Abstract
Protein palmitoylation or, more specifically, S-acylation is a reversible post-translational lipid modification. Despite the identification of several proteins that are altered in this way, our understanding of the enzymology of this process has been hampered by the lack of well-characterized acyltransferases. We now know of three proteins in Saccharomyces cerevisiae that promote palmitoylation: effector of Ras function (Erf2), ankyrin-repeat-containing protein (Akr1) and the SNARE protein Ykt6. Erf2 and Akr1 are integral membrane proteins that contain a cysteine-rich domain and an Asp-His-His-Cys motif, both of which catalyse acylation at the carboxyl terminus of their target proteins. Recently, we discovered that Ykt6 mediates the amino-terminal acylation of the fusion protein Vac8. Even though these three proteins differ in sequence, topology, size and substrate specificity, they might function in a similar manner. In this review, we discuss these observations in the context of a potential general mechanism of acylation.
Keywords: autoacylation, DHHC proteins, palmitoylation, S-acylation, Ykt6
Introduction
Lipid modifications are required for the membrane targeting of proteins and for their enrichment in microdomains on organelles (Resh, 1999; Berthiaume, 2002; Hancock, 2003). Protein palmitoylation is the addition of fatty acids (mainly palmitic acid) through an N-amide bond or a thioester (S-acylation). S-acylation is unique in that it is the only reversible lipid modification (Linder & Deschenes, 2003; Smothrys & Linder, 2004). In this review, we use the term palmitoylation exclusively to describe S-acylation.
Similar to other lipid modifications, protein palmitoylation is thought to be an enzymatic reaction, which is mediated by a protein that is known as a palmitoyltransferase (PAT) or an acyltransferase. However, the existence of such proteins has long been questioned for several reasons: first, although biochemical studies were successful in enriching PAT activity, they either failed to identify the respective protein (Kasinathan et al, 1990; Berthiaume & Resh, 1995; Das et al, 1997) or identified false positives (Liu et al, 1996); second, palmitoylated proteins have no clear consensus sequence for the modification—the common denominator for most palmitoylated proteins is a membrane-targeting sequence in the vicinity of the target cysteines that consists of positive charges, adjacent lipid anchors or transmembrane domains (Bijlmakers & Marsh, 2003); third, proteins containing a target cysteine can be autoacylated in vitro in the presence of palmitoyl-CoA (Pal-CoA; Duncan & Gilman, 1996; Veit et al, 1998; Veit, 2000; Bizzozero et al, 2001). The spontaneous nature of this modification raised doubts as to whether an enzyme was required by cells. However, during the past two years, three putative acyltransferases have been identified in the yeast Saccharomyces cerevisiae: two proteins with a conserved Asp-His-His-Cys (DHHC) motif—effector of Ras protein (Erf2) and ankyrin-repeat-containing protein (Akr1)—and the SNARE protein Ykt6 (Table 1; Lobo et al, 2002; Roth et al, 2002; Dietrich et al, 2004). In this review, we compare the recent findings on these acyltransferases and discuss a potential general mechanism of acylation.
Table 1.
Overview of characterized PATs and their substrates
| Palmitoyl transfer protein |
Substrate |
|||||
|---|---|---|---|---|---|---|
| Name | Localization | Anchored by | Domain | Name | Target cysteines | Reference |
| Akr1 | Golgi | 4–5 transmembrane domains | DHHC–CRD | Yck2 | —CC | Roth et al, 2002 |
| Erf2 | Endoplasmic reticulum | 4–5 transmembrane domains | DHHC–CRD | Ras2 | —C...CIIS | Lobo et al, 2002 |
| Ykt6 | Ubiquitous | Farnesylated Palmitoylated | Longin domain | Vac8 | MGSCCSC— | Dietrich et al, 2004 |
Akr1, ankyrin repeat-containing protein 1; CRD, cysteine-rich domain; DHHC, an Asp-His-His-Cys motif that is found in many putative acyltransferases; Erf2, eukaryotic peptide chain-release factor GTP-binding subunit 2; Ras2, Ras-like protein 2; Vac8, vacuolar protein 8; Yck2, yeast casein kinase 2.
Palmitoyltransferases in yeast
The DHHC proteins. The identification of Erf2 and Akr1 as acyltransferases brought into focus a family of proteins with a DHHC consensus sequence and a cysteine-rich domain (CRD; Lobo et al, 2002; Roth et al, 2002). Both Erf2 and Akr1 are polytopic membrane proteins with four predicted transmembrane domains. The traits that identify these proteins as acyltransferases are summarized below.
In mammalian cells, two members of the RAS superfamily, N-RAS and H-RAS, are farnesylated and require palmitoylation for their localization and function at the plasma membrane. Yeast has two redundant Ras homologues: Ras-like protein 1 (Ras1) and Ras2. Deschenes and colleagues identified the proteins Erf2 and Erf4 as necessary for the targeting of Ras2 to membranes (Bartels et al, 1999). Both proteins seemed to be involved in Ras2 palmitoylation, as this modification was suppressed in erf2- and erf4-deletion mutants (Bartels et al, 1999). Erf2 is a transmembrane protein that recruits Erf4 to the endoplasmic reticulum (Zhao et al, 2002). Subsequent purification of the Erf2/4 complex from yeast and Escherichia coli showed that it has acyltransferase activity for farnesylated yeast Ras2 (Lobo et al, 2002). Erf2 contains a DHHC box, and mutations in this sequence inhibit the in vitro palmitoylation activity of Erf2/4 and interfere with Ras2 palmitoylation in vivo. These findings indicate that Erf2 might act as the acyltransferase. Erf2 mediates palmitoylation in a substratespecific manner: mammalian H-RAS and the amino (N)-terminal Src-homology 4 (SH4) domain of Gαi are not acylated by Erf2 in vitro. Less specificity is observed for the lipid moiety, with palmitate (C16) and oleate (C18) being preferred over myristate (C14) and shorter length CoA derivatives (Lobo et al, 2002). The specificity of Erf2 for Ras2 is, at least in part, determined by lysine residues that are upstream of the target cysteine (Dong et al, 2003).
The second DHHC protein, Akr1, was initially identified as being required for plasma-membrane targeting of two casein kinase I homologues: Yck1 and Yck2 (Feng & Davis, 2000). Both of these proteins have a carboxy (C)-terminal CCsequence and were initially thought to be prenylated (Feng & Davis, 2000). However, Davis and co-workers showed that the Yck2 C-terminus is, in fact, palmitoylated in an Akr1-dependent manner (Roth et al, 2002). Akr1 was shown to palmitoylate Yck2 at the Golgi, which then moved to the plasma membrane by vesicular transport (Babu et al, 2004). Purified Akr1 from yeast promoted the palmitoylation of both Yck2 and itself; the self-acylation was independent of substrate palmitoylation as it remained unaltered irrespective of whether Yck2 was added to the reaction, and both activities were lost on mutating the DHHC box of Akr1. Interestingly, in vitro acylation was stimulated by ATP, although the reason for this is unclear. Davis and colleagues noted that Akr1 has no clear consensus sequence for ATP binding and other nucleotide analogues were not tested in the reaction. It therefore remains to be seen whether ATP is a specific effector in the Akr1-mediated reaction.
These examples could mark the DHHC box as a motif of palmitoyltransferases. Indeed, further DHHC proteins have recently been identified and implicated in protein palmitoylation. In neuronal cells, the Golgi-specific DHHC zinc-finger protein (GODZ) is required for the palmitoylation of the γ2-subunit of the GABAA receptor (Keller et al, 2004). In addition to Erf2 and Akr1, five DHHC proteins have been identified through sequence homology in yeast (Linder & Deschenes, 2003). One of these, the hypothetical zinc-finger membrane protein Ynl326c, has been localized to the vacuoles (Huh et al, 2003) to which palmitoylation activity has also previously been mapped (Veit et al, 2003). However, identifying the precise role of each of these DHHC proteins and their substrate specificities will require further studies.
The longin Ykt6. It has been known for some time that Pal-CoA stimulates yeast vacuole fusion (Haas & Wickner, 1996), which indicates a requirement for protein palmitoylation. The fusion factor Vac8 was subsequently identified as a target of palmitoylation on the yeast vacuole (Veit et al, 2001; Wang et al, 2001). Vac8 contains an N-terminal SH4 domain that is myristoylated at a glycine residue and is palmitoylated at up to three cysteine residues (Fleckenstein et al, 1998; Pan & Goldfarb, 1998; Wang et al, 1998). Palmitoylation of Vac8 occurs during an early stage of in vitro vacuole fusion and is required for completion of the process (Veit et al, 2001; Wang et al, 2001). We showed recently that Ykt6 is required for the acylation of Vac8 (Dietrich et al, 2004). Ykt6 is a highly conserved SNARE (Tochio et al, 2001) that consists of three domains: an N-terminal longin domain, which is a tightly folded domain that is found in a subset of v/RsNAREs; the coiled-coil/SNARE domain; and a farnesylation consensus sequence at the C-terminus (Filippini et al, 2001). Sequence comparison indicates that the longin domain has some similarity to the human β-ketoacyl synthase, which is a subunit of the fatty-acid synthase that conjugates acetyl-CoA and malonyl-CoA. Consistent with this finding, the Ykt6 longin domain is able to bind Pal-CoA, CoA or palmitate. Antibodies to the longin domain inhibit both fusion and palmitoylation (Dietrich et al, 2004); we therefore reasoned that Ykt6 could function in the palmitoylation reaction. This theory was verified by palmitoylating recombinant Vac8 in vitro in the presence of recombinant Ykt6 or its longin domain, all of which were purified from E. coli. Surprisingly, palmitoylation required a roughly equimolar ratio of Vac8 and Ykt6. This finding is not consistent with a typical catalytic mechanism, in which the enzyme can be reused in several reactions. Interestingly, Akr1- and Erf2-mediated protein acylation were also performed at equimolar concentrations of substrate and enzyme (Lobo et al, 2002; Roth et al, 2002). The following section offers a possible explanation for these observations in the context of mechanistic studies on protein palmitoylation.
An integrated model for protein palmitoylation
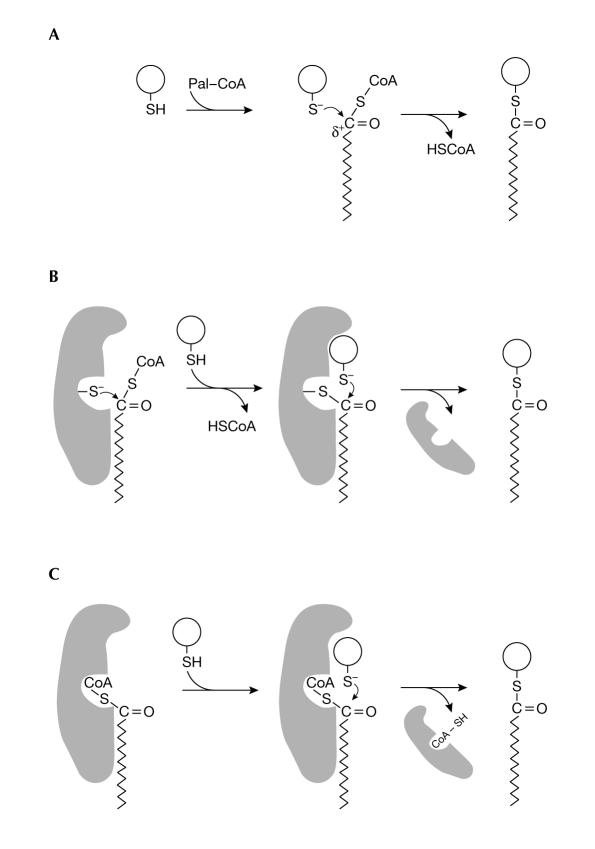
It has been proposed that acyltransferases might act as enzymes that recognize specific sequences on proteins and transfer lipids to the target cysteine. This reaction is thought to occur through a thioester intermediate, which is similar to known acyltransferases in lipid metabolism (Fig 1B). However, research on protein palmitoylation has been plagued by an inherent property of the target proteins: under appropriate conditions, they can be autopalmitoylated in the absence of an apparent enzyme (Duncan & Gilman, 1996; Veit et al, 1998; Dunphy et al, 2000; Veit, 2000; also reviewed by Bijlmakers & Marsh, 2002; Smotrys & Linder, 2004). Autoacylation of purified proteins occurs at the same specific sites as in the presumably enzyme-mediated reaction in vivo, albeit at a slower rate (Leventis et al, 1997; Bano et al, 1998; Veit et al, 1998; Dunphy et al, 2000). This has led to the speculation that protein palmitoylation is not necessarily enzyme mediated (Bano et al, 1998; Bizzozero et al, 2001). Consistent with this theory, Bizzozero and colleagues noted that, under appropriate conditions, the activation energy that is required for transferring palmitate from Pal-CoA to a peptide is only one-fifth of the energy that is required for enzyme-catalysed acyl-transfer reactions (Bharadwaj & Bizzozero, 1995). Therefore, palmitoylation can occur spontaneously. The crucial aspect of this transfer reaction is the formation of a reduced deprotonated cysteine (a thiolate) as the target for palmitate (Bizzozero et al, 2001). The thiolate anion can then act as a nucleophile on the thioester bond in Pal-CoA to catalyse the generation of the palmitoylated protein (Fig 1A).
Figure 1.
Potential mechanisms of palmitoylation. (A) Basic mechanism. The reduced sulphydryl group on the target protein is deprotonated to form a thiolate. The thioester bond between the protein and palmitate is formed as a consequence of a nucleophilic attack of the thiolate on the α-carbon of palmitoyl-CoA (Pal-CoA). (B) Formation of a thioester intermediate. The palmitoyltransferase forms a thioester intermediate with palmitate, then binds to a target protein and catalyses the transfer of palmitate. For simplicity, the targeting of the substrate protein to the membrane is not shown. (C) Transfer protein-assisted palmitoylation. The Pal transfer protein binds to CoA or Pal-CoA through a binding pocket and presents Pal-CoA to a target protein, which might then bind to the transfer protein. Nucleophilic attack of the thiolate of the target protein on the α-carbon of bound Pal-CoA allows the formation of a thioester bond. After this reaction, the CoA might remain bound to the transfer protein.
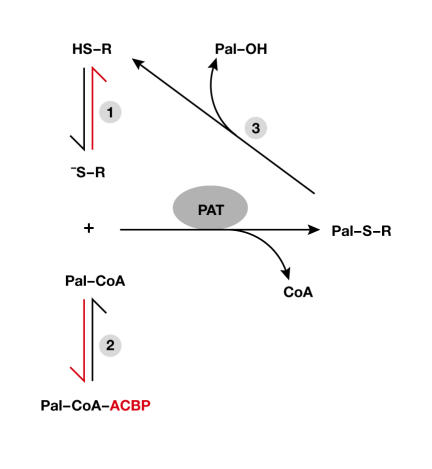
If autoacylation is indeed the basis for S-acylation, this raises the question of how the spontaneous transfer of palmitate to target proteins is regulated in cells. Control might occur at two levels: the formation of the thiolate and the Pal-CoA availability (Fig 2).
Figure 2.
Regulation of palmitoylation: thiolate formation and the availability of palmitoyl-CoA. (1) Thiolate formation on the substrate protein (R) could be counteracted by changes in the redox environment; that is, the formation of disulphides or oxidation of the sulphydryl group. (2) The available palmitoyl-CoA (Pal-CoA) pool is buffered by the acyl-CoA binding protein (ACBP). (3) Pal transfer protein (PAT)-mediated protein palmitoylation is counteracted by a thioesterase.
Focusing on the first level, what is known about the formation of thiolates? The sulphydryl group of a cysteine has an ionization constant pKA of 8.5, which makes the formation of a thiolate under the cytosolic conditions of the cell (pH 7.2–7.4) unlikely. However, in the context of a peptide or a protein, proximal polar or charged side chains can notably modulate the pKa of a cysteine and, therefore, its potential to form a thiolate. Indeed, the pKa of a cysteine can be reduced by as much as six orders of magnitude to a value of 3 (Mossner et al, 2000), which makes the formation of thiolates highly likely. The dependence of the pKa on the position of a cysteine in a protein could also explain the peculiar observation that autoacylation targets the same cysteines that are acylated in vivo (O'Brien et al, 1987; Quesnel & Silvius, 1994; Bharadwaj & Bizzozero, 1995; Schroeder et al, 1996, 1997; Bano et al, 1998).
It is tempting to speculate that, similar to the modulation of cysteines by intramolecular effects, protein–protein interactions could affect the pKa of a cysteine in trans. In this context, it is interesting to note that the SNARE SNAP25 is acylated approximately 100-fold more efficiently when it is bound to the SNARE syntaxin (Veit, 2000) and that Gα palmitoylation is favoured in the presence of Gβγ (Duncan & Gilman, 1996). It is possible that interaction with a protein partner might influence protein stability and, therefore, the accessibility of the sulphydryl group, or it might change the local pKa of the target cysteine. It is also noteworthy that not only palmitate (C16) but also palmitoleate (C16:1), stearate (C18) and oleate (C18:1) have been found on target cysteines (Hallak et al, 1994; Schroeder et al, 1996; Liang et al, 2002, 2004). These findings indicate that the respective enzyme must be less specific in its substrate recognition in vivo than, for example, the cytosolic N-myristoyl transferase (Bhatnagar et al, 1997, 1998).
The second level at which spontaneous acylation might be regulated is the availability of Pal-CoA in the cell (Fig 2). Eukaryotic cells maintain a low free Pal-CoA concentration in the cytoplasm; the conserved acyl-CoA binding protein (ACBP) binds free Pal-CoA and maintains the intracellular concentration within the nanomolar range (Faergeman & Knudsen, 1997). This buffering effect of ACBP might be crucial in preventing uncontrolled palmitoylation. Consistent with this idea, ACBP has been used as a tool to quench autoacylation. Interestingly, PAT-mediated acylation is largely resistant to ACBP (Leventis et al, 1997; Dunphy et al, 2000), which indicates that a factor can compete with ACBP for Pal-CoA and then transfer the palmitate moiety to the target cysteine. For this reaction to occur, the factor does not necessarily need to form a thioester intermediate (Fig 1B). It would be sufficient to bind and present Pal-CoA to the thiolate of the target protein, thereby facilitating an efficient nucleophilic attack (transfer protein-assisted acylation; Fig 1C).
A crucial difference between the two models is the nature of palmitate binding to the PAT. Whereas a thioester intermediate constitutes a covalent interaction, a transfer protein might noncovalently associate with the palmitate and CoA moiety. After the transfer of palmitate, CoA could remain associated with the PAT, thereby preventing the incorporation of another Pal-CoA molecule for a further round of fusion. This is consistent with our observation that Ykt6 binds not only Pal-CoA but also CoA alone (Dietrich et al, 2004). Furthermore, the transfer-protein model would provide a possible explanation for our intriguing finding that, in vitro, Ykt6 is required in a 1:1 ratio with its substrate Vac8 for efficient acylation. In vivo, further control mechanisms might allow an efficient release of CoA from Ykt6.
In this context, it is notable that Erf2 and Akr1, even though they are assumed to be enzymes, have not been shown to act as such—kinetics and titrations have not been presented so far. The strongest arguments for their specificity are based on mutations in the DHHC box that inactivate the proteins (Lobo et al, 2002; Roth et al, 2002), which indicate that crucial amino acids might be involved in the formation of a potential thioester intermediate during the reaction. The autoacylation of DHHC proteins, as reported for Erf2 (Lobo et al, 2002), might point to such an intermediate. However, these residues and, consequently, autoacylation could also be required for protein stability, as the DHHC box has similarity to a zinc-finger domain. Taking into consideration the fact that the autoacylation of some proteins is strongly increased in the presence of their interaction partners (Duncan & Gilman, 1996; Veit, 2000), it is conceivable that the binding of Erf2 to Ras2, or of Akr1 to Yck2, might facilitate autoacylation.
These speculations on the regulation of protein palmitoylation are consistent with findings on another cysteine-based reversible protein modification: S-nitrosylation. This post-translational process is implicated in the control of several physiological reactions, such as smooth-muscle relaxation (Davis et al, 2001; Hess et al, 2001; Stamler et al, 2001; Boehning & Snyder, 2003). S-nitrosylation occurs on the sulphydryl groups of many target proteins and is not catalysed by enzymes. It might therefore be predicted that nitrosylation targets cysteine residues randomly (Davis et al, 2001). However, only certain cysteine side chains in target proteins are modified by S-nitrosylation (Lander et al, 1995; Xu et al, 1998; Sun et al, 2001). This indicates that the local environment of the target sequence controls the availability of sulphydryl groups (Hess et al, 2001), which is similar to our view of the availability of certain cysteines for palmitoylation. Interestingly, S-nitrosylation usually occurs in the vicinity of hydrophobic surfaces, either at membranes or in proteins (Stamler et al, 2001), and the autoacylation of peptides is facilitated under the same conditions (Quesnel & Silvius, 1994).
It is important to note that our discussion of S-acylation might not reflect the mechanism of palmitoylation for the signalling molecules Hedgehog and Wnt (reviewed by Linder & Deschenes, 2004; Mann & Beachy, 2004). The modification of these proteins occurs in the oxidizing environment of the Golgi and, at least for Hedgehog, results in an N-amide linkage (Mann & Beachy, 2004). It is therefore unlikely to reflect the mechanism of cytosolic S-acylation.
Our transfer protein-based model of S-acylation integrates several conflicting observations that have been made on the palmitoylation of proteins. It proposes that a PAT mediates specific acylation (consistent with an enzymatic mechanism) in a manner that is not dependent on a thioester intermediate (as predicted by studies on autoacylation). As an enzymatic function has not yet been shown, we propose that the protein that mediates this reaction should be known as a Pal transfer protein (and might therefore retain the acronym PAT).
We speculate that the specificity of palmitoylation is determined by the target sequence; that is, the formation of the thiolate anion. The function of a Pal transfer protein is to present Pal-CoA to the thiolate of the target protein. Ykt6 fulfils the criteria for such an activity: on vacuoles, Ykt6 is present in close proximity to Vac8 and is able to bind Pal-CoA through its longin domain (Dietrich et al, 2004). On the basis of our present knowledge, we consider it unlikely that Ykt6 contains an active site, and it remains possible that Erf2 and Akr1 act by a similar mechanism. As the activities of all three yeast PATs can be reconstituted in vitro, we are now in a good position to address directly many of the unresolved questions and to arrive at a general mechanism of protein palmitoylation.
Lars E.P. Dietrich & Christian Ungermann, who is the recipient of an EMBO Young Investigator Award
Acknowledgments
This work has been supported by the Nachwuchsgruppen in den Biowissenschaften (DFG; UN111/2-3), the SFB 638 Collaborative Research Centre, the Fonds der Chemischen Industrie, the EMBO Young Investigator Programme (C.U.) and a predoctoral fellowship from the Boehringer Ingelheim Fonds (L.E.P.D.).
References
- Babu P, Deschenes RJ, Robinson LC (2004) Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J Biol Chem 279: 27138–27147 [DOI] [PubMed] [Google Scholar]
- Bano MC, Jackson CS, Magee AI (1998) Pseudo-enzymatic S-acylation of myristoylated Yes protein tyrosine kinase peptide in vitro may reflect non-enzymatic S-acylation in vivo. Biochem J 330: 723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels DJ, Mitchell DA, Dong X, Deschenes RJ (1999) Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol Cell Biol 19: 6775–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume LG (2002) Insider information: how palmitoylation of Ras makes it a signaling double agent. Sci STKE 2002: PE41. [DOI] [PubMed] [Google Scholar]
- Berthiaume L, Resh MD (1995) Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem 270: 22399–22405 [DOI] [PubMed] [Google Scholar]
- Bharadwaj M, Bizzozero OA (1995) Myelin P0 glycoprotein and a synthetic peptide containing the palmitoylation site are both autoacylated. J Neurochem 65: 1805–1815 [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Schall OF, Jackson-Machelski E, Sikorski JA, Devadas B, Gokel GW, Gordon JI (1997) Titration calorimetric analysis of AcylCoA recognition by myristoylCoA:protein N-myristoyltransferase. Biochemistry 36: 6700–6708 [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Futterer K, Farazi TA, Korolev S, Murray CL, Jackson-Machelski E, Gokel GW, Gordon JI, Waksman G (1998) Structure of N-myristoyltransferase with bound myristoylCoA and peptide substrate analogs. Nat Struct Biol 5: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M (2003) The on–off story of protein palmitoylation. Trends Cell Biol 13: 32–42 [DOI] [PubMed] [Google Scholar]
- Bizzozero OA, Bixler HA, Pastuszyn A (2001) Structural determinants influencing the reaction of cysteine-containing peptides with palmitoyl-coenzyme A and other thioesters. Biochim Biophys Acta 1545: 278–288 [DOI] [PubMed] [Google Scholar]
- Boehning D, Snyder SH (2003) Novel neural modulators. Annu Rev Neurosci 26: 105–131 [DOI] [PubMed] [Google Scholar]
- Das AK, Dasgupta B, Bhattacharya R, Basu J (1997) Purification and biochemical characterization of a protein-palmitoyl acyltransferase from human erythrocytes. J Biol Chem 272: 11021–11025 [DOI] [PubMed] [Google Scholar]
- Davis KL, Martin E, Turko IV, Murad F (2001) Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol 41: 203–236 [DOI] [PubMed] [Google Scholar]
- Dietrich LE, Gurezka R, Veit M, Ungermann C (2004) The SNARE Ykt6 mediates protein palmitoylation during an early stage of homotypic vacuole fusion. EMBO J 23: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Mitchell DA, Lobo S, Zhao L, Bartels DJ, Deschenes RJ (2003) Palmitoylation and plasma membrane localization of Ras2p by a nonclassical trafficking pathway in Saccharomyces cerevisiae. Mol Cell Biol 23: 6574–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG (1996) Autoacylation of G protein α subunits. J Biol Chem 271: 23594–23600 [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Schroeder H, Leventis R, Greentree WK, Knudsen JK, Silvius JR, Linder ME (2000) Differential effects of acyl-CoA binding protein on enzymatic and non-enzymatic thioacylation of protein and peptide substrates. Biochim Biophys Acta 1485: 185–198 [DOI] [PubMed] [Google Scholar]
- Faergeman NJ, Knudsen J (1997) Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J 323: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Davis NG (2000) Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol Cell Biol 20: 5350–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini F, Rossi V, Galli T, Budillon A, D'Urso M, D'Esposito M (2001) Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci 26: 407–409 [DOI] [PubMed] [Google Scholar]
- Fleckenstein D, Rohde M, Klionsky DJ, Rudiger M (1998) Yel013p (Vac8p), an armadillo repeat protein related to plakoglobin and importin-α is associated with the yeast vacuole membrane. J Cell Sci 111: 3109–3118 [DOI] [PubMed] [Google Scholar]
- Haas A, Wickner W (1996) Homotypic vacuole fusion requires Sec17p (yeast αsNAP) and Sec18p (yeast NSF). EMBO J 15: 3296–3305 [PMC free article] [PubMed] [Google Scholar]
- Hallak H, Muszbek L, Laposata M, Belmonte E, Brass LF, Manning DR (1994) Covalent binding of arachidonate to G protein αsubunits of human platelets. J Biol Chem 269: 4713–4716 [PubMed] [Google Scholar]
- Hancock JF (2003) Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol 4: 373–384 [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Nudelman R, Stamler JS (2001) S-nitrosylation: spectrum and specificity. Nat Cell Biol 3: E46–E49 [DOI] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425: 686–691 [DOI] [PubMed] [Google Scholar]
- Kasinathan C, Grzelinska E, Okazaki K, Slomiany BL, Slomiany A (1990) Purification of protein fatty acyltransferase and determination of its distribution and topology. J Biol Chem 265: 5139–5144 [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panazanelli P, Martin ML, Alldred M, Sassoè-Pognetto M, Lüscher B (2004) The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J Neurosci 24: 5881–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander HM, Ogiste JS, Pearce SF, Levi R, Novogrodsky A (1995) Nitric oxidestimulated guanine nucleotide exchange on p21ras. J Biol Chem 270: 7017–7020 [DOI] [PubMed] [Google Scholar]
- Leventis R, Juel G, Knudsen JK, Silvius JR (1997) Acyl-CoA binding proteins inhibit the nonenzymic S-acylation of cysteinyl-containing peptide sequences by long-chain acyl-CoAs. Biochemistry 36: 5546–5553 [DOI] [PubMed] [Google Scholar]
- Liang X, Lu Y, Neubert TA, Resh MD (2002) Mass spectrometric analysis of GAP-43/neuromodulin reveals the presence of a variety of fatty acylated species. J Biol Chem 277: 33032–33040 [DOI] [PubMed] [Google Scholar]
- Liang X, Lu Y, Wilkes M, Neubert TA, Resh MD (2004) The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J Biol Chem 279: 8133–8139 [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ (2003) New insights into the mechanisms of protein palmitoylation. Biochemistry 42: 4311–4320 [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ (2004) Model organisms lead the way to protein palmitoylation. J Cell Sci 117: 521–526 [DOI] [PubMed] [Google Scholar]
- Liu L, Dudler T, Gelb MH (1996) Purification of a protein palmitoyltransferase that acts on H-Ras protein and on a C-terminal N-Ras peptide. J Biol Chem 271: 23269–23276; erratum in J Biol Chem (1999) 274: 3252 [DOI] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ (2002) Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem 277: 41268–41273 [DOI] [PubMed] [Google Scholar]
- Mann RK, Beachy PA (2004) Novel lipid modifications of secreted protein signals. Annu Rev Biochem 73: 891–923 [DOI] [PubMed] [Google Scholar]
- Mossner E, Iwai H, Glockshuber R (2000) Influence of the pK(a) value of the buried, activesite cysteine on the redox properties of thioredoxin-like oxidoreductases. FEBS Lett 477: 21–26 [DOI] [PubMed] [Google Scholar]
- O'Brien PJ, St Jules RS, Reddy TS, Bazan NG, Zatz M (1987) Acylation of disc membrane rhodopsin may be nonenzymatic. J Biol Chem 262: 5210–5215 [PubMed] [Google Scholar]
- Pan X, Goldfarb DS (1998) YEB3/VAC8 encodes a myristylated armadillo protein of the Saccharomyces cerevisiae vacuolar membrane that functions in vacuole fusion and inheritance. J Cell Sci 111: 2137–2147 [DOI] [PubMed] [Google Scholar]
- Quesnel S, Silvius JR (1994) Cysteine-containing peptide sequences exhibit facile uncatalyzed transacylation and acyl-CoA-dependent acylation at the lipid bilayer interface. Biochemistry 33: 13340–13348 [DOI] [PubMed] [Google Scholar]
- Resh MD (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451: 1–16 [DOI] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG (2002) The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 159: 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H, Leventis R, Shahinian S, Walton PA, Silvius JR (1996) Lipid-modified, cysteinyl-containing peptides of diverse structures are efficiently S-acylated at the plasma membrane of mammalian cells. J Cell Biol 134: 647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H, Leventis R, Rex S, Schelhaas M, Nagele E, Waldmann H, Silvius JR (1997) S-acylation and plasma membrane targeting of the farnesylated carboxyl-terminal peptide of N-RAS in mammalian fibroblasts. Biochemistry 36: 13102–13109 [DOI] [PubMed] [Google Scholar]
- Smotrys JE, Linder ME (2004) Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem 73: 559–587 [DOI] [PubMed] [Google Scholar]
- Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106: 675–683 [DOI] [PubMed] [Google Scholar]
- Sun J, Xin C, Eu JP, Stamler JS, Meissner G (2001) Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci USA 98: 11158–11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochio H, Tsui MM, Banfield DK, Zhang M (2001) An autoinhibitory mechanism for nonsyntaxin SNARE proteins revealed by the structure of Ykt6p. Science 293: 698–702 [DOI] [PubMed] [Google Scholar]
- Veit M (2000) Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem J 345: 145–151 [PMC free article] [PubMed] [Google Scholar]
- Veit M, Sachs K, Heckelmann M, Maretzki D, Hofmann KP, Schmidt MF (1998) Palmitoylation of rhodopsin with S-protein acyltransferase: enzyme catalyzed reaction versus autocatalytic acylation. Biochim Biophys Acta 1394: 90–98; erratum in (1999) 1436: 630 [DOI] [PubMed] [Google Scholar]
- Veit M, Laage R, Dietrich L, Wang L, Ungermann C (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J 20: 3145–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Dietrich LEP, Ungermann C (2003) Biochemical characterization of the vacuolar palmitoyl acyltransferase. FEBS Lett 540: 101–105 [DOI] [PubMed] [Google Scholar]
- Wang YX, Catlett NL, Weisman LS (1998) Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J Cell Biol 140: 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Kauffman EJ, Duex JE, Weisman LS (2001) Fusion of docked membranes requires the armadillo repeat protein Vac8p. J Biol Chem 276: 35133–35140 [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279: 234–237 [DOI] [PubMed] [Google Scholar]
- Zhao L, Lobo S, Dong X, Ault AD, Deschenes RJ (2002) Erf4p and Erf2p form an endoplasmic reticulum-associated complex involved in the plasma membrane localization of yeast Ras proteins. J Biol Chem 277: 49352–49359 [DOI] [PubMed] [Google Scholar]