Abstract
Mutations in the death domain of the death receptor CD95 (APO-1/Fas) cause lymphoproliferation and autoimmune disease in both lprcg mice and in patients with autoimmune lymphoproliferative syndrome (ALPS) type Ia. By testing lymphocytes from ALPS type Ia patients, comparing heterozygous with homozygous lprcg mice and coexpressing wild-type and mutant CD95 receptors, we demonstrate that induction of apoptosis requires two wild-type alleles of CD95. By contrast, nuclear factor-κB (NF-κB) can be fully activated in cells expressing both a mutant and a wild-type CD95 allele, suggesting different thresholds to activate the two signalling pathways. This was confirmed by testing lymphocytes from heterozygous lpr mice, which showed reduced sensitivity to CD95-mediated apoptosis but normal activation of NF-κB when compared with wild-type mice. Mutations in CD95 may eliminate the tumour-suppressive function of CD95, at the same time allowing induction of survival or proliferative pathways, which could contribute to the increased risk for lymphoma seen in ALPS type Ia patients.
Keywords: autoimmune lymphoproliferative syndrome, Fas, lprcg, MAPK, NF-κB
Introduction
On binding of CD95 ligand (CD95L), CD95 recruits the adaptor protein FADD, the initiator caspases 8 and 10 and the apoptosis regulator c-FLIP, forming the death-inducing signalling complex (DISC; Peter & Krammer, 2003). This process requires an intact death domain (DD) in the cytoplasmic tail of CD95. Mutations in the DD have been shown to abrogate induction of apoptosis (Fisher et al, 1995). In addition to serving a critical role in mediating lymphocyte apoptosis, stimulation of CD95 has been shown to result in activation of the transcription factor nuclear factor-κB (NF-κB) and of mitogen-activated protein (MAP) kinases (Desbarats et al, 2003; Wajant et al, 2003). The mechanisms that differentially or coordinately regulate the engagement of the apoptosis pathway by CD95 on the one hand, or antiapoptotic pathways on the other, are poorly understood.
Mutations in the DD of CD95 are naturally found both in mice (lprcg mice; Kimura & Matsuzawa, 1994) and in many patients suffering from autoimmune lymphoproliferative syndrome (ALPS) type Ia (Rieux-Laucat et al, 1995, 1999; Drappa et al, 1996; Martin et al, 1999; Straus et al, 2001). We demonstrated previously that mutated CD95 found in these patients can form a DISC, but only very inefficiently (Martin et al, 1999). Consequently, both lprcg mice and such human patients suffer from a disease that is characterized by defective lymphocyte apoptosis, lymphocyte accumulation and humoral autoimmune symptoms. This has been attributed primarily to their defect in lymphocyte apoptosis. However, it has not been determined whether the mutant CD95 receptor in these patients might still activate critical cell signalling pathways that are independent of apoptosis.
We now demonstrate that cells carrying heterozygous mutations in the CD95 DD are deficient in induction of apoptosis but can still efficiently activate the transcription factor NF-κB and the MAP kinases Erk1/2 and p38. Importantly, none of these pathways can be activated in cells expressing exclusively mutant CD95 alleles. Thus, apoptosis requires two functional CD95 alleles, whereas activation of NF-κB and MAP kinases may only require one functional CD95 allele. Comparison of homozygous lpr mice, which express almost no CD95, with heterozygous lpr mice, which express severely reduced levels of CD95, demonstrated that far less intact CD95 is required to activate NF-κB than to induce apoptosis. Our data have uncovered a major difference in the way proapoptotic and antiapoptotic pathways are engaged by CD95.
Results and Discussion
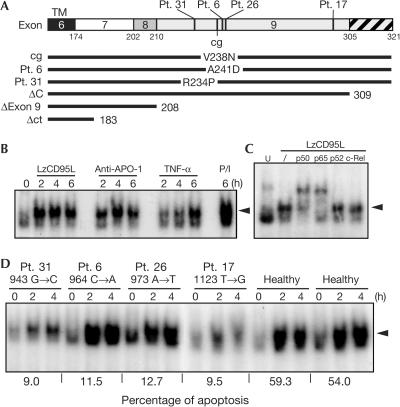
Most patients with ALPS type Ia carry mutations in exon 9, which encodes the CD95 DD (Martin et al, 1999; Straus et al, 2001; Fig 1A), and these patients have up to 51-fold increased risk of developing lymphomas (Straus et al, 2001). We recently demonstrated that CD95 has a role in inducing increased motility and invasiveness in tumour cells that resist its proapoptotic activity (Barnhart et al, 2004). One of the pathways that was found to be required for this activity was mediated through NF-κB. To test whether peripheral blood lymphocytes (PBLs) activate NF-κB when stimulated through CD95, we incubated them with phytohaemagglutinin (PHA) for 24 h followed by interleukin-2 (IL-2) for 6 days, a treatment that activates and sensitizes T cells to CD95-mediated apoptosis (Peter et al, 1997). Aliquots of these stimulated T cells were treated with either highly active leucine zipper-tagged CD95L (LzCD95L), anti-APO-1 monoclonal antibody (mAb), tumour necrosis factor α (TNF-α) or phorbol 12-myristate 13-acetate (PMA) and ionomycin (Fig 1B). As determined by electrophoretic mobility shift assay (EMSA), NF-κB was profoundly activated by LzCD95L, anti-APO-1 and TNF-α. Supershift analysis identified the canonical p50/p65 heterodimer as the predominant complex induced by CD95 in the activated PBLs (Fig 1C). We then tested PBLs from ALPS type Ia patients 6, 17, 26 and 31, all of whom possess discrete heterozygous mutations in the CD95 DD (Fig 1A). As reported previously, the apoptotic response to CD95 stimulation of cells from these four ALPS patients was severely blunted when compared with cells from healthy volunteers (Fig 1D; Martin et al, 1999). The observed impairment of apoptosis is consistent with the inability of the CD95 receptor of these patients to form a functional DISC efficiently (Martin et al, 1999). Despite this defect in apoptosis, NF-κB activation was detected in PBLs from all four ALPS patients. In fact, after treatment with LzCD95L for 2–4 h, the extent of NF-κB activation in cells from patients 6 and 26 was at least as great as that achieved with control cells (Fig 1D). Cells from patients 17 and 31 only moderately activated NF-κB, probably reflecting some variability in responses in humans.
Figure 1.
Activation of NF-κB in ALPS type Ia patients despite inhibition of apoptosis. (A) Location of mutations in the cytoplasmic tail of CD95. The CD95 mutant constructs used in the following experiments are depicted below. cg, CD95 carrying a point mutation in the DD homologous to the mutation found in CD95 of lprcg mice; mutations in patients (Pt.) 6, 17, 26 and 31 were described previously (Martin et al, 1999). ΔExon 9, CD95 lacking exon 9; ΔC, CD95 lacking the C-terminal part that follows DD (hatched); Δct, CD95 lacking most of its cytoplasmic tail, as indicated. (B) EMSA analysis of PHA/IL-2-activated PBLs from healthy volunteers stimulated with 1 μg/ml LzCD95L, 1 μg/ml anti-APO-1, 1,000 U/ml TNF-α or PMA (10 ng/ml)/ionomycin (1 μg/ml) (P/I). (C) Supershift EMSA of PBLs stimulated for 4 h with LzCD95L followed by addition of indicated antibodies specific for NF-κB components. (D) EMSA analysis of PHA/IL-2-activated PBLs from healthy donors and from four ALPS patients stimulated for the indicated times with 1 μg/ml LzCD95L. Percentage of specific apoptosis of PBLs after 16 h incubation with LzCD95L (1 μg/ml) is given below. The arrowheads mark the migration of the p50/p65 NF-κB heterodimer.
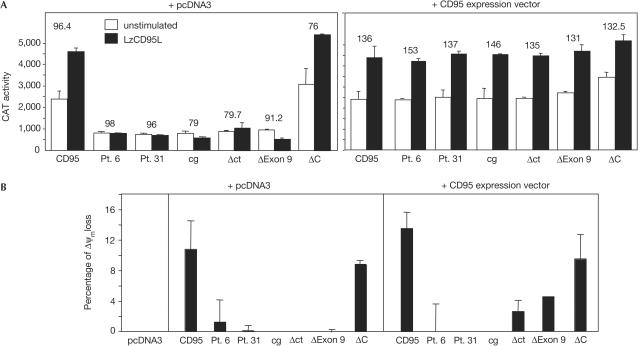
To compare the effects of different CD95 mutants on the relative strengths of activation of apoptosis and NF-κB, we used CD95 plasmid constructs bearing the point mutations found in patients 6 and 31, or the humanized equivalent of murine lprcg CD95 (Fig 1A; Martin et al, 1999). We also constructed truncation mutants lacking only the carboxy-terminal amino acids outside DD (ΔC), lacking exon 9 (ΔExon 9) or lacking most of the CD95 cytoplasmic tail (Δct). We transfected wild-type and mutant CD95 receptors into MCF-7 cells, which express very little endogenous CD95, and monitored CD95-induced activation of NF-κB using a CAT gene reporter assay (Fig 2A). Under conditions that resulted in the expression of mostly exogenous receptors (Fig 2A, left panel), only the wild-type receptor and the ΔC construct allowed an increase in CD95-induced transcriptional activity of NF-κB, suggesting that wild-type CD95 is required for NF-κB to be activated.
Figure 2.
The ratio of mutant to wild-type CD95 protein expressed determines whether apoptosis and/or NF-κB is activated by CD95L. (A) CAT activity of MCF-7 cells transfected with either CD95 constructs and control vector (left) or CD95 mutant constructs and the same amount of wild-type CD95 construct (right) treated with LzCD95L/zVAD-fmk for 16 h. Values for cells transfected with vector only were subtracted. The mean fluorescence intensity (MFI) of the stained overexpressed proteins is given above each pair of columns. The MFI of the endogenous CD95 was between 2 and 3. Equal expression of co-transfected mutant and wild-type receptors was ensured by both flow cytometry and immunoprecipitation of the truncation mutants after surface biotinylation (data not shown). (B) MCF7 cells were transfected with the combination of the indicated CD95 constructs and pcDNA3 (left panel) or with wild-type CD95 (right panel). At 24 h after transfection, living cells were purified by Ficoll centrifugation, incubated for 18 h with 1 μg/ml of LzCD95L and analysed for a change in mitochondrial transmembrane potential. Background apoptosis was subtracted for each condition.
To better emulate the relative levels of mutant and wild-type CD95 expressed in ALPS patients, we co-transfected cells with equal amounts of wild-type and mutant receptors (Fig 2A, right panel). In all cases, increased NF-κB transcriptional activity could be detected on stimulation of cells with LzCD95L. The known ability of the mutant receptors to interfere dominantly with the apoptosis-inducing function of wild-type CD95 was confirmed by coexpression of mutant CD95 receptors either with control vector (Fig 2B, left panel) or with wild-type CD95 (Fig 2B, right panel). The ability of all mutant proteins, except for that encoded by the ΔC mutant, to inhibit apoptosis induced through wild-type CD95 was confirmed by transfecting the mutant receptors into Jurkat cells, which express endogenous CD95 (supplementary Fig 1 online). The data confirmed that all of the DD mutants acted as dominant-negative inhibitors of apoptosis induced by wild-type CD95, consistent with a previous report in which it was demonstrated that all CD95 mutant receptors found in ALPS type Ia patients contain an amino-terminal preligand assembly domain (PLAD) responsible for the dominant interference of mutants with wild-type receptors (Siegel et al, 2000). The data suggest that both induction of apoptosis and activation of NF-κB by CD95 critically depend on the presence of some level of CD95 containing an intact DD. However, as with cells from ALPS patients, dominant interference by mutant receptors blocked apoptosis but not activation of NF-κB.
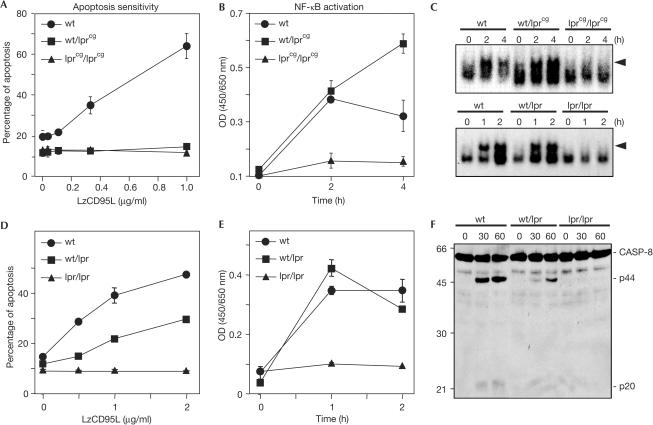
To further explore the relative requirement for a functional DD in inducing apoptosis versus activating NF-κB and MAP kinases, we studied splenocytes from C3Hwt/wt, C3Hwt/lpr(cg) and C3Hlpr(cg)/lpr(cg) mice, each of which expresses relatively predictable and consistent levels of wild-type and mutant CD95 receptors. We demonstrate that, as with PBLs from our ALPS type Ia patients, splenocytes from heterozygous lprcg mice are resistant to CD95-mediated apoptosis, whereas cells from wild-type mice are highly sensitive (Fig 3A). Using an enzyme-linked immunosorbent assay (ELISA) specific for p65, EMSA and western blot analysis, we documented the activation of NF-κB, Erk1/2 and p38 in cells from both wild-type and wt/lprcg mice, but not in cells from lprcg/lprcg mice (Fig 3B,C and supplementary Fig 2A online).
Figure 3.
Different thresholds are required to activate NF-κB and apoptosis through CD95. (A) Activated splenocytes from C3H (wt), homozygous C3Hlpr(cg)/lpr(cg) (lprcg/lprcg) and heterozygous C3Hwt/lpr(cg) (wt/lprcg) mice were incubated with 1 μg/ml LzCD95L for 16 h and DNA fragmentation was quantified. (B,C) Cells were treated as in (A), but nuclear extracts were subjected to a p65specific ELISA to quantify activation of NF-κB (B) or an NF-κB-specific EMSA analysis (C). Note: Experiments in (A) and (B) were performed in duplicate with splenocytes from 4-week-old mice (before the onset of autoimmunity) and 8-week-old mice (after the onset of autoimmunity), respectively. The means of these two experiments and the standard deviations from these means are shown. The experiment in (C) shows the results obtained with cells from the spleens of 4-week-old mice. The results from the 8-week-old mice were virtually identical. The splenocytes consisted of 99% CD3+ cells after 4 days of activation with no clear differences between mouse strains in cellular composition. (D,E) Apoptosis assay (D) and p65 ELISA (E) of CD95-stimulated splenocytes of the indicated mice performed as in (A) and (B). (F) Western blot analysis of caspase-8 in lysates of splenocytes stimulated as in (D).
The cumulative data suggest that the induction of apoptosis, when compared with the activation of NF-κB, requires different signalling mechanisms or has very different thresholds. To test directly whether induction of apoptosis has a higher threshold than activation of NF-κB, we reduced the amount of CD95 expressed in mouse cells by crossing wild-type mice with homozygous lpr mice, which express undetectable amounts of CD95 on splenocytes (supplementary Fig 2B online). As shown by flow cytometry, surface expression of CD95 on cells from the resulting heterozygous mice was about half of that from wild-type mice. Splenocytes from these heterozygous lpr/wt mice showed a marked reduction in sensitivity to apoptosis mediated by CD95. Specifically, both DNA fragmentation (Fig 3D) and processing of procaspase-8 (Fig 3F) were strongly reduced in cells from these heterozygous mice. In contrast, activation of NF-κB was indistinguishable between wt/wt and lpr/wt mice, as determined both by EMSA (Fig 3C) and an ELISA specific for p65 (Fig 3E). The data on CD95 signalling complexes containing mutant CD95 and identical cell types expressing different CD95 levels confirmed that the threshold for CD95 to activate NF-κB is much lower than to activate apoptosis.
Our data speak to the possibility that the preserved activation of NF-κB in ALPS patients could contribute to their risks of lymphoma. So far, we have analysed 178 subjects from 113 families who fall within the ALPS spectrum. ALPS type Ia was confirmed clinically and by sequencing of CD95 DNA in 131 patients from 69 families. These patients carry heterozygous mutations in CD95, mostly clustered in the DD, and carry about a 10% lifetime risk of lymphoma (Straus et al, 2001). Furthermore, among the families with ALPS type Ia we have analysed, 78% have mutations in the DD and 22% have extracellular and other non-DD mutations. That eight out of nine cases of lymphoma documented thus far have arisen in our patients with DD mutations suggests that lymphoma is more likely to develop in subjects with DD mutations, but the cohort sample size is not large enough for this difference to achieve statistical significance.
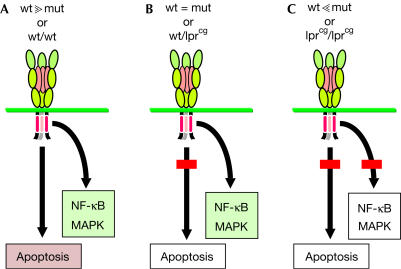
Altogether, our data suggest the following model (Fig 4): when wild-type CD95 is expressed and cells encounter CD95L, at least two sets of cellular pathways can be activated—those associated with apoptosis and those associated with activation of NF-κB/MAPK (Fig 4A). When cells express roughly similar levels of mutated and wild-type CD95, the mutant allele dominantly inhibits induction of apoptosis by impairing formation of DISC. These conditions occur in ALPS type Ia patients and certain tumour cells (Fig 4B). Under these very conditions, however, NF-κB/MAPK can still be activated, possibly contributing to the growth and survival of cells. Under conditions of large excess of mutated CD95 or in homozygous lprcg mice with no wild-type receptor, neither apoptosis nor NF-κB/MAPK can be induced (Fig 4C). Our findings on the lack of activation of Erk1/2 in cells expressing two CD95 mutant alleles differ from those in a study that reported that in certain neurons from lprcg/lprcg mice, triggering of CD95 can still activate Erk1/2 (Desbarats et al, 2003). This discrepancy points to a possible difference in the way CD95 activates MAP kinases in different tissues but could also be explained by a difference in the genetic background of the lprcg mice used.
Figure 4.
Dependence of different CD95 signalling pathways on a functional DD. Cells expressing primarily wild-type CD95 in the presence of stimulating CD95 ligand activate both apoptosis and NF-κB/MAPK pathways. Under these conditions, the apoptosis pathway prevails by restraining NF-κB activation through robust caspase activity (A). In cells expressing one wild-type and one mutant allele with a mutation in DD (X in DD) to comparable levels (a situation often found in ALPS type Ia patients), or under conditions of low receptor expression, apoptosis cannot be induced efficiently owing to the inability of the receptor to form an efficient DISC. However, NF-κB/MAPK pathways are activated normally (B). When mutant CD95 receptor is in large excess (achievable only in overexpression experiments) or when two CD95 mutant alleles are expressed (as in the lprcg mice), neither apoptosis nor NF-κB/MAPK pathways can be activated (C).
Speculation
Activation of NF-κB promotes tumour growth through transcriptional activation of a number of target genes that exert proliferative, antiapoptotic and/or tumorigenic functions (Lin & Karin, 2003). Moreover, activation of NF-κB by treating tumour cells with CD95 ligand was shown recently to render apoptosis-resistant tumour cells more invasive (Barnhart et al, 2004). Cells found in the lymph nodes of ALPS patients often show activation markers (Lim et al, 1998), which would suggest that they also express CD95L. As chronic activation of NF-κB in lymphoid cells contributes to lymphomagenesis (Ruland & Mak, 2003), the stimulation of mutant CD95 in ALPS type Ia patients associated with enhanced CD95L expression could further increase the risk of lymphoma.
Methods
Patient samples and other cells All patient samples were studied at the National Institutes of Health under approved protocols with informed consent. Peripheral blood mononuclear cells were obtained from buffy coats of healthy donors or from ALPS patients. Erythrocytes were removed by Ficoll (Cambrex Bio Science, Walkersville, MD, USA) centrifugation, whereas monocytes were removed by adherence to plastic dishes overnight. The resulting lymphocytes were stimulated for 20 h with 1 μg/ml PHA-L (Sigma, St Louis, MO, USA), washed and then incubated for 6 days in medium containing 50 U/ml IL-2.
Plasmid construction CD95 mutations from patients 6 and 31 and human CD95-translated lprcg mutation have been described previously (Martin et al, 1999). Truncated CD95 complementary DNAs were constructed by incorporating nonsense mutations using pKEX-CD95 WT as a template for site-directed mutagenesis (Quick-Change II, Stratagene, La Jolla, CA, USA). CD95 constructs ΔIC, CD95 ΔExon 9 and CD95 ΔC were made by introducing nonsense mutations at nucleotide 549 (Cys 183), 623 (Leu 208) or 927 (Ser 309), respectively. Transfections were performed as described in the legend to supplementary Fig 1 online.
EMSA and p65 ELISA For EMSA and MAPK analysis, all cells were preincubated for 1 h with 40 μM zVAD-fmk, which allowed a direct comparison of activation of NF-κB between wild-type, CD95 apoptosis-sensitive and mutated, apoptosis-resistant cells. A total of 5 × 106 PBLs were washed in cold phosphate-buffered saline (PBS) and incubated on ice for 5 min in modified lysis buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.4% NP-40, 0.1 mM EDTA, 0.5 mM dithiothreitol (DTT), 1.5 mM MgCl2, 0.5 mM PMSF, containing protease inhibitor cocktail (Sigma)). The cells were centrifuged at 2,500 r.p.m. for 3 min at 4°C, resuspended and incubated for 10 min at 4°C in high-salt buffer (20 mM HEPES pH 7.9, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT, 1.5 mM MgCl2, 0.5 mM PMSF, containing protease inhibitor cocktail (Sigma)), centrifuged at 12,000 r.p.m. for 10 min at 4°C and the supernatants were collected. EMSA, supershift analysis and p65 ELISA were performed as previously described (Barnhart et al, 2004).
CAT assay MCF-7 cells were transfected with an HIV-κB-CAT reporter plasmid driven by the κB sites in the HIV LTR (Franzoso et al, 1992) using Polyfect from Qiagen (Maryland, USA) according to the manufacturer's protocols. HIV-κB-CAT/pCI or pCI-CD95/ mutated CD95 constructs were transfected at the ratio 1:3:3. A total of 105 cells were harvested after 16–24 h and left untreated or treated with 1 μg/ml LzCD95L/40 μM zVAD-fmk. CAT analysis was performed as previously described (Barnhart et al, 2004).
Apoptosis assay Cells treated with LzCD95L (1 μg/ml) were washed in PBS and fixed/permeabilized using BD Cytofix/Cytoperm™ (BD Biosciences, San Jose, CA, USA), following the recommendations of the manufacturer. Cells were incubated for 30 min at 4°C with anti-active caspases-3 (BD Biosciences), washed in cold PBS and incubated for 30 min at 4°C with phycoerythrin-conjugated anti-rabbit IgG (Southern Biotechnology, Birmingham, AL, USA) and analysed on a FACScan flow Cytometer (BD Biosciences, San Jose, CA, USA). To quantify mitochondrial depolarization, transfected Jurkat cells were treated for 2 h with LzCD95L or left untreated, then washed and directly incubated for 30 min with MitoShiftTM using the manufacturer's recommendations (Trevigen, Gaithersburg, MD, USA). For each staining assay, the sensitivity of the green fluorescent protein-positive cells to CD95 was analysed. Quantification of DNA fragmentation of untransfected cells was carried out as previously described (Barnhart et al, 2004). Western blot analysis of mouse caspase-8 was performed as described in the legend to supplementary Fig 2 online.
Mice and splenocyte purification C3H lprcg mice (Yasuda et al, 2000) were bred with C3H wild-type mice (Jackson Laboratories, Bar Harbor, ME, USA) to generate heterozygous mice. C3H wt/lpr mice were generated in the same way. Spleens were isolated and mechanically dissociated, followed by passage through 70 μm nylon filters. The splenocytes were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 550 μM 2-mercaptoethanol (Gibco). Cells were incubated on plates coated with anti-mouse CD3ɛ mAb (Pharmingen, San Diego, CA, USA) for 16 h, then the cells were harvested, washed and incubated in 50 U/ml IL-2 for 3 days. Viable cells were isolated using Ficoll centrifugation, washed and resuspended in IL-2/2-mercaptoethanol containing culture medium.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v5/n11/extref/7400280s1.pdf and http://www.nature.com/embor/journal/v5/n11/extref/7400280s2.pdf).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
We thank Dr P. Krammer and Dr H. Walczak for providing us with anti-APO-1 and LzCD95L, respectively. We are thankful to Dr K. Sayama and Dr A. Strasser for providing C3H lprcg mice and the anti-mouse caspase-8 antibody, respectively. B.C.B. was supported by the DOD Breast Cancer Research Program DAMD17-03-1-0200 and P.L. by the Committee on Cancer Biology, University of Chicago.
References
- Barnhart BC, Legembre P, Pietras EM, Bubici C, Franzoso G, Peter ME (2004) CD95 ligand induces motility and invasiveness of apoptosis resistant tumor cells. EMBO J 23: 3175–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK (2003) Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol 5: 118–125 [DOI] [PubMed] [Google Scholar]
- Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB (1996) Fas gene mutations in the Canale–Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med 335: 1643–1649 [DOI] [PubMed] [Google Scholar]
- Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM (1995) Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81: 935–946 [DOI] [PubMed] [Google Scholar]
- Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U (1992) The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature 359: 339–342 [DOI] [PubMed] [Google Scholar]
- Kimura M, Matsuzawa A (1994) Autoimmunity in mice bearing lprcg: a novel mutant gene. Int Rev Immunol 11: 193–210 [DOI] [PubMed] [Google Scholar]
- Lim MS et al. (1998) Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol 153: 1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Karin M (2003) NF-κB in cancer: a marked target. Semin Cancer Biol 13: 107–114 [DOI] [PubMed] [Google Scholar]
- Martin DA et al. (1999) Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci USA 96: 4552–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10: 26–35 [DOI] [PubMed] [Google Scholar]
- Peter ME, Kischkel FC, Scheuerpflug CG, Medema JP, Debatin KM, Krammer PH (1997) Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur J Immunol 27: 1207–1212 [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F et al. (1999) Lymphoproliferative syndrome with autoimmunity: a possible genetic basis for dominant expression of the clinical manifestations. Blood 94: 2575–2582 [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP (1995) Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268: 1347–1349 [DOI] [PubMed] [Google Scholar]
- Ruland J, Mak TW (2003) From antigen to activation: specific signal transduction pathways linking antigen receptors to NF-κB. Semin Immunol 15: 177–183 [DOI] [PubMed] [Google Scholar]
- Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ (2000) Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science 288: 2354–2357 [DOI] [PubMed] [Google Scholar]
- Straus SE et al. (2001) The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood 98: 194–200 [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P (2003) Non-apoptotic Fas signaling. Cytokine Growth Factor Rev 14: 53–66 [DOI] [PubMed] [Google Scholar]
- Yasuda T, Zhang Y, Nagase H, Kaneko T, Sayama K, Hashimoto H, Matsuzawa A (2000) Immunological characterization of C3H mice congenic for Fas(lprcg), C3h/HeJ-Fas(lprcg)/Fas(lprcg). Lab Anim 34: 46–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2