Abstract
Botulinum neurotoxins (BoNTs) block neurotransmitter release through their specific proteolysis of the proteins responsible for vesicle exocytosis. Paradoxically, two serotypes of BoNTs, A and E, cleave the same molecule, synaptosome-associated protein with relative molecular mass 25K (SNAP-25), and yet they cause synaptic blockade with very different properties. Here we compared the action of BoNTs A and E on the plasma membrane fusion machinery composed of syntaxin and SNAP-25. We now show that the BoNT/A-cleaved SNAP-25 maintains its association with two syntaxin isoforms in vitro, which is mirrored by retention of SNAP-25 on the plasma membrane in vivo. In contrast, BoNT/E severely compromises the ability of SNAP-25 to bind the plasma membrane syntaxin isoforms, leading to dissociation of SNAP-25. The distinct properties of botulinum intoxication, therefore, can result from the ability of shortened SNAP-25 to maintain its association with syntaxins—in the case of BoNT/A poisoning resulting in unproductive syntaxin/SNAP-25 complexes that impede vesicle exocytosis.
Keywords: botulinum toxin, syntaxin, SNAP-25, SNARE, PC12 cells
Introduction
Botulinum neurotoxins (BoNTs) are produced by Clostridium botulinum and consist of two functionally distinct subunits (Rossetto et al, 2004). The heavy chain is responsible for neuronal targeting, whereas the smaller, light chain acts as a proteolytic enzyme. The light chains of BoNTs (BoNT LCs) specifically cleave the proteins responsible for the fusion of synaptic vesicles with the plasma membrane: synaptobrevin, syntaxin and synaptosome-associated protein with relative molecular mass 25K (SNAP-25; Schiavo et al, 2000). The resulting inability of vesicles to fuse with the plasma membrane causes the blockade of neurotransmission. The severity and duration of BoNT intoxication vary widely among the seven known serotypes, labelled A–G in accordance with the chronological order of their discovery. Especially intriguing are BoNT/A and BoNT/E, the former being widely used in medicine due to its ability to provide long-lasting blockade of neurotransmission (Johnson, 1999; Rossetto et al, 2004). These two neurotoxins proteolyse SNAP-25: BoNT/A removes nine amino-acid residues from the carboxyl terminus, whereas BoNT/E removes 26 C-terminal amino-acid residues (Schiavo et al, 1993; Binz et al, 1994). As BoNT/A and BoNT/E cleave the same molecule, SNAP-25, it would be intuitive to predict that these BoNTs have identical effects on neurotransmitter release. However, the blockade of exocytosis is less severe in the case of BoNT/A and manipulations such as high calcium concentrations can overcome the damaging effects of BoNT/A but not BoNT/E intoxication (Banerjee et al, 1996; Huang et al, 2001). BoNT/A poisoning is characterized by persistence of the BoNT/A-cleaved SNAP-25 in presynaptic endings, whereas the BoNT/E-cleaved molecule is cleared and replaced by intact SNAP-25 relatively quickly, suggesting that SNAP-25 retention contributes to the difference in the properties of intoxication by these neurotoxins (Eleopra et al, 1998; Keller & Neale, 2001; Foran et al, 2003; Meunier et al, 2003).
In this study, we sought to determine the molecular basis for the differential retention of SNAP-25 on the plasma membrane following intoxication by botulinum toxins. Syntaxin1 is believed to be the main interacting partner for SNAP-25 on the plasma membrane of neuroendocrine cells (Chen et al, 1999; Vogel et al, 2000; Washbourne et al, 2001). This is based on several independent lines of evidence including colocalization of syntaxin1 with SNAP-25 on the plasma membrane (Ohara-Imaizumi et al, 2004; Rickman et al, 2004) and stoichiometric co-immunoprecipitation of the two proteins from neuronal tissues (McMahon et al, 1995; Rickman & Davletov, 2003). Indeed, on immunoprecipitation of all SNAP-25 from the brain, only syntaxin1 shows robust enrichment among molecules reported to bind monomeric SNAP-25, whereas, for example, endosomal Hrs-2 is not readily detectable (Tsujimoto et al, 1999; Hu et al, 2002). Importantly, in cell lines lacking syntaxin1, SNAP-25 accumulates in the cytosol before being cleared by intracellular proteases on a timescale of hours (Vogel et al, 2000). If, however, syntaxin is present on the plasma membrane, then SNAP-25 accumulates at the plasma membrane (Vogel et al, 2000; Washbourne et al, 2001).
Syntaxin1, being both a plasma membrane protein and capable of high-affinity SNAP-25 binding (Rickman et al, 2004), would be a natural candidate to account for the differential SNAP-25 retention in the cases of BoNT/A and BoNT/E intoxication; however, this has not yet been demonstrated. We recently showed that BoNT/E has the ability not only to cleave SNAP-25 but also to disrupt the syntaxin1/SNAP-25 dimers at the plasma membrane of chromaffin cells (Rickman et al, 2004). We now tested whether, in the case of BoNT/A-cleaved SNAP-25, the SNAP-25 molecule can maintain its association with syntaxin1. Our study provides the first direct evidence that the ability of SNAP-25 to interact not only with syntaxin1 but also syntaxin2 mirrors the differential SNAP-25 retention at the plasma membrane during BoNT intoxication.
Results And Discussion
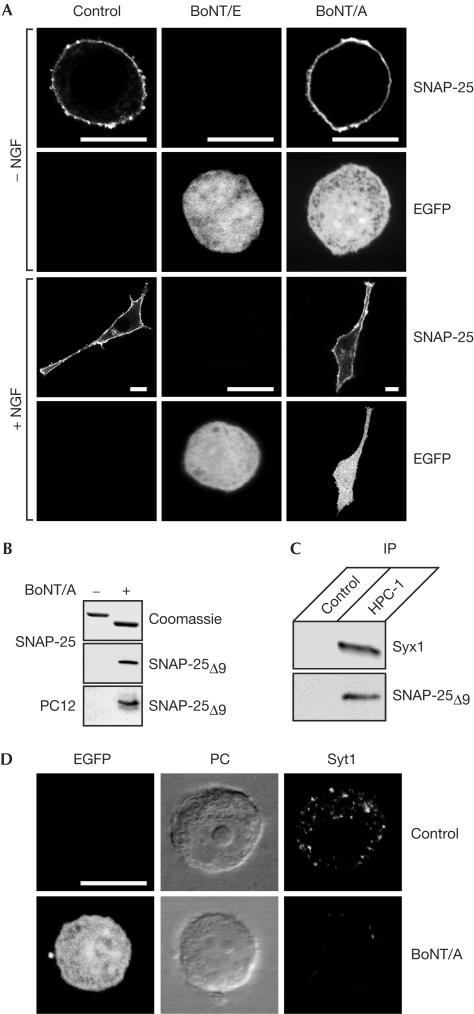
To study the effects of BoNT/A and BoNT/E in vivo, we used PC12 cells, a popular model for neuronal exocytosis, transfected with plasmids carrying the corresponding light chains (LCs) and a fluorescent marker protein enhanced green fluorescent protein (EGFP). This approach ensures a potent blockade of exocytosis and allows identification of transfected cells (Aguado et al, 1997; Graham et al, 2000). At 2 days after transfection, cells were immunostained with an amino-terminal antisNAP-25 antibody. In good agreement with the behaviour of SNAP-25 in neuromuscular junctions (Raciborska et al, 1998; Meunier et al, 2003), SNAP-25 was cleared from the plasma membrane on expression of BoNT/E LCs but not BoNT/A LCs (Fig 1A). A similar effect on SNAP-25 retention was observed with BoNT LC-transfected cells treated with nerve growth factor (NGF), which normally induces neurite extensions (Fig 1A). In agreement with previous reports (Morihara et al, 1999; Martinez-Arca et al, 2000), PC12 cells expressing BoNT/E LCs failed to grow neurites, whereas neurite extension was still observable on expression of BoNT/A LCs. As studies of vesicle exocytosis use PC12 cells without NGF treatment (Banerjee et al, 1996; Chen et al, 1999) and as the differential retention of SNAP-25 on the plasma membrane is evident in nontreated cells (Fig 1A), we conducted all further experiments without using NGF treatment. To prove that the expressed BoNT/A was active, we used an antibody that specifically recognizes the BoNT/A-cleaved end of SNAP-25 (Meunier et al, 2003). This rabbit antibody, developed against a short SNAP-25 peptide, detected BoNT/A-cleaved SNAP-25 (SNAP-25Δ9) in transfected cells (Fig 1B), confirming the proteolytic ability of the expressed LCs. To analyse whether the cleaved SNAP-25 is capable of interaction with syntaxin1, we performed immunoprecipitation from a PC12 cell extract using a monoclonal antisyntaxin1 antibody and analysed the bound material using rabbit antibodies against both syntaxin1 and SNAP-25Δ9. Fig 1C shows that syntaxin1 was able to bind the BoNT/A-cleaved SNAP-25. Next, we examined the blockade of exocytosis by following the uptake of an antibody that recognizes the intravesicular part of synaptotagmin1 (Angaut-Petit et al, 1995). Whereas control, nontransfected cells showed a high level of uptake of the intravesicular antibody, transfection with BoNT/A LCs inhibited vesicle cycling, as evidenced by a significant decrease in the uptake of the antibody (Fig 1D). This is in agreement with the observations that neuroendocrine cells expressing BoNT/A LCs lose their ability to support exocytosis (Aguado et al, 1997; Huang et al, 2001).
Figure 1.
SNAP-25 is retained at the plasma membrane of PC12 cells on expression of BoNT/A LCs but not BoNT/E LCs. (A) PC12 cells were transfected with BoNT LCs in the presence of pEGFP-C1 and grown without (−) or with (+) NGF. Cells were immunostained using a polyclonal antisNAP-25 antibody. (B) BoNT/A LC cleaves SNAP-25 in transfected PC12 cells. Recombinant SNAP-25 before and after cleavage by BoNT/A LCs was analysed by Coomassie staining and immunoblotting. The antibody specifically recognizes BoNT/A-cleaved SNAP-25 (SNAP-25Δ9) and detects the cleavage product in BoNT/A LC-transfected PC12 cells. (C) Syntaxin1's ability to bind SNAP-25Δ9 demonstrated by immunoprecipitation (IP) of syntaxin1 from the PC12 extract using a monoclonal HPC-1 antibody. Proteins bound to protein G beads without (control) or with HPC-1 antibody were analysed by western immunoblotting using rabbit antibodies against syntaxin1 and SNAP-25Δ9. (D) BoNT/A LC-transfected PC12 cells show diminished exocytosis/endocytosis vesicle cycling in the presence of 55 mM KCl as measured by anti-synaptotagmin1 (Syt1) luminal antibody uptake. Phase contrast (PC) and fluorescence (EGFP) images of the cells are also shown. Scale bars, 10 μm.
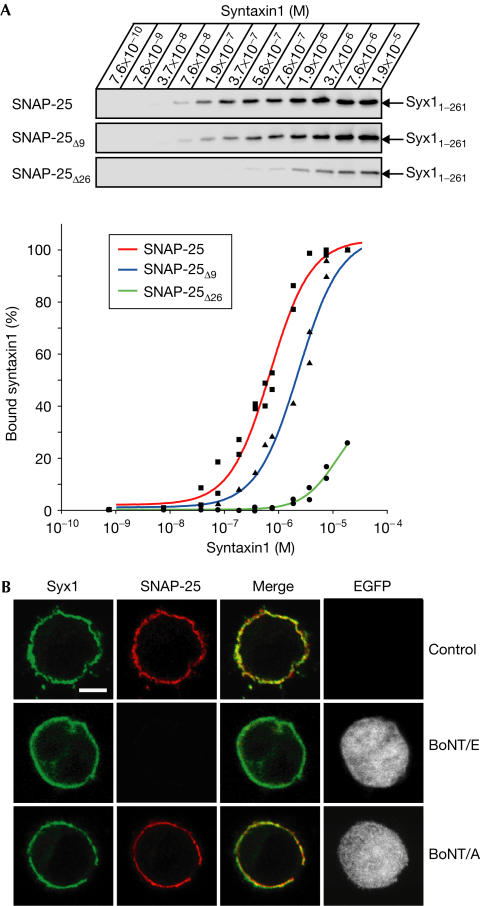
To analyse possible differences in the affinities of syntaxin1 for BoNT-cleaved versions of SNAP-25 in vitro, we immobilized 10 ng of GST–SNAP-25B on Sepharose beads and, following BoNT cleavage, added increasing amounts of syntaxin1 ranging from 3 ng to 70 μg in a 100 μl reaction. The binding of syntaxin1 showed saturable binding to SNAP-25Δ9, whereas the binding to BoNT/E-cleaved SNAP-25 (SNAP-25Δ26) was so weak that even at high syntaxin1 concentrations only a small fraction was able to bind (Fig 2A). Thus, syntaxin1 can interact with SNAP-25Δ9 at nano- and micromolar concentrations, whereas BoNT/E cleavage of SNAP-25 severely compromises its ability to associate with syntaxin1.
Figure 2.
SNAP-25 interaction with syntaxin1 persists after cleavage by BoNT/A LCs but not by BoNT/E LCs. (A) Increasing concentrations of syntaxin1 (Syx1–261) were incubated with either GST–SNAP-25, SNAP-25Δ9 or BoNT/E-cleaved GST–SNAP-25 (SNAP-25Δ26). The immunoblot for syntaxin1 and the quantification of the syntaxin1 signals plotted as graphs are shown. (B) Control PC12 cells or cells transfected with either BoNT/E or BoNT/A were immunostained for syntaxin1 (Syx1) and SNAP-25. Scale bar, 5 μm.
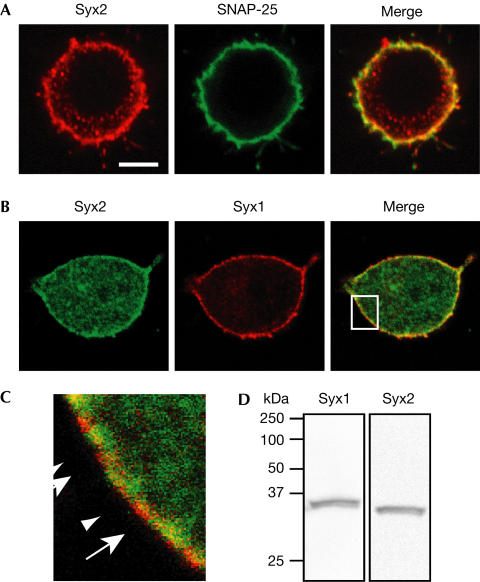
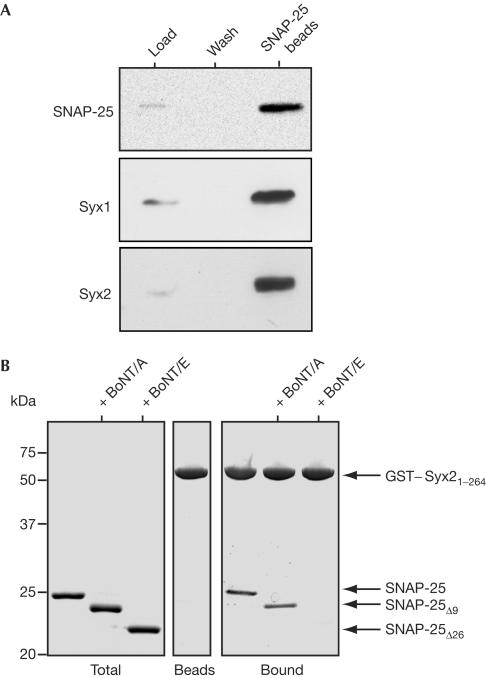
Next, we investigated whether syntaxin1 alone is responsible for the plasma membrane localization of SNAP-25. We co-immunostained the transfected and control cells using mouse anti-syntaxin1 and rabbit anti-SNAP-25 antibodies. Consistent with recent observations in PC12 cells (Lang et al, 2001; Ohara-Imaizumi et al, 2004), the two proteins showed only partial colocalization (Fig 2B). As SNAP-25 is known to interact with several members of the syntaxin family (Bennett et al, 1993; Fasshauer et al, 1999), we analysed whether an additional syntaxin isoform is present at the plasma membrane of PC12 cells. We specifically looked at syntaxin2 because a previous study reported an abundance of syntaxin2 mRNA in this cell line with a weak signal detectable at the protein level (Quinones et al, 1999). The syntaxin2 antibody used here readily recognized a single band in a PC12 cell extract, demonstrating the antibody specificity (Fig 3D). Confocal microscopy demonstrated an abundance of syntaxin2 at the plasma membrane with partial colocalization of SNAP-25 with syntaxin2 in the merged images (Fig 3A). This partial colocalization was maintained in BoNT/A LC-transfected cells, whereas expression of BoNT/E LCs led to full clearance of SNAP-25 immunoreactivity (supplementary Fig 1 online). The availability of mouse anti-syntaxin1 and rabbit anti-syntaxin2 antibodies allowed us to determine the relative distribution of the two isoforms at the plasma membrane. It was evident that areas of the plasma membrane showed a patchwork of the two isoforms with extensive coverage of the membrane (Fig 3B,C). Next, we immunoprecipitated SNAP-25 using a monoclonal antibody from the cell extracts and analysed the bound material for the two syntaxin isoforms using the corresponding rabbit antibodies. Fig 4A shows that syntaxin2 was enriched in the immunoprecipitate to the same degree as the more extensively studied syntaxin1 isoform. It was, therefore, possible that the complete clearance of SNAP-25 from the plasma membrane of PC12 cells (Fig 1A), following BoNT/E expression, is due to disruption of SNAP-25 association not only with syntaxin1 but also with syntaxin2. Consequently, we analysed the ability of syntaxin2 to interact with SNAP-25 following BoNT treatment. At 2 μM, the concentration sufficient to cause saturable binding of SNAP-25 to syntaxin1 (Fig 2), syntaxin2 associated with both the intact SNAP-25 molecule and the cleavage product of BoNT/A (Fig 4). In contrast, cleavage of SNAP-25 by BoNT/E abolished the ability of SNAP-25 to bind syntaxin2.
Figure 3.
Syntaxin2 is present at the plasma membrane of PC12 cells in a complementary manner to syntaxin1. Double immunostaining for syntaxin2 and either SNAP-25 (A, scale bar, 5 μm) or syntaxin1 (B, magnification as in A). (C) Magnification (× 5) of the boxed area in (B). The arrows and arrowheads indicate syntaxin2- and syntaxin1-rich patches, respectively. (D) Immunoblot analysis of PC12 cell extracts using antibodies against syntaxin1 and syntaxin2 confirms their specificity.
Figure 4.
Syntaxin2 interacts with SNAP-25 in PC12 cells and this interaction shows differential sensitivity to BoNT/A and BoNT/E. (A) Immunoprecipitation of PC12 cell extracts using monoclonal antisNAP-25 Sepharose beads shows both syntaxin1 (Syx1) and syntaxin2 (Syx2) as SNAP-25-interacting partners. (B) GST–syntaxin2 attached to beads was incubated with SNAP-25, SNAP25Δ9 or SNAP-25Δ26. SNAP-25Δ26 does not bind syntaxin2, whereas control and SNAP25Δ9 show similar binding.
The continuing interest in the mechanism of BoNT/A action is due to the growing use of BoNT/A in the treatment of neuromuscular disorders (Johnson, 1999; Rossetto et al, 2004). It was suggested that the specific properties of BoNT/A-induced blockade of exocytosis are due to mild destabilization of ternary soluble N-ethylmaleimidesensitive factor attachment protein receptor (SNARE) complexes that include vesicular synaptobrevin (Banerjee et al, 1996). However, this theory was recently undermined by findings that BoNT/E is able to (i) clear BoNT/A-damaged SNAP-25 from the neurons and (ii) restore neurotransmission on the timescale of recovery from BoNT/E intoxication, (Eleopra et al, 1998; Keller & Neale, 2001; Meunier et al, 2003). Indeed, biochemical studies previously demonstrated that BoNT/E is incapable of cleaving SNAP-25 upon ternary complex formation (Otto et al, 1995; Pellegrini et al, 1995), making it unclear how BoNT/E can override BoNT/A toxicity if SNARE complexes have been assembled. We now propose that the plasma membrane syntaxins alone provide the molecular basis for specific retention of SNAP-25 at the plasma membrane in the presence of BoNT/A (Fig 1A). We recently demonstrated that the syntaxin1/SNAP-25 dimers can be attacked by BoNT/E, leading to the release of the BoNT/E-cleaved SNAP-25 both in vitro and in vivo (Rickman et al, 2004). This marked effect of BoNT/E was obtained with preassembled syntaxin1/SNAP-25 dimers in the absence of free SNAP-25 molecules. Our current results (Fig 2A) can explain, without invoking ternary complexes, why a short treatment of permeabilized PC12 cells with BoNT/E, but not BoNT/A, leads to a decrease in co-immunoprecipitation of syntaxin and SNAP-25 (Banerjee et al, 1996). The existence of the plasma membrane SNAP-25, which is not in association with syntaxin1 (Fig 2B), seems to indicate that another protein or lipid factor can maintain SNAP-25 on the plasma membrane. However, we demonstrated that SNAP-25 colocalizes and interacts in PC12 cells not only with syntaxin1 but also with syntaxin2, the two syntaxins showing complementary spatial organization at the plasma membrane. Together, our results demonstrate that BoNT/A and BoNT/E differentially affect the ability of SNAP-25 to bind the plasma membrane syntaxins, providing a new explanation for the differences in the BoNT intoxication.
Methods
Plasmids and antibodies. Plasmids encoding the LCs of BoNT/A and BoNT/E, syntaxins 1 and 2, and SNAP-25B were described previously (Vaidyanathan et al, 1999; Kauppi et al, 2002; Zhang et al, 2002; Hu et al, 2004; Rickman et al, 2004). The pEGFP-C1 vector was purchased from BD Biosciences (Oxford, UK). Antibodies against full-length SNAP-25 and SNAP-25 cleaved by BoNT/A were described previously (Hu et al, 2002; Meunier et al, 2003). Antisyntaxin2 and anti-synaptotagmin1 were purchased from Synaptic Systems (Göttingen, Germany) and anti-syntaxin1 (clone HPC-1) was from Sigma (Gillingham, UK).
Cell culture, immunofluorescence staining and SNARE immunoprecipitation. PC12 cells were maintained as described previously (Rudolf et al, 2001). Cells were transfected by electroporation using an Amaxa kit with an efficiency of 35–50%. After transfection, the cells were plated and incubated with or without 50 ng/ml NGF (Sigma). Exocytosis/endocytosis was stimulated by application of 55 mM KCl for 20 min. Cells were fixed and stained as described previously (Rickman et al, 2004). Cell extracts were prepared using buffer A (20 mM HEPES (pH 7.3), 100 mM NaCl, 1 mM EDTA, 0.1% Triton X-100) containing 2% Triton X-100. The cell material was centrifuged at 20,000g for 30 min at 4°C, and the supernatant was used for immunoprecipitation. Sepharose beads with covalently attached antisNAP-25 antibody (clone SMI81, Sternberger Monoclonals, Lutherville, USA) or protein G–agarose beads (Sigma) with attached HPC-1 monoclonal antibody were incubated with the cell detergent extract for 1 h at 4°C, and then extensively washed in buffer A. Protein bands on western immunoblots were detected using SNARE-specific antibodies and a West Dura enhanced chemiluminescence kit (Pierce, Tattenhall, UK).
Quantitative protein binding assays The His6-tagged LCs of BoNT/E and BoNT/A, and GST-tagged syntaxins and SNAP-25 were expressed and purified as previously described (Vaidyanathan et al, 1999; Rickman et al, 2004). To compare the effects of BoNTs on the affinity of SNAP-25 for syntaxin1, 0.2 pmol of GST–SNAP-25B was immobilized on glutathione–Sepharose beads and, where necessary, cleaved using the LCs of BoNT/A or BoNT/E for 1 h at 22°C. Following washing to remove toxins, SNAP-25 affinity for syntaxin1 was determined as described previously (Rickman et al, 2004). For analysis of the syntaxin2/SNAP-25 interaction, 3 μg of GST–syntaxin2, immobilized on glutathione–Sepharose beads, was mixed with 2 μM SNAP-25B, intact or precleaved with BoNT/A or BoNT/E. Following a 30 min incubation at 22°C and washing, bound protein was eluted in SDS-containing sample buffer and analysed by SDS–polyacrylamide gel electrophoresis and Coomassie staining.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor).
Supplementary Material
supplementary material
Acknowledgments
We thank Y.-K. Shin for the SNAP-25B plasmid, V. Olkkonen for the syntaxin2 plasmid and F. Meunier for the anti-SNAP-25 antibody.
References
- Aguado F, Gombau L, Majo G, Marsal J, Blanco J, Blasi J (1997) Regulated secretion is impaired in AtT-20 endocrine cells stably transfected with botulinum neurotoxin type A light chain. J Biol Chem 272: 26005–26008 [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D, Juzans P, Molgo J, Faille L, Seagar MJ, Takahashi M, Shoji-Kasai Y (1995) Mouse motor nerve terminal immunoreactivity to synaptotagmin II during sustained quantal transmitter release. Brain Res 681: 213–217 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Kowalchyk JA, DasGupta BR, Martin TF (1996) SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J Biol Chem 271: 20227–20230 [DOI] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH (1993) The syntaxin family of vesicular transport receptors. Cell 74: 863–873 [DOI] [PubMed] [Google Scholar]
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H (1994) Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem 269: 1617–1620 [PubMed] [Google Scholar]
- Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH (1999) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell 97: 165–174 [DOI] [PubMed] [Google Scholar]
- Eleopra R, Tugnoli V, Rossetto O, De Grandis D, Montecucco C (1998) Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett 256: 135–138 [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R (1999) Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J Biol Chem 274: 15440–15446 [DOI] [PubMed] [Google Scholar]
- Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, Smith L, Aoki KR, Dolly JO (2003) Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem 278: 1363–1371 [DOI] [PubMed] [Google Scholar]
- Graham ME, Fisher RJ, Burgoyne RD (2000) Measurement of exocytosis by amperometry in adrenal chromaffin cells: effects of clostridial neurotoxins and activation of protein kinase C on fusion pore kinetics. Biochimie 82: 469–479 [DOI] [PubMed] [Google Scholar]
- Hu K, Carroll J, Rickman C, Davletov B (2002) Action of complexin on SNARE complex. J Biol Chem 277: 41652–41656 [DOI] [PubMed] [Google Scholar]
- Hu K, Rickman C, Carroll J, Davletov B (2004) A common mechanism for the regulation of vesicular SNAREs on phospholipid membranes. Biochem J 377: 781–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Kang YH, Pasyk EA, Sheu L, Wheeler MB, Trimble WS, Salapatek A, Gaisano HY (2001) Ca2+ influx and cAMP elevation overcame botulinum toxin A but not tetanus toxin inhibition of insulin exocytosis. Am J Physiol Cell Physiol 281: C740–C750 [DOI] [PubMed] [Google Scholar]
- Johnson EA (1999) Clostridial toxins as therapeutic agents: benefits of nature's most toxic proteins. Annu Rev Microbiol 53: 551–575 [DOI] [PubMed] [Google Scholar]
- Kauppi M, Wohlfahrt G, Olkkonen VM (2002) Analysis of the Munc18bsyntaxin binding interface. Use of a mutant Munc18b to dissect the functions of syntaxins 2 and 3. J Biol Chem 277: 43973–43979 [DOI] [PubMed] [Google Scholar]
- Keller JE, Neale EA (2001) The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J Biol Chem 276: 13476–13482 [DOI] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R (2001) SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 20: 2202–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T (2000) Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol 149: 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Sudhof TC (1995) Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83: 111–119 [DOI] [PubMed] [Google Scholar]
- Meunier FA, Lisk G, Sesardic D, Dolly JO (2003) Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: the extent and duration are dictated by the sites of SNAP-25 truncation. Mol Cell Neurosci 22: 454–466 [DOI] [PubMed] [Google Scholar]
- Morihara T et al. (1999) Distribution of synaptosomal-associated protein 25 in nerve growth cones and reduction of neurite outgrowth by botulinum neurotoxin A without altering growth cone morphology in dorsal root ganglion neurons and PC-12 cells. Neuroscience 91: 695–706 [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Kumakura K, Nakamichi Y, Nagamatsu S (2004) Site of docking and fusion of insulin secretory granules in live MIN6 beta cells analyzed by TAT-conjugated antisyntaxin 1 antibody and total internal reflection fluorescence microscopy. J Biol Chem 279: 8403–8408 [DOI] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Chapman ER, Blasi J, Jahn R (1995) Poisoning by botulinum neurotoxin A does not inhibit formation or disassembly of the synaptosomal fusion complex. Biochem Biophys Res Commun 212: 945–952 [DOI] [PubMed] [Google Scholar]
- Pellegrini LL, O'Connor V, Lottspeich F, Betz H (1995) Clostridial neurotoxins compromise the stability of a low energy SNARE complex mediating NSF activation of synaptic vesicle fusion. EMBO J 14: 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones B, Riento K, Olkkonen VM, Hardy S, Bennett MK (1999) Syntaxin 2 splice variants exhibit differential expression patterns, biochemical properties and subcellular localizations. J Cell Sci 112: 4291–4304 [DOI] [PubMed] [Google Scholar]
- Raciborska DA, Trimble WS, Charlton MP (1998) Presynaptic protein interactions in vivo: evidence from botulinum A, C, D and E action at frog neuromuscular junction. Eur J Neurosci 10: 2617–2628 [DOI] [PubMed] [Google Scholar]
- Rickman C, Davletov B (2003) Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J Biol Chem 278: 5501–5504 [DOI] [PubMed] [Google Scholar]
- Rickman C, Meunier FA, Binz T, Davletov B (2004) High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J Biol Chem 279: 644–651 [DOI] [PubMed] [Google Scholar]
- Rossetto O, Rigoni M, Montecucco C (2004) Different mechanism of blockade of neuroexocytosis by presynaptic neurotoxins. Toxicol Lett 149: 91–101 [DOI] [PubMed] [Google Scholar]
- Rudolf R, Salm T, Rustom A, Gerdes HH (2001) Dynamics of immature secretory granules: role of cytoskeletal elements during transport, cortical restriction, and F-actin-dependent tethering. Mol Biol Cell 12: 1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C (1993) Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett 335: 99–103 [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C (2000) Neurotoxins affecting neuroexocytosis. Physiol Rev 80: 717–766 [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Pelto-Huikko M, Aitola M, Meister B, Vik-Mo EO, Davanger S, Scheller RH, Bean AJ (1999) The cellular and developmental expression of hrs-2 in rat. Eur J Neurosci 11: 3047–3063 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan VV, Yoshino K, Jahnz M, Dorries C, Bade S, Nauenburg S, Niemann H, Binz T (1999) Proteolysis of SNAP-25 isoforms by botulinum neurotoxin types A, C, and E: domains and amino acid residues controlling the formation of enzyme–substrate complexes and cleavage. J Neurochem 72: 327–337 [DOI] [PubMed] [Google Scholar]
- Vogel K, Cabaniols JP, Roche PA (2000) Targeting of SNAP-25 to membranes is mediated by its association with the target SNARE syntaxin. J Biol Chem 275: 2959–2965 [DOI] [PubMed] [Google Scholar]
- Washbourne P, Cansino V, Mathews JR, Graham M, Burgoyne RD, Wilson MC (2001) Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting. Biochem J 357: 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Chen Y, Kweon DH, Kim CS, Shin YK (2002) The four-helix bundle of the neuronal target membrane SNARE complex is neither disordered in the middle nor uncoiled at the C-terminal region. J Biol Chem 277: 24294–24298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary material