Abstract
The p38 mitogen-activated protein kinase (MAPK) cascade is an evolutionarily conserved signalling mechanism involved in processes as diverse as apoptosis, cell fate determination, immune function and stress response. Aberrant p38 signalling has been implicated in many human diseases, including heart disease, cancer, arthritis and neurodegenerative diseases. To further understand the role of p38 in these processes, we generated a Drosophila strain that is null for the D-p38a gene. Mutants are homozygous viable and show no observable developmental defects. However, flies lacking D-p38a are susceptible to some environmental stresses, including heat shock, oxidative stress and starvation. These phenotypes only partially overlap those caused by mutations in D-MEKK1 and dTAK1, suggesting that the D-p38a gene is required to mediate some, but not all, of the functions ascribed to p38 signalling.
Keywords: Drosophila, p38 MAPK, stress signalling
Introduction
p38/HOG1 was first identified in yeast as a gene required for osmotic maintenance (Brewster et al, 1993). p38 signalling has since been shown to be important in organisms as diverse as plants and humans, and in processes including apoptosis, cell fate determination, immunity and stress responses (Ono & Han, 2000; Johnson & Lapadat, 2002). Aberrant p38 signalling has been implicated in many human diseases, including heart disease, cancer, arthritis and neurodegenerative disease, making it an attractive target for pharmaceutical intervention (Obata et al, 2000). For this reason, defining the processes regulated by p38 in vivo is essential.
The mitogen-activated protein kinase (MAPK) signalling cascade consists of three components: MAPK, MAPK kinase (MAPKK) and MAPKK kinase (MAPKKK). The main families of MAPKs are the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs) and p38 MAPKs (Kyriakis & Avruch, 1996; Ip & Davis, 1998; Pearson et al, 2001). Like all MAPKs, p38s are serine/threonine kinases, which contain a canonical TGY dual phosphorylation motif (Hanks & Hunter, 1995).
Four vertebrate p38 genes have been identified: α, β, δ and γ. However, the requirements for p38 in development and homeostasis remain the least clearly defined of the MAPKs. Murine p38α knockout mice are embryonic lethal and show defects in angiogenesis (Adams et al, 2000; Allen et al, 2000; Tamura et al, 2000). Single gene knockouts for MKK3 (Lu et al, 1999; Wysk et al, 1999) and MKK6 (Tanaka et al, 2002) are viable and lack developmental defects, whereas the phenotype of double MKK3 and MKK6 knockouts mimics the p38α mutant (Brancho et al, 2003), making detailed in vivo analysis difficult. Mutations that disrupt p38 pathway signalling in Caenorhabditis elegans result in mild defects in olfactory neuron cell fate determination (Sagasti et al, 2001; Tanaka-Hino et al, 2002), sensitivity to high metal ion concentrations and starvation (Koga et al, 2000), or pathogen resistance (Kim et al, 2002; Aballay et al, 2003).
Two Drosophila p38 MAPK genes, D-p38a and D-p38b, have been identified. D-p38 activity is increased in Drosophila cell lines in response to a variety of environmental stimuli, including osmotic shock, heat shock, oxidative stress, immune stimulation, serum starvation and UV radiation (Han SJ et al, 1998; Han ZS et al, 1998). The MAPKKs D-MKK3/licorne and D-MKK4 have been shown to activate both p38 isoforms in vitro (Han SJ et al, 1998; Han ZS et al, 1998). A small deletion that removes several genes including D-MKK3/licorne results in embryos with axis determination defects, although interpretation of these data is complicated by the presence of other genes in the deletion (Suzanne et al, 1999). Additionally, the Drosophila genome contains two MAPKKK genes implicated in p38 signalling. Both D-MEKK1 and dTAK1 (TGF-Activated Kinase) mutants have been isolated, and are homozygous viable and fertile, with no observed developmental defects. D-MEKK1 mutants show stress response phenotypes including sensitivity to heat shock and osmotic stress, but have a normal immune response (Inoue et al, 2001), whereas dTAK1 mutants have a strong immune response deficiency phenotype (Vidal et al, 2001). Overexpression screens indicate that TGF-β/dTAK1 signalling during development may act through JNK, D-p38a or D-p38b, depending on the overexpression system and tissue used (Adachi-Yamada et al, 1999; Takatsu et al, 2000; Mihaly et al, 2001). The relationship between D-MEKK1 and the p38 kinases is complex; phosphorylation of p38 in response to heat shock and osmotic stress is reduced but not abolished in D-MEKK1 null mutants, indicating that another MAPKKK is also partially responsible for p38 phosphorylation in the stress response (Inoue et al, 2001).
Importantly, no mutations in either Drosophila p38 gene have been reported so far. In this paper, we report the phenotypes of a complete deletion of D-p38a. Mutants show only partial phenotypic overlap with D-MEKK1, sharing susceptibility to some environmental stresses but not to others. D-p38a mutants show no detectable developmental defects, suggesting that D-p38a and D-p38b may be partially redundant as mediators of at least some aspects of MAPKK/MAPKKK activity.
Results
Generation of a D-p38a null strain
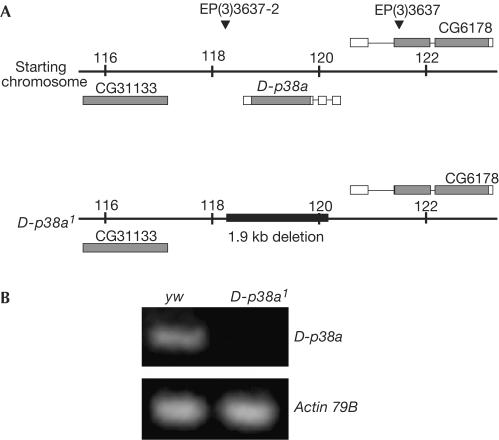
To address the role of p38 signalling in vivo, we generated a D-p38a null strain, D-p38a1, using a series of P-element-mediated mutagenesis strategies (Fig 1A). We mobilized the EP(3)3637 P-element (820 base pairs (bp) upstream of the D-p38a locus; Rörth et al, 1998), and recovered a new line that contained a second P-element insertion, EP(3)3637-2, 263 bp downstream of the D-p38a locus. These two elements were then simultaneously excised, creating a complete deletion of the D-p38a locus. The original EP(3)3637 was precisely excised, leaving both flanking genes intact, as determined by direct sequencing. Reverse transcription–PCR (RT–PCR) confirmed that this strain is null for the D-p38a locus (Fig 1B).
Figure 1.
Mutagenesis of the D-p38a locus. (A) Boxes indicate gene structure; shaded boxes indicate coding regions. Triangles indicate P-element insertion sites. EP(3)3637 was mobilized and the local hop EP(3)3637-2 recovered. Next, both P-elements were simultaneously excised, resulting in complete deletion of D-p38a. The black box indicates the size and position of the deletion, as determined by genomic DNA sequencing. Neither neighbouring gene was disrupted. (B) RT–PCR analysis confirms that D-p38a1 is null for the locus. No D-p38a transcript is detected in mutants. Actin (control) levels are unchanged.
D-p38a mutant strain is viable
Unlike mouse p38α mutants, which are embryonic lethal (Adams et al, 2000; Allen et al, 2000; Tamura et al, 2000), D-p38a1 flies are viable and fertile, and show no developmental abnormalities. No gross defects in patterning or apoptosis were observed in developing embryonic or larval tissues, including the embryonic nervous system, or the leg, wing and eye imaginal discs, in contrast to mutants of the Drosophila EGFR/Ras/MAPK and JNK pathways. Expected mendelian ratios of viable progeny were observed from crosses of mutants to flies heterozygous for a chromosomal deficiency of the D-p38a region (48% D-p38a1/+ versus 52% D-p38a1/deficiency, n=353). In addition, no interaction was observed when one allele of jnk/bsk1 or jnk/bsk2 was introduced into a D-p38a1 background; deficiencies removing one copy of D-p38b or both MKK3/lic and MKK7/hep also had no effect. Reducing D-p38b activity by overexpression of a D-p38b antisense construct (Adachi-Yamada et al, 1999) in a wild-type or D-p38a1 background similarly resulted in no developmental defects. Loss of one copy of D-p38a suppressed the ommatidial polarity phenotype induced by overexpression of the planar polarity gene disheveled (dsh), as previously reported (Paricio et al, 1999); however, removal of both copies of D-p38a1 failed to modify the hypomorphic phenotype of dsh1, making the significance of this observation unclear.
D-p38a mutants are susceptible to certain stresses
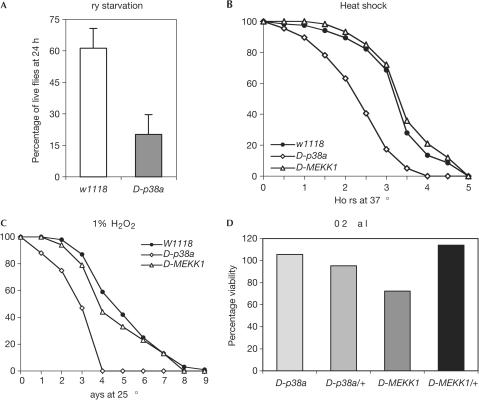
On the basis of previous work demonstrating the role of p38 signalling in environmental stress responses, we tested the susceptibility of D-p38a mutants to a variety of such stresses. Adult D-p38a1 mutants showed reduced resistance to dry starvation (n=220; Fig 2A). In addition, D-p38a1 mutant flies are susceptible to 37°C heat shock (n=480; Fig 2B). This susceptibility seems to be a specific defect in the heatshock response, as the average lifespan of D-p38a1 flies was unchanged compared with wild type at both 25 and 29°C (data not shown, n⩾380). This finding is in contrast to the phenotype observed for adult D-MEKK1 mutant flies, which were largely wild type in their response to heat shock. Lastly, D-p38a1 mutants are susceptible to oxidative stress; flies placed on medium containing 1% H2O2 showed a reduced lifespan compared with both wild-type and D-MEKK1 mutant flies (n=373; Fig 2C).
Figure 2.
D-p38a mutants are sensitive to some environmental stresses. (A) D-p38a1 mutants show reduced resistance to dry starvation. Adult Drosophila were placed in empty culture vials, and placed at 25°C. Live flies were counted twice daily. (B) D-p38a1 flies are vulnerable to 37°C heat shock, whereas D-MEKK1 mutants are not. Adult Drosophila were placed in vials containing normal growth medium, and placed at 37°C. Live flies were counted every 30 min. (C) D-p38a1 mutants are susceptible to oxidative stress, whereas D-MEKK1 mutants are not. Adult Drosophila were placed in vials containing a modified growth medium, consisting of 1.3% low-melting agarose, 1% sucrose and 1% H2O2, and placed at 25°C. Live flies were counted daily. (D) D-p38a mutants are not sensitive to high osmolarity, but D-MEKK1 mutants show reduced resistance (P=0.05). Homozygous D-p38a1 flies were crossed to flies heterozygous for a deficiency in the region, and balanced D-MEKK1ur36 flies in two genetic backgrounds were crossed to each other. Flies were allowed to lay eggs on normal food containing 0.2 M NaCl, and the total numbers of homozygous and heterozygous offspring were counted.
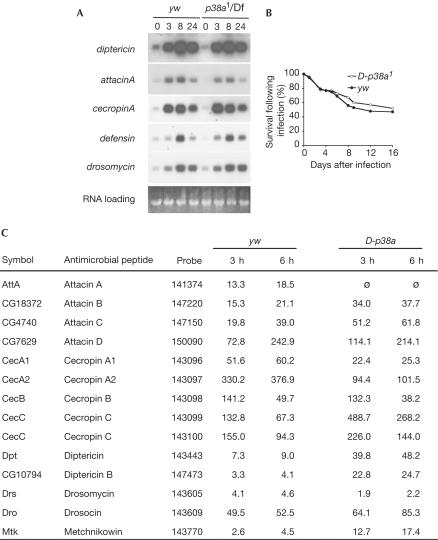
Curiously, unlike D-MEKK1 mutant flies, D-p38a1 mutants were not susceptible to osmotic shock. The viability of D-p38a1 homozygous and heterozygous flies was equivalent when reared on food containing 0.2 M NaCl, whereas the viability of D-MEKK1 homozygous flies was reduced (n=368), as previously reported (Inoue et al, 2001; Fig 2D). We observed a smaller reduction in D-MEKK1ur36 viability than previously reported (Inoue et al, 2001); the line has been subsequently outcrossed and this disparity in sensitivity to osmotic shock may reflect a difference in the genetic background. Although dTAK1 mutants were immune response deficient (Vidal et al, 2001), and overexpression of p38a in vivo impaired the innate immune response of larvae (Han ZS et al, 1998), no immune response deficiency was observed in D-p38a1 mutant adults. Northern analysis showed that following septic injury, the inducible expression of five representative antimicrobial peptide genes, diptericin, attacinA, cecropinA, defensin and drosomycin, was similar in control and mutant flies (Fig 3A). Moreover, survival of D-p38a mutants following bacterial infection was not compromised (Fig 3B). Additionally, wound healing appeared normal in adults and embryos (data not shown; B. Stramer and P. Martin, personal communication). Finally, we used Affymetrix oligonucleotide arrays to provide a more global measure of gene regulation after infection. Microarray analysis indicated that antimicrobial peptide gene expression was comparable to controls, as was the expression of other genes important in immune and stress responses: although some variation in expression levels was observed between control and mutant flies, such variation is normal for immune response genes (Fig 3C; also see supplementary information online for the full microarray data set).
Figure 3.
D-p38a mutants are not sensitive to septic injury. (A) Following infection, there were no changes in RNA levels for diptericin, cecropinA, defensin and drosomycin, as assessed by northern analysis; attacinA showed at most a small decrease. (B) Survival following infection was also unaffected. Adult Drosophila were inoculated with a bacterial cocktail of E. coli 055:B5, M. luteus and E. carotovora 15, and allowed to recover at 25°C. Live flies were counted every 24 h. (C) DNA microarray experiments confirm that the immune response profile of D-p38a mutants is largely normal.
One potential exception is induction of attacinA, which was consistently reduced on our microarrays for D-p38a flies. However, subsequent attempts to confirm this result using RT–PCR and northern analysis did not clearly confirm this result (Fig 3A). There are four attacin genes (attacinA–D) in the Drosophila genome, and cross-hybridization of the attacinA probe to these other genes makes it difficult to determine which (if any) of them is defective in the response to septic injury. Because the viability of the D-p38a flies after septic injury is normal, any defect in the induction of the single attacinA gene may not have a significant effect on the overall immune response of the mutants.
Overexpression of D-p38a alters the heat stress response
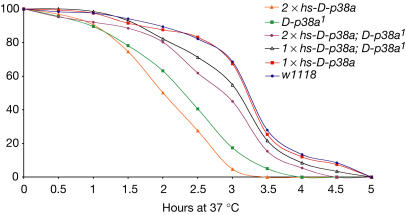
To demonstrate that the stress responses reported above are indeed due to loss of D-p38a function, we performed rescue experiments by expressing D-p38a on an inducible ubiquitous heat-shock promoter. Ectopic expression of D-p38a from a single copy of the transgene in a wild-type background did not change survival of 37°C heat shock (Fig 4). However, similar expression of D-p38a in a D-p38a1 mutant background was successful in rescuing mutant survival to 86.8% of wild-type levels.
Figure 4.
Ectopic expression of p38a rescues heat-shock sensitivity. D-p38a1 mutants are sensitive to 37°C heat shock, as previously shown. Expression of D-p38a with one copy of the transgene in a wild-type background has no effect. Expression of D-p38a in one copy in a D-p38a1 background rescues heat sensitivity to 86.8% of wild type, demonstrating that the D-p38a1 heat-shock sensitivity phenotype is due to the loss of D-p38a. However, expression of D-p38a with two copies of the transgene in a D-p38a1 background rescues heat sensitivity to only 75.4% of wild type. Expressing D-p38a with two copies of the transgene in a wild-type background reduced the survival rate to just 33.6% of control, suggesting that high levels of D-p38a also inhibit the ability of flies to adapt to heat shock. An average of 200 flies was tested for each genotype.
Surprisingly, a further increase in expression derived from two copies of the transgene in a D-p38a mutant background resulted in a less robust rescue of heat stress, to 75.4% of wild-type levels. In addition, expressing D-p38a with two copies of the transgene in a wild-type background further reduced the survival rate to just 33.6% of control (Fig 4). This suggests that levels of D-p38a above a certain threshold can inhibit the ability of flies to adapt to heat shock. Therefore, although these experiments confirm that signalling through D-p38a mediates heatshock response, it is also clear that tight regulation of D-p38a is required for appropriate stress response signalling.
Discussion
Our genotypically null D-p38a1 strain lacks defects in development or apoptosis. D-p38a mutants are sensitive to some environmental stresses, such as heat shock, dry starvation and H2O2 exposure. D-p38a flies are not sensitive to high osmolarity or infection, distinguishing them from D-MEKK1 and dTAK1 flies, respectively. Another MAPK, such as D-p38b or JNK, may mediate these processes. Significantly, reduction of D-p38b or JNK did not show any additional phenotypes in a D-p38a null background, although we cannot rule out the ability of residual D-p38b or JNK to provide full function. Conversely, D-p38a mutants are more susceptible to acute heat shock and oxidative stress, whereas D-MEKK1 mutants responded in a manner similar to wild type. This difference suggests that at least for some responses, D-p38a activity is independent of D-MEKK1. These phenotypes highlight the complexity of signalling in this pathway; phosphorylation of p38 in response to heat and osmotic stress is reduced but not abolished in D-MEKK1 mutants, indicating that another MAPKKK may be partially responsible for phosphorylating the p38 MAPKs (Inoue et al, 2001). To assess the p38 pathway function more completely, mutations in the D-MKK3/licorne, D-MKK4 and D-p38b loci will be required.
p38 signalling is a potential target for pharmacological intervention in many inflammatory diseases, including pulmonary disease, Crohn's disease and Alzheimer's disease. Clinical trials are presently underway to study the efficacy of p38 inhibitors in rheumatoid arthritis and asthma, among others. Given the chronic nature of these diseases, understanding the consequences of long-term loss of p38 signalling in vivo may be important in predicting potential negative outcomes in patients. Indeed, in one example, clinical development was abandoned after adverse neurological effects were seen in animal models, although it is unclear whether these effects were specific to the loss of p38 function or another unintended target (Kumar et al, 2003). We demonstrate that in vivo loss of D-p38a in Drosophila does not have any developmental consequences or affect longevity, but does confer sensitivity to specific environmental stresses.
Methods
Fly strains. EP(3)3637, Df(3R)crbF894, CyOΔ23/EgfrBc, dsh1, jnk/bsk1 and jnk/bsk2 were obtained from the Bloomington Stock Center. Df(2L)b82a2 was obtained from the Umeå Stock Center. UASp38bantisense and D-MEKK1ur36 flies were a gift from K. Matsumoto. hs-p38a has been described (Han ZS et al, 1998). sevDsh were provided by M. Mlodzik.
P-element excisions. EP(3)3637 flies were crossed to transposase-expressing lines, and male progeny carrying both elements were mated to w; TM3Sb/TM6BTb females. EP(3)3637-2 flies, containing a second P-element insertion 3′ of the D-p38a locus, were then crossed to transposase-expressing lines, and male progeny carrying all three elements were collected, mated to w; TM3Sb/TM6BTb females, and progeny with white eyes were collected and analysed. The D-p38a1 mutation was subsequently recombined onto the chromosome FRT82B to remove a lethal background mutation.
Genomic DNA was isolated as described (Liao et al, 2000). Insertion lines were screened by PCR for proximity to the original P-element insertion. Excisions of the tandem P-elements in EP(3)36372 were analysed by PCR using a series of primers that recognized genomic sequences flanking the D-p38a locus.
RT–PCR analysis of mutants. Total RNA was isolated from adult flies using TRI Reagent (Molecular Research Center Inc.). Complementary DNA was synthesized with random primers and SuperScript II Reverse Transcriptase RT–PCR System (Invitrogen) and analysed by PCR with the following primer sequences: p38a, 5′-TGGAAAAGATGTTGGAG-3′ and 5′-TATCCTCGAAGCTGTGATCG-3′, and Actin79B, 5′-CATCCGCAAGGATCTGTATG-3′ and 5′-TTCCTTTTGCATACGGTCAG-3′.
Stress tests. Adult flies of the genotypes shown below were aged 3–5 days and subjected to various environmental stresses, and live flies were counted, on the basis of movement following manipulation of the vial. All tests were performed at 25°C unless otherwise stated.
w1118
w; FRT82B, D-p38a1/FRT82B, D-p38a1
w; hs-p38a/+; FRT82B, D-p38a1/FRT82B, D-p38a1
w; hs-p38a/hs-p38a; FRT82B, D-p38a1/FRT82B, D-p38a1
w; hs-p38a/+
w; hs-p38a/hs-p38a
w; D-MEKK1ur36-old/D-MEKK1ur36-new
Oxidative stress: Adult Drosophila were placed in vials containing a modified growth medium, consisting of 1.3% low-melting agarose, 1% sucrose and 1% H2O2, and live flies were counted daily (Monnier et al, 2002).
Dry starvation: Adult Drosophila were placed in empty culture vials, and live flies were counted twice daily (Kang et al, 2002).
Heat shock: Adult Drosophila were placed in vials containing normal growth medium, and placed in a 37°C water bath. Live flies were counted every 30 min.
Osmotic shock: Progeny from the crosses w; FRT82B, D-p38a1/FRT82B, D-p38a1 × w; Df(3R)crb-F89-4,st1e/TM3,Sb or D-MEKK1ur36-old/TM3,SbSer × D-MEKK1ur36-new/TM3,SbSer were grown from embryos on medium containing 0.2 or 0.5 M NaCl (Inoue et al, 2001). Genotypes were scored on eclosion. Viability was calculated by dividing the number of observed progeny by the number of predicted progeny for each genotype. χ2 analysis was used to test for fit.
Infection experiments. Adult flies (yw and yw; D-p38a/Df(3R)crb-F89-4,st1e1, to control for background mutations) were aged 3–5 days at 18°C. Bacterial infections were performed by pricking CO2-anaesthetized flies in the thorax, using a needle dipped in a bacterial cocktail containing Escherichia coli 055:B5, Micrococcus luteus and Erwinia carotovora 15. Flies were allowed to recover at 25°C, and live flies were counted daily.
Northern analysis. Following infection, adult flies were homogenized and total RNA was isolated using TRI Reagent (Molecular Research Center Inc.). Northern blots were performed as previously described (Han ZS et al, 1998).
DNA microarray analysis. Infections were performed and RNA was isolated as above. Double-stranded cDNA was prepared, and biotin-labelled cRNA made using the BioArray High Yield Kit according to the manufacturer's protocol (Affymetrix). cRNA (30 μg) was hybridized to Drosophila GeneChips (Affymetrix). Fluorescence intensity measurements and data analysis were carried out using GeneChip software. Baseline values were obtained from the 0 h condition for each genotype. Each condition was performed in duplicate. Transcripts that were changed at least twofold at two or more time points were considered significant.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor).
Supplementary Material
Supplementary Information
Acknowledgments
We thank S. Tanenbaum and A. DiAntonio for helpful advice. We thank K. Matsumoto, M. Mlodzik, the Bloomington Stock Center and the Umeå Stock Center for materials. This work was supported by NIH-2R01-EY11495.
References
- Aballay A, Drenkard E, Hilbun LR, Ausubel FM (2003) Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol 13: 47–52 [DOI] [PubMed] [Google Scholar]
- Adachi T et al. (1999) p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol 19: 2322–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH et al. (2000) Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 6: 109–116 [PubMed] [Google Scholar]
- Allen M, Svensson L, Roach M, Hambor J, McNeish J, Gabel CA (2000) Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med 191: 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancho D et al. (2003) Mechanism of p38 MAP kinase activation in vivo. Genes Dev 17: 1969–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC (1993) An osmosensing signal transduction pathway in yeast. Science 259: 1760–1763 [DOI] [PubMed] [Google Scholar]
- Han SJ, Choi KY, Brey PT, Lee WJ (1998) Molecular cloning and characterization of a Drosophila p38 mitogen-activated protein kinase. J Biol Chem 273: 369–374 [DOI] [PubMed] [Google Scholar]
- Han ZS et al. (1998) A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol Cell Biol 18: 3527–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576–596 [PubMed] [Google Scholar]
- Inoue H et al. (2001) A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J 20: 5421–5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol 10: 205–219 [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912 [DOI] [PubMed] [Google Scholar]
- Kang HL, Benzer S, Min KT (2002) Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci USA 99: 838–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH et al. (2002) A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297: 623–626 [DOI] [PubMed] [Google Scholar]
- Koga M, Zwaal R, Guan KL, Avery L, Ohshima Y (2000) A Caenorhabditis elegans MAP kinase kinase, MEK-1, is involved in stress responses. EMBO J 19: 5148–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC (2003) p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2: 717–726 [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J (1996) Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays 18: 567–577 [DOI] [PubMed] [Google Scholar]
- Liao GC, Rehm EJ, Rubin GM (2000) Insertion site preferences of the P transposable element in Drosophila melanogaster. Proc Natl Acad Sci USA 97: 3347–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HT et al. (1999) Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J 18: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly J, Kockel L, Gaengel K, Weber U, Bohmann D, Mlodzik M (2001) The role of the Drosophila TAK homologue dTAK during development. Mech Dev 102: 67–79 [DOI] [PubMed] [Google Scholar]
- Monnier V, Girardot F, Audin W, Tricoire H (2002) Control of oxidative stress resistance by IP3 kinase in Drosophila melanogaster. Free Radic Biol Med 33: 1250–1259 [DOI] [PubMed] [Google Scholar]
- Obata T, Brown GE, Yaffe MB (2000) MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med 28: N67–N77 [DOI] [PubMed] [Google Scholar]
- Ono K, Han J (2000) The p38 signal transduction pathway: activation and function. Cell Signal 12: 1–13 [DOI] [PubMed] [Google Scholar]
- Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M (1999) The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J 18: 4669–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G et al. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183 [DOI] [PubMed] [Google Scholar]
- Rörth P et al. (1998) Systematic gain-of-function genetics in Drosophila. Development 125: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Sagasti A, Hisamoto N, Hyodo J, Tanaka-Hino M, Matsumoto K, Bargmann CI (2001) The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell 105: 221–232 [DOI] [PubMed] [Google Scholar]
- Suzanne M et al. (1999) The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev 13: 1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu Y et al. (2000) TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol Cell Biol 20: 3015–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M (2000) Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102: 221–231 [DOI] [PubMed] [Google Scholar]
- Tanaka N et al. (2002) Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep 3: 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Hino M et al. (2002) SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep 3: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B (2001) Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev 15: 1900–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysk M, Yang DD, Lu HT, Flavell RA, Davis RJ (1999) Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA 96: 3763–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information