Summary
How are human and veterinary medicines in soils and water bodies affecting human and environmental health?
Medicines have an important role in the treatment and prevention of disease in both humans and animals. But it is because of the very nature of medicines that they may also have unintended effects on animals and microorganisms in the environment. Although the side effects on human and animal health are usually investigated in thorough safety and toxicology studies, the potential environmental impacts of the manufacture and use of medicines are less well understood and have only recently become a topic of research interest. Some of the effects of various compounds—most notably anthelmintics from veterinary medicine and antibacterial therapeutics—are already known (Daughton & Ternes, 1999; Boxall et al, 2003a, 2004a; Floate et al, 2005), but there are many other substances that can affect organisms in the environment. This is further complicated by the fact that some pharmaceuticals can cast effects on bacteria and animals well below the concentrations that are usually used in safety and efficacy tests. In addition, breakdown products and the combination of different biologically active compounds may have unanticipated effects on the environment. Although it may be safe to assume that these substances do not substantially harm humans, we have only recently begun to research whether and how they affect a wide range of organisms in the environment and what this means for environmental health.
...we have only begun to research whether and how they affect a wide range of organisms in the environment and what this means for environmental health
...recent monitoring studies have detected low levels of a wide range of pharmaceuticals ... in soils, surface waters and groundwaters
The scope of this potential problem is not to be underestimated. More than 10 million women in the USA alone use oral contraceptives, which eventually find their way into the environment. A wide range of human medicines, including antibiotics, statins or cytotoxins used in cancer treatment, are produced and used, some in the range of thousands of tons per year. It is hard to obtain information on the amount of human medicines used, but recent data from Canada indicates that high-use drugs include acetominophen, acetylsalicylic acid, ibuprofen, naproxen and carbamazepine (Metcalfe et al, 2004). Large amounts of veterinary medicines, such as antibacterials, antifungals and parasiticides from aquaculture and agriculture, may also contribute to the stress on the environment, particularly as they often find their way directly into soils and surface waters unlike human medicines, which usually go through a water treatment plant first. The use of antibacterials in aquaculture in the USA alone is estimated to be between 92,500 and 196,400 kg per year (Benbrook, 2002), while estimates for the total use of antibacterials in US agriculture range between 8.5 and 11.2 million kg annually (Nawaz et al, 2001; Mellon et al, 2001).
These human and veterinary therapeutics are released to the environment by various routes (Fig 1). Residues released during the manufacturing process may ultimately enter surface waters. After administration, human medicines are absorbed, metabolized and then excreted to the sewer system. They usually go through a treatment works before they find their way into receiving waters or land by the application of sewage sludge. Antibacterials for the treatment of fish or shrimp in aquaculture are directly released to surface waters. Veterinary medicines used to treat pasture animals are excreted to soils or surface waters. In intensive livestock treatments, these medicines are likely to enter the environment indirectly through the application of slurry and manure as fertilizers. Other minor routes of entry include emissions to air and through the disposal of unused medicines and containers.
Figure 1.
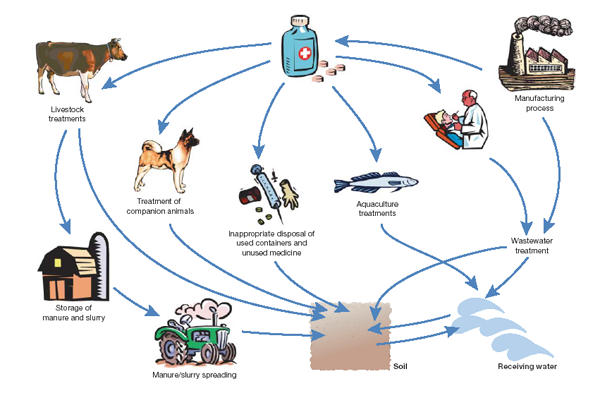
Routes of pharmaceuticals entering the environment
Although pharmaceuticals have been released into the environment for decades, researchers have only recently begun to quantify their levels in the environment. Using information from different countries and on various usage patterns, several prioritization exercises have identified those pharmaceuticals that are most likely to be released into the environment (Box 1). For example, data from the UK on annual usage of veterinary drugs was combined with information on administration routes, metabolism and ecotoxicity to identify medicines that should be monitored in a national reconnaissance programme (Boxall et al, 2003b). Hilton et al (2003) performed a similar exercise for human medicines using information on annual usage and therapeutic dose along with predictive models. Although these studies are generally based on countryspecific information, they still provide an indication of those substances that should be investigated at the international level. New analytical techniques, such as liquid chromatography coupled with tandem mass spectrometry (LC-MS-MS), have allowed us to develop a better understanding of how medicines behave in the environment and to determine concentrations in wastewater treatment plants, soils, surface waters and groundwaters.
Once released into the environment, pharmaceuticals will be transported and distributed to air, water, soil or sediment. A range of factors, such as the physico-chemical properties of the compound and the characteristics of the receiving environment, will affect their distribution. The degree to which a pharmaceutical is transported between the different environmental media primarily depends on the sorption behaviour of the substance in soils, sediment-water systems and treatment plants, which varies widely across pharmaceuticals. Reported sorption coefficients for several veterinary medicines in soils range from less than 1 litre per kilogram to more than 6,000 litres per kilogram (Boxall et al, 2004b). Additionally, there is a large variability in the sorption behaviour within an individual matrix—for example, partition coefficients for enrofloxacin in a range of soil types vary by up to a factor of 30 (Boxall et al, 2004b). Moreover, unlike other organic substances, such as pesticides and industrial chemicals, the sorption behaviour of many pharmaceuticals cannot be simply derived from the substance's hydrophobicity or the organic carbon content of the solid material (Tolls, 2001).
Pharmaceutical substances may also be degraded by biological organisms in treatment systems, water bodies and soils as well as abiotic reactions. Generally, these processes reduce the potency of medicines; however, some breakdown products have similar toxicity to their parent compounds (Hallingsörensen et al, 2002). Furthermore, degradation varies significantly depending on chemistry, biology and climatic conditions. For example, the half-life of the antiparasitic ivermectin under winter conditions is six times greater than in the summer and the compound degrades faster in sandy soils than in sandy loam soils (Halley et al, 1993). The natural estrogens 17β-estradiol and estrone degrade in the aerobic and anoxic tanks of activated sludge systems, whereas 17α-ethinylestradiol only degrades under aerobic conditions (Ternes et al, 2004). All this adds to the complexity of the problem and calls for individual solutions for individual pharmaceuticals and applications.
Not surprisingly, recent monitoring studies have detected low levels of a wide range of pharmaceuticals, including hormones, steroids, antibiotics and parasiticides, in soils, surface waters and groundwaters (Hirsch et al, 1999; Kolpin et al, 2002; Table 1). The reported concentrations are generally low—usually less then 1 μg l−1 in surface waters—but what is more worrisome is that many therapeutic substances have been found across a wide variety of hydrological, climatic and land-use settings, and many of the substances have been detected throughout the year. These findings have raised questions about how this mixture of veterinary and human medicines abundant in soils and surface waters has an impact on beneficial organisms in the environment and on human health.
Table 1.
Pharmaceuticals detected in surface water monitoring studies
| Medicine class | Substances detected | Maximum concentration (ng l−1) |
|---|---|---|
| Antibiotics | Chloramphenicol | 355 |
| Chlortetracycline | 690 | |
| Ciprofloxacin | 30 | |
| Lincomycin | 730 | |
| Norfloxacin | 120 | |
| Oxytetracycline | 340 | |
| Roxithromycin | 180 | |
| Sulphadimethoxine | 60 | |
| Sulphamethazine | 220 | |
| Sulphamethizole | 130 | |
| Sulphamethoxazole | 1,900 | |
| Tetracycline | 110 | |
| Trimethoprim | 710 | |
| Tylosin | 280 | |
| Antacid | Cimetidine | 580 |
| Ranitidine | 10 | |
| Analgesic | Codeine | 1,000 |
| Acetylsalicylic acid | 340 | |
| Carbamazepine | 1,100 | |
| Diclofenac | 1,200 | |
| Aminopyrine | 340 | |
| Indomethacine | 200 | |
| Ketoprofen | 120 | |
| Naproxen | 390 | |
| Phenazone | 950 | |
| Antianginal | Dehydronifedipine | 30 |
| Antihypertensive | Diltiazem | 49 |
| Antidepressant | Fluoxetine | 12 |
| Antihyperlipidemic | Gemfibrozil | 790 |
| Antidiabetic | Metformin | 150 |
| Antipyretic | Acetaminophen | 10,000 |
| Anti-inflammatory | Ibuprofen | 3,400 |
| Antiseptic | Triclosan | 150 |
| Beta blockers | Betaxolol | 28 |
| Bisoprolol | 2,900 | |
| Carazolol | 110 | |
| Metoprolol | 2,200 | |
| Propanolol | 590 | |
| Timolol | 10 | |
| Bronchodilator | Clenbuterol | 50 |
| Fenoterol | 61 | |
| Salbutamol | 35 | |
| Contraceptive | 17a-Ethinylestradiol | 4.3 |
| Ectoparasiticides | Cypermethrin | 85,100 |
| Diazinon | 580,000 | |
| Emamectin benzoate | 1,060 | |
| Lipid regulator | Bezafibrate | 3,100 |
| Clofibrate | 40 | |
| Gemfibrozil | 510 | |
| Stimulant | Caffeine | 6,000 |
| X-ray contrast media | Diatrizoate | 100,000 |
Data taken from Daughton & Ternes, 1999; Kolpin et al, 2002; Boxall et al, 2004a.
Comparison of these data with therapeutic dose information, drinking water limits and health advisories indicates that the concentrations of therapeutic compounds in surface waters are well below levels that would be of concern to human health (Webb, 2001; Kolpin et al, 2002). It therefore seems that indirect exposure to pharmaceuticals through the water supply is unlikely to pose a risk to humans. However, risks through other routes of exposure, such as uptake from soils into crops and biomagnification through the food chain have yet to be quantified and cannot be ruled out completely.
The impacts on environmental health are more difficult to assess. Since 1980, the US Food and Drug Administration (FDA) requires environmental risk assessments of human and veterinary medicines on the effects on aquatic and terrestrial organisms before they allow a product to the market (Breton & Boxall, 2003), and the EU introduced similar requirements in 1997. These environmental impact studies investigate the potential negative effects on fish, daphnids, algae, bacteria, earthworms, plants and dung invertebrates. Much of the data are publicly accessible—many of the environmental assessments are published on the FDA's web site—and provide a reasonable body of data for further study (Boxall et al, 2004a). However, there are valid questions about the real-world value of these studies. Risk assessments usually use standard ecotoxicity tests, which are often short-lived and focus predominantly on mortality as the endpoint. Moreover, aquatic tests tend to focus on the water compartment and do not take into account pharmaceuticals residing in sediments. In general, the effects observed in these studies occur at much higher concentrations than those that are measured in the environment. What is less known are the more subtle effects that therapeutically active substances can have on organisms in the environment, such as growth, fertility or behaviour.
It is clear that current standard ecotoxicity tests are probably inappropriate for assessing the impacts of many pharmaceuticals
Pharmaceutical compounds are designed either to be highly active and interact with receptors in humans and animals or to be toxic for many infectious organisms, including bacteria, fungi and parasites. But this does not mean that they affect only these living forms. Many lower animals have receptor systems similar to humans and animals used in agriculture. Furthermore, many groups of organisms that affect human and animal health, which are targeted by pharmaceuticals, have a crucial role in the functioning of ecosystems. It is therefore possible that pharmaceuticals may cause subtle effects on aquatic and terrestrial organisms that are not detected in standard studies. And as human medicines are almost continuously released to the environment, wildlife organisms are exposed for much longer durations than those used in standard tests. Researchers have therefore begun to look into some of the more subtle effects caused by long-term, low-level exposure to pharmaceuticals. A wide range of subtle impacts has been reported so far (Table 2), including effects on oocytes and testicular maturation, impacts on insect physiology and behaviour, effects on dung decomposition, inhibition or stimulation of growth in aquatic plant and algae species, and the development of antibacterial resistance in soil microbes. Steroids from contraceptives are strongly suspected to affect the fertility and development of fish, reptiles and aquatic invertebrates. Equally, antibiotics from human and veterinary use have an effect on soil microbes and algae. Macrocyclic lactones can affect invertebrate larvae in dung at fairly low concentrations; earthworms appear sensitive to the parasiticides used in veterinary medicine and plants may be sensitive to many antibiotics. In addition, macrocyclic lactones have also been shown to elicit many sub-lethal responses in dung invertebrates, such as reduced feeding, disruption of water balances, reduction of growth rate, inhibition of pupation and the disruption of mating. As dung from livestock contains diverse fauna and provides a fruitful foraging habitat for other species, macrocyclic lactones may therefore indirectly affect other species by depleting the quality and quantity of their food source. The impacts of sediment-associated pharmaceuticals have also been considered. Using life-cycle studies, Nentwig et al (2004) showed that carbamazepine affects the emergence of chironomids, an aquatic midge. While many of these observations have been seen at environmentally realistic concentrations, the significance in terms of environmental health has yet to be established—in fact, this will be one of the challenges in the coming years.
Table 2.
Reported subtle effects of pharmaceutical compounds on aquatic and terrestrial organisms
| Substance(s) | Medicine class | Reported effect | Reference |
|---|---|---|---|
| Fenfluramine | Anorexic | Enhances release of serotonin (5-HT) in crayfish which in turn triggers the release of ovary-simulating hormone resulting in larger oocytes with enhances amounts of vitellin In fiddler crabs, stimulates the production of gonad-stimulating hormone accelerating testicular maturation | Daughton & Ternes, 1999 |
| 17a-Ethinylestradiol | Synthetic steroid | Endocrine-disrupting effects on fish, reptiles and invertebrates | Young et al, 2002 |
| Methyltestosterone | Synthetic steroid | Impersex, reduced fecundity, oogenesis, spermatogenesis in snails | Schulte-Oehlmann et al, 2004 |
| Avermectins | Parasiticide | Adults insects: loss of water balance, disruption of feeding and reduced fat accumulation, delayed ovarian development, decreased fecundity and impaired mating Juvenile insects: delayed development, reduced growth rates, development of physical abnormalities, impairment of pupariation or emergence and a loss of developmental symmetry | Floate et al, 2005 |
| Tetracyclines, macrolides and streptomycin | Antibacterials | Antibacterial resistance measured in soil bacteria obtained from sites treated with pig slurry | Sengelov et al, 2003 |
| Cypermethrin | Ectoparasiticide | Impact on dung decomposition | Sommer & Bibby, 2002 |
| Fenbendazole | Parasiticide | Impact on dung decomposition | Sommer & Bibby, 2002 |
| Tylosin | Antibacterial | Impacts on the structure of soil microbial communities | Westergaard et al, 2003 |
| Erythromycin | Antibacterial | Inhibition of growth cyanobacteria and aquatic plants | Pomati et al, 2004 |
| Tetracycline | Antibacterial | Inhibition of growth cyanobacteria and aquatic plants | Pomati et al, 2004 |
| Ibuprofen | Anti-inflammatory | Stimulation of growth of cyanobacteria and inhibition of growth of aquatic plants | Pomati et al, 2004 |
| Fenofibrate | Lipid regulator | Inhibition of basal EROD activity in cultures of rainbow trout hepatocytes | Laville et al, 2004 |
| Carbamazepine | Analgesic | Inhibition of basal EROD activity in cultures of rainbow trout hepatocytes Inhibition of emergence of Chironomus riparius | Laville et al, 2004; Nentwig et al, 2004 |
| Diclofenac | Analgesic | Inhibition of basal EROD activity in cultures of rainbow trout hepatocytes | Laville et al, 2004 |
| Propanolol | Beta blocker | Weak EROD inducer in cultures of rainbow trout hepatocytes | Laville et al, 2004 |
| Sulphamethazole | Antibacterial | Inhibition of basal EROD activity in cultures of rainbow trout hepatocytes | Laville et al, 2004 |
| Clofibrate | Lipid regulator | Inhibition of basal EROD activity in cultures of rainbow trout hepatocytes | Laville et al, 2004 |
| Diazepam | Antianxiety drug | Inhibition in the ability of dissected polyps from the cnidarian Hydra Vulgaris to regenerate a hypostome, tentacles and a foot | Pascoe et al, 2003 |
| Digoxin | Cardiac glycoside | Inhibition in the ability of dissected polyps from the cnidarian Hydra Vulgaris to regenerate a hypostome, tentacles and a foot | Pascoe et al, 2003 |
| Amlodipine | Calcium channel blocker | Inhibition in the ability of dissected polyps from the cnidarian Hydra Vulgaris to regenerate a hypostome, tentacles and a foot | Pascoe et al, 2003 |
Furthermore, pharmaceutical substances are not the only contaminants in environmental systems. Aquatic and terrestrial organisms are exposed to a mixture of medicines and other substances, including pesticides, biocides and general industrial chemicals. A recent US monitoring study (Kolpin et al, 2002) detected the antibacterial agent lincomycin in combination with up to 27 additional chemicals. The study looked only for selected compounds, so many other synthetic substances may also have been present. Interactive effects, such as additivity of substances with similar modes of action and synergism, are therefore possible. As current environmental risk assessments focus on single substances, it is possible that these assessments are underestimating the impacts.
When we begin to consider these interactions, it is important that we do not just focus on toxicological endpoints. It is also possible that the environmental behaviour of a substance could change in the presence of other substances. Antibacterials, for example, have been shown to affect soil microbes, which have an important role in breaking down pesticides. For example, studies indicate that veterinary antibacterials may affect sulphate reduction in soil and inhibit the decomposition of dung (Westergaard et al, 2001). If a veterinary antibacterial were to be applied in slurry to an agricultural field before the application of a pesticide, it is quite possible that the environmental impact of the pesticide could be radically changed.
As very little is known about the impacts of pharmaceuticals on ecological health and the interactions of different compounds, some workers are taking a precautionary approach and are developing methods to reduce the releases of these substances to the environment. Various approaches have been advocated, including the control of pharmaceuticals at the source, the segregation of sources, the treatment of waste products to remove pharmaceutical compounds, the introduction of husbandry practices and the improvement of disposal systems for out-of-date medicines and waste containers (Table 3; Ternes et al, 2002, 2004; Daughton, 2003a,b). Source controls include labelling, controlled disposal and urine separation. Segregating sources of pharmaceuticals, such as hospital wastewater, which is likely to be heavily contaminated with pharmaceuticals and antibiotic-resistance bacteria, should make it possible to focus treatment resources on the most contaminated waters.
Table 3.
Approaches to reduce amounts of pharmaceuticals released to the environment
| Human medicines | Veterinary medicines | |
|---|---|---|
| Source control | Medicine labels provide information on possible environmental impact allowing physicians and veterinary surgeons to consider potential environmental impact when prescribing | |
| Source separation | Separation of hospital waste from other wastes to allow targeted treatment; separation of urine from solid material | Separation of treated animals from untreated animals |
| Changes in treatment and husbandry practices | – | Advice to user on usage and husbandry methods to reduce environmental exposure; for example, pasture animals should not be allowed near water bodies for X days after administration |
| Treatment of water and slurry | Wastewater treatment Sorption removes fluoroquinolones Biodegradation removes bezafibrate, sulphamethoxazole, ibuprofen, ethinylestradiol, diclofenac Ozonation removes estrone, beta blockers, antiphlogistics, antibiotics | Slurry and sludge storage; for example, tylosin degrades very rapidly during slurry storage so this might be a mechanism to reduce amounts released to soils |
| Good farming practice | For example, sludge and slurry should be applied to land as good farming practice and they then reduce the potential for drugs to contaminate surface and groundwaters | |
| Disposal | Advice on, and mechanisms for, appropriate disposal of unused medicines and containers | |
Pharmaceuticals can be removed when treated through physical processes, such as sorption or volatilization, biological degradation or chemical reactions, for instance, through treatment with ozone. The suitability of different options are likely to be highly specific for each substance. For example, the antibiotic ciprofloxacin is removed by strong sorption onto suspended solids of sewage sludge whereas diclofenac and 17α-ethinylestradiol undergo significant biodegradation in aged activated sludge. It is therefore likely that a range of measures will be required to reduce emissions. Many of the treatment methods, whilst removing the pharmaceuticals, may also produce transformation products that are more persistent and mobile than the parent compounds, some of which may also have similar or enhanced toxicity. Little work has been performed to assess the environmental impacts of these transformation products on the environment (Boxall et al, 2004c).
It is clear that during the past few years a wealth of data has become available on the levels of pharmaceuticals in the environment and on their effects on aquatic and terrestrial organisms. There are, however, still many questions that need to be addressed before we can eventually determine whether residues in the environment are a threat to human and environmental health. First, what are the risks of substances that have yet to be studied? Due to resource limitations, only a small proportion of pharmaceuticals in use today have so far been investigated, and there is a great need to understand how other substances affect the environment. Second, how can we better assess ecotoxicity? Current standard ecotoxicity tests are probably inappropriate for assessing the impacts of many pharmaceuticals. The use of more subtle endpoints, such as changed behaviour, physiology and biochemistry, seem to show some merit. Further work should be performed to identify these subtle effects. It is likely that many of the technologies now used by molecular biologists—for instance, proteomics and genomics techniques or large-scale DNA or protein arrays—could greatly help with this task.
Third, what do these ecotoxicity data mean in the real world? Although many subtle effects have been shown after exposure to pharmaceuticals at environmentally realistic concentrations, we need to establish what these data mean in terms of ecological functioning. Fourth, what are the risks of mixtures? Pharmaceuticals are unlikely to appear in the environment on their own so the current 'single-substance' approach to risk assessment could be underestimating environmental impacts. This also includes possible indirect effects. Little work has been done to determine the uptake of pharmaceuticals into organisms and through the food chain. Such studies are crucial to determine the potential indirect effects of environmental exposure on ecological and human health. A related question is whether we should worry about transformation products. Most work so far has focused on the parent compounds; however, we know that transformation products are produced in the environment and in treatment processes. It is important that we begin to understand the potential impacts of these substances.
Future work must therefore focus on understanding the biotic and abiotic processes underlying the release, environmental fate and effects of pharmaceuticals
Finally, does environmental exposure result in more antibacterial resistance? A wide range of antibacterials has been observed in waters and soils and many of these persist for some time. It is possible that such exposure will result in the formation of resistant microbes, which could pose a serious threat to human and animal health.
It will be impossible to design and carry out studies to answer each of these questions for every single substance that is in use today. Future work must therefore focus on understanding the biotic and abiotic processes underlying the release, environmental fate and effects of pharmaceuticals. Such an understanding should ultimately allow the development of new modelling approaches. For instance, Huggett et al (2004) have proposed a comparative plasma concentration model that bridges mammalian and fish species, which could provide useful information on the probable impacts of pharmaceuticals on fish. Other modelling approaches, such as quantitative structure–activity relationships, could allow us to estimate the environmental impacts of pharmaceuticals from their chemical structure. Read-across approaches, where data from closely related compounds are used to identify the impacts of an untested compound, may also help to improve environmental assessment studies. These improved tools should allow us to understand better the impacts of pharmaceuticals on the environment. In the meantime, we should strive to refine the ways in which we use, handle and treat medicines to minimize their releases to the environment.
Pharmaceuticals that have been identified as a priority for further study.
Human
Aminophylline, Beclametasone, theophylline, Paracetamol, Norethisterone, codeine, furosemide, Atenolol, Bendroflumethiazide, chlorphenamine, lofepramine, Dextropropoxyphene, Procyclidene, Tramadol, Clotrimazole, Thiridazine, Mebeverine, Terbinafine, tamoxifen, Trimethoprim, Sulfamethoxazole, Fenofibrate, diclofenac (Hilton et al, 2003)
Veterinary
Amitraz, Amoxicillin, Amprolium, Baquiloprim, Cephalexin, Chlortetracycline, Clavulanic acid, Clindamycin, clopidol, Cypermethrin, Cyromazine, Decoquinate, Deltamethrin, Diazinon, Diclazuril, Dihydrostreptomycin, Dimethicone, Emamectin benzoate, Enrofloxacin, Fenbendazole, Flavomycin, Flavophospholipol, florfenicol, Flumethrin, Ivermectin, Lasalocid Na, levamisole, Lido/lignocaine, lincomycin, Maduramicin, moensin, Morantel, Neomycin, Nicarbazin, Nitroxynil, Oxolinic acid, Oxytetracycline, Phosmet, Piperonyl butoxide, Poloxalene, Procaine benzylpencillin, Procaine penicillin, Robenidine HCL, Salinomycin Na, Sarafloxicin, Sulphadiazine, Tetracycline, Tiamulin, tilmicosin, Toltrazuril, Triclabendazole, Trimethoprim, Tylosin (Boxall et al, 2003b)

Acknowledgments
The author is grateful to the European Commission, the UK Environment Agency, DEFRA and English Nature, who have funded the work that has made this paper possible.
References
- Benbrook CM (2002) Antibiotic drug use in U.S. aquaculture. www.iatp.org/library/antibiotics
- Boxall ABA, Kolpin DW, Hallingsorensen B, Tolls J (2003a) Are veterinary medicines causing environmental risks? Environ Sci Technol 37: 286A–294A [DOI] [PubMed] [Google Scholar]
- Boxall ABA, Fogg LA, Kay P, Blackwell PA, Pemberton EJ, Croxford A (2003b) Prioritisation of veterinary medicines in the UK environment. Toxicol Lett 142: 207–218 [DOI] [PubMed] [Google Scholar]
- Boxall ABA, Fogg LA, Blackwell PA, Kay P, Pemberton EJ, Croxford A (2004a) Veterinary medicines in the environment. Rev Environ Contam Toxicol 180: 1–91 [DOI] [PubMed] [Google Scholar]
- Boxall ABA, Kay P, Blackwell PA, Fogg LA (2004b) Fate of veterinary medicines applied to soils. In Pharmaceuticals in the Environment Kummerer K (ed), pp 165–180. Heidelberg, Germany: Springer [Google Scholar]
- Boxall ABA, Sinclair CJ, Fenner K, Kolpin D, Maund SJ (2004c) When synthetic chemicals degrade in the environment. Environ Sci Technol 38: 368A–375A [DOI] [PubMed] [Google Scholar]
- Breton R, Boxall ABA (2003) Pharmaceuticals and personal care products in the environment: Regulatory drivers and research needs. QSAR Comb Sci 22: 399–409 [Google Scholar]
- Daughton CG (2003a) Cradle-to-cradle stewardship of drugs for minimizing the deposition whilst promoting human health. I. Rationale for and avenues toward a green pharmacy. Environ Health Perspect 111: 757–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton CG (2003b) Cradle-to-cradle stewardship of drugs for minimizing the deposition whilst promoting human health. II. Drug disposal, waste reduction and future directions. Environ Health Perspect 111: 775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107: 907–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floate KD, Wardhaugh KG, Boxall ABA, Sherratt TN (2005) Fecal residues of veterinary parasiticides: nontarget effects in the pasture environment. Annu Rev Entomol 50: 153–179 [DOI] [PubMed] [Google Scholar]
- Halley BA, VanHeuval WJA, Wislocki PG (1993) Environmental effects of the usage of avermectins in livestock. Vet Parasitol 49: 109–125 [DOI] [PubMed] [Google Scholar]
- Hallingsörensen B, Sengelöv G, Tjörnelund J (2002) Toxicity of tetracycline and tetracycline degradation products to environmentally-relevant bacteria, including, selected tetracycline-resistant bacteria. Arch Environ Contam Toxicol 42: 263–271 [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Thomas KV, Ashton D (2003) Targeted monitoring programme for pharmaceuticals in the aquatic environment. R&D Technical Report P6-012/06/TR, UK Environment Agency, Bristol, UK
- Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225: 109–118 [DOI] [PubMed] [Google Scholar]
- Huggett DB, Ericson JF, Cook JC, Williams RT (2004) Plasma concentrations of human pharmaceuticals as predictors of pharmacological responses in fish. In Pharmaceuticals in the Environment Kummerer K (ed), pp 373–386. Heidelberg, Germany: Springer [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36: 1202–1211 [DOI] [PubMed] [Google Scholar]
- Laville N, Ait-Aissa S, Gomez E, Casellas C, Porcher JM (2004) Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 196: 41–55 [DOI] [PubMed] [Google Scholar]
- Mellon M, Benbrook C, Benbrook KL (2001) Hogging It: Estimates of Antimicrobial Abuse in Livestock. Union of Concerned Scientists, Cambridge, MA, USA. www.ucsusa.org/publications [Google Scholar]
- Metcalfe C, Miao Xs, Hua W, Letcher R, Servos M (2004) Pharmaceuticals in the Canadian Environment. In Pharmaceuticals in the Environment Kummerer K (ed), pp 67–90. Heidelberg, Germany: Springer [Google Scholar]
- Nawaz MS, Erickson BD, Khan AA, Khan SA, Pothuluri JV, Rafii F, Sutherland JB, Wagner D, Cerniglia CE (2001) Human health impact and regulatory issues involving antimicrobial resistance in the food animal production environment. Regul Res Perspect 1: 1–10 [Google Scholar]
- Nentwig G, Oetken M, Oehlmann J (2004) Effects of pharmaceuticals on aquatic invertebrates—the example of carbamazepine and clofibric acid. In Pharmaceuticals in the Environment Kummerer K (ed), pp 195–208. Heidelberg, Germany: Springer [Google Scholar]
- Pascoe D, Karntanut W, Muller CT (2003) Do pharmaceuticals affect freshwater invertebrates? A study with the cnidarian Hydra vulgaris. Chemosphere 51: 521–528 [DOI] [PubMed] [Google Scholar]
- Pomati F, Netting AG, Calamari D, Neilan BA (2004) Effects of erythromycin and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat Toxicol 67: 387–396 [DOI] [PubMed] [Google Scholar]
- Schulte-Oehlmann U, Oetken M, Bachmann J, Oehlmann J (2004) Effects of ethinylestradiol and methyltestosterone in prosobranch snails. In Pharmaceuticals in the Environment Kummerer K (ed), pp 233–246. Heidelberg, Germany: Springer [Google Scholar]
- Sengelov G, Agerso Y, Hallingsorensen B, Baloda SB, Andersen JS, Jensen LB (2003) Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environ Int 28: 587–595 [DOI] [PubMed] [Google Scholar]
- Sommer C, Bibby BM (2002) The influence of veterinary medicines on the decomposition of dung organic matter in soil. Eur J Soil Biol 38: 155–159 [Google Scholar]
- Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Gulde BH, Preuss G, Wilme U, Seibert NZ (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36: 3855–3863 [DOI] [PubMed] [Google Scholar]
- Ternes TA, Stuber J, Herrmann N, McDowall D, Ried A, Kampmann M, Teiser B (2003) Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res 37: 1976–1982 [DOI] [PubMed] [Google Scholar]
- Ternes TA, Joss A, Siegrist H (2004) Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ Sci Technol 38: 393–398 [DOI] [PubMed] [Google Scholar]
- Tolls J (2001) Sorption of veterinary pharmaceuticals—a review. Environ Sci Technol 35: 3397–3406 [DOI] [PubMed] [Google Scholar]
- Webb SF (2001) A data based perspective on the environmental risk assessment of human pharmaceuticals. III. Indirect human exposure. In Pharmaceuticals in the Environment Kummerer K (ed), pp 221–230. Heidelberg, Germany: Springer [Google Scholar]
- Westergaard K, Muller AK, Christensen S, Bloem J, Sorensen SJ (2001) Effects of tylosin on the soil microbial community. J Soil Biol Biochem 33: 2061–2071 [Google Scholar]
- Young WF, Whitehouse P, Johnson I, Sorokin N (2002) Proposed predicted no-effect concentrations (PNECs) for natural and synthetic steroid oestrogens in surface waters. Environment Agency R&D Technical Report P2-T04/1, UK Environment Agency, Bristol, UK


