lipids. In contrast to H1, H2 temporarily remains near YidC during elongation of 40–60 amino acids. However, like H1, H2 leaves the direct vicinity of the Sec/YidC insertion site and becomes lipid embedded well before termination of translation.
Discussion
The crosslink data presented in this paper show that the two TMs of the simple polytopic IMP Lep, H1 and H2, integrate into the lipid bilayer independently of each other during synthesis through a sequence of highly specific and transient interactions with the Sec/YidC complex. Previous experiments of this kind have shown that insertion of H1 is characterized by early and specific contacts with both SecY and YidC (at 50 amino acids nascent chain length; Houben et al, 2002). Consequently, a model for the insertion of 50Lep has been proposed (Fig 4).
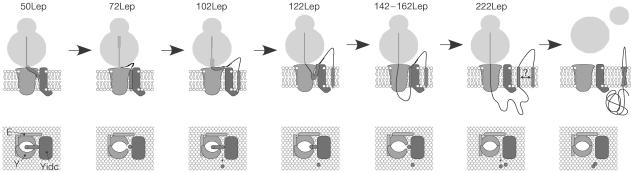
Figure 4.
Model for the membrane insertion of H1 and H2 of Lep. Both side- and top-view models are depicted and discussed in the text. E, SecE; Y, SecY.
In this study, analysis of even shorter Lep nascent chains demonstrates that YidC receives position 10 in H1 without an earlier intimate contact with SecY/E, suggesting an early role of YidC in membrane integration of H1 (Fig 1C). Recently, the crystal structure of the SecYEG complex from Methanococcus jannaschii has been resolved at a resolution of 3.2 Å (van den Berg et al, 2004). The structure suggests that one copy of the heterotrimer serves as a functional translocation channel. The ten TMs of SecY form the channel that seems divided in two halves, connected at the back of the molecule by an external loop and the single TM of SecE. A hinge motion in the back region is suggested to result in the opening of the channel at the front side, allowing TMs to partition into the lipid phase. Interpreting our crosslink data in the context of the crystal structure, a model is presented in which YidC is located at the front side of the Sec-translocon (Fig 4). During insertion of H1, the ribosome is most likely docked on the Sec channel (Prinz et al, 2000). Strikingly, the fact that YidC is adjacent to H1 at the very initial insertion step suggests that SecY laterally opens towards YidC before H1 has fully emerged from the ribosomal exit site (depicted for 50Lep). It is tempting to speculate that the presence of H1 is already sensed in the ribosome, which prepares the translocon for the forthcoming integration event through a sequence of conformational changes. A similar early role for the ribosome in defining the ‘operational mode' of the translocon has been proposed by Johnson and co-workers (Liao et al, 1997).
A crucial phase in the insertion of H1 occurs when the nascent chain reaches a length of ∼70 amino acids (72Lep; Fig 4). At this stage, H1 starts to contact lipids and is optimally integrated in the membrane (Houben et al, 2002). Position 10 in H1 is still close to YidC but position 15 has moved from SecY to lipids, again suggesting a laterally open translocon at this stage. Presumably, lipids are present at the Sec/YidC interface and YidC assists the lipid integration of H1. The observation that position 10 only contacts YidC, whereas position 15 is not near YidC during H1 insertion, suggests that H1 is kept in a fixed orientation during these early steps in the integration process.
At a nascent chain length of 102 amino acids, H1 has completely left the vicinity of the Sec/YidC complex and is lipid embedded. At this stage, H2 has only partly emerged from the ribosomal exit site and contacts SecY. At a nascent chain length of 122 amino acids, H2 subsequently has shifted from SecY to YidC and lipids. H2 continues to be near YidC until a nascent chain length of 162 amino acids has been reached, and has finally moved away from YidC at 222 amino acids. It is not clear why H2 is kept longer at YidC than H1. Notably, H2 is less hydrophobic than H1 and has a different topology in the native protein. In conclusion, the data suggest that H1 and H2 insert one by one via the Sec/YidC site into the lipid phase, where they subsequently assemble during late stages or after termination of polypeptide synthesis.
Lep has also been used as a model protein for membrane integration into the mammalian endoplasmic reticulum (ER) in the context of the eukaryotic counterpart of the Sec-translocon, the Sec61 complex (Mothes et al, 1997; Heinrich et al, 2000; Heinrich & Rapoport, 2003; McCormick et al, 2003). In one set of studies, H1 crosslinked to Sec61α (a homologue of SecY) and lipids with a timing very similar to that reported in this study, suggesting a conserved mechanism of membrane insertion (Mothes et al, 1997; Heinrich et al, 2000). Interestingly, H1 seemed to move back to the Sec61 complex when H2 started to insert in the membrane and was proposed to assist in the lipid integration of the weakly hydrophobic H2 (Heinrich & Rapoport, 2003). In E. coli membranes, this function may be fulfilled by YidC, which has no homologue in the ER membrane. The ER membrane protein TRAM, which has been proposed to have a YidC-like function, did not contact H1 during insertion in the ER membrane unless the hydrophobicity of the TM had been decreased (Heinrich et al, 2000). This indicates that YidC and TRAM at least differ in their requirements for substrate recognition.
In conclusion, our data are consistent with a linear insertion model of TMs in polytopic membrane proteins. The transient interaction of YidC with the TMs of Lep during this process suggests a role for YidC in the recognition and lateral transfer of TMs from the Sec-translocon into the lipid bilayer. A different insertion model of polytopic IMPs at Sec/YidC has been put forward by Beck et al (2001). They proposed that YidC assists in the assembly of TMs before release en bloc into the lipid bilayer, which is based on simultaneous YidC crosslinking to the first three TMs of another, more complex model IMP: mannitol permease. It remains unclear whether these observed differences in the contacts of TMs with YidC are due to the different model proteins used (the two-TM Lep versus the more complex six-TM mannitol permease) or to differences in the experimental set-up. Clearly, the consecutive interactions of more polytopic IMPs need to be analysed to define generic or disparate mechanisms for lipid partitioning of TMs in the E. coli inner membrane.
Methods
Reagents, antisera against YidC, SecY and SecE and strains used for preparation of translation lysate and inner membrane vesicles have been described previously (Scotti et al, 2000; Houben et al, 2002). Antiserum against the Myc sequence was obtained from Sigma-Aldrich Chemical Co (Zwijndrecht). The Myc sequence EQKLISEEDL was introduced in between the HindIII and the ClaI site downstream from the four methionines in the pC4Meth vector, resulting in plasmid pCMM. Plasmids pC4Meth40–50LepTAG10 and pCMM72–222LepTAG10, 15, 71 and 73 were constructed by PCR using pC4Meth100LepTAG10 and pGem3-Lep, respectively, as templates (Houben et al, 2000). Preparation of truncated mRNA, in vitro translation in the presence of the artificial tRNA carrying a photoactivatable amino acid L[3-(trifluoromethyl)-3-diazirin-3H-yl] phenylalanine ((Tmd)Phe-tRNAsup), targeting to inner membrane vesicles, photo-crosslinking, carbonate extraction, immunoprecipitation and PLA2 treatment of nascent Lep derivatives were carried out as described previously (Scotti et al, 2000; Houben et al, 2002).
Acknowledgments
We thank N. Harms for critical reading of the manuscript. This work was supported by the Earth and Life Sciences Foundation (ALW), which is subsidized by the Netherlands Organisation of Scientific Research (NWO).
References
- Beck K, Eisner G, Trescher D, Dalbey RE, Brunner J, Müller M (2001) YidC, an assembly site for polytopic Escherichia coli membrane proteins located in immediate proximity to the SecYE translocon and lipids. EMBO Rep 2: 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier J-WL, Luirink J (2001) Biogenesis of inner membrane proteins in Escherichia coli. Mol Microbiol 40: 314–322 [DOI] [PubMed] [Google Scholar]
- Hardesty B, Kramer G (2001) Folding of a nascent peptide on the ribosome. Prog Nucleic Acid Res Mol Biol 66: 41–66 [DOI] [PubMed] [Google Scholar]
- Heinrich SU, Rapoport TA (2003) Cooperation of transmembrane segments during the integration of a doublespanning protein into the ER membrane. EMBO J 22: 3654–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich SU, Mothes W, Brunner J, Rapoport TA (2000) The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell 102: 233–244 [DOI] [PubMed] [Google Scholar]
- Houben ENG, Scotti PA, Valent QA, Brunner J, de Gier J-WL, Oudega B, Luirink J (2000) Nascent Lep inserts into the Escherichia coli inner membrane in the vicinity of YidC, SecY and SecA. FEBS Lett 476: 229–233 [DOI] [PubMed] [Google Scholar]
- Houben ENG, Urbanus ML, van der Laan M, ten Hagen-Jongman CM, Driessen AJ, Brunner J, Oudega B, Luirink J (2002) YidC and SecY mediate membrane insertion of a Type I transmembrane domain. J Biol Chem 277: 35880–35886 [DOI] [PubMed] [Google Scholar]
- Liao S, Lin J, Do H, Johnson AE (1997) Both lumenal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell 90: 31–41 [DOI] [PubMed] [Google Scholar]
- McCormick PJ, Miao Y, Shao Y, Lin J, Johnson AE (2003) Cotranslational protein integration into the ER membrane is mediated by the binding of nascent chains to translocon proteins. Mol Cell 12: 329–341 [DOI] [PubMed] [Google Scholar]
- Mori H, Ito K (2001) The Sec protein-translocation pathway. Trends Microbiol 9: 494–500 [DOI] [PubMed] [Google Scholar]
- Mothes W, Heinrich SU, Graf R, Nilsson I, von Heijne G, Brunner J, Rapoport TA (1997) Molecular mechanism of membrane protein integration into the endoplasmic reticulum. Cell 89: 523–533 [DOI] [PubMed] [Google Scholar]
- Prinz A, Behrens C, Rapoport TA, Hartmann E, Kalies KU (2000) Evolutionarily conserved binding of ribosomes to the translocation channel via the large ribosomal RNA. EMBO J 19: 1900–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson JC, Chen M, Jiang F, Möller I, Wiedmann M, Kuhn A, Phillips GJ, Dalbey RE (2000) YidC mediates membrane protein insertion in bacteria. Nature 406: 637–641 [DOI] [PubMed] [Google Scholar]
- Scotti PA, Urbanus ML, Brunner J, de Gier J-WL, von Heijne G, van der Does C, Driessen AJM, Oudega B, Luirink J (2000) YidC, the Escherichia coli homologue of motochondrial Oxa1p, is a component of the Sec translocase. EMBO J 19: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B, Clemons WM Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA (2004) X-ray structure of a protein-conducting channel. Nature 427: 36–44 [DOI] [PubMed] [Google Scholar]