Abstract
The assembly of spliceosomal U-rich small nuclear ribonucleoproteins (U snRNPs) is an ATP-dependent process mediated by the coordinated action of the SMN and the PRMT5 complex. Here, we provide evidence that the activity of this assembly machinery is regulated by means of post-translational modification. We show that two main components of the SMN/PRMT5 system, namely the survival motor neuron (SMN) protein (reduced levels thereof causing spinal muscular atrophy) and pICln, are phosphorylated in vivo. Both proteins share a previously unknown motif containing either one or two phosphoserines. Alteration of these residues in SMN (serines 28 and 31) significantly impairs the activity of the SMN complex. Despite the presence of SMN in both the nucleus and cytoplasm, we find that only the latter promotes efficient SMN-mediated U snRNP assembly activity. As cytoplasmic SMN is phosphorylated to a much larger extent, we hypothesize that this modification is a key activator of the SMN complex.
Keywords: survival motor neuron (SMN) protein, phosphorylation, splicing, RNP biogenesis
Introduction
The biogenesis of spliceosomal U-rich small nuclear ribonucleoproteins (U snRNPs) is a stepwise process, the hallmark of which is the formation of a heptameric protein ring on the Sm site common to snRNAs U1, U2, U5 and U4. Although this assembly reaction occurs spontaneously in vitro (Raker et al, 1996), it requires the activity of the survival motor neuron (SMN) protein in vivo (Meister et al, 2002; Yong et al, 2004). Its functional entity, the SMN complex, comprises eight distinct proteins (termed SMN, Gemins 2–7 and the unr-interacting protein unrip) and the seven spliceosomal substrate proteins SmB/B′, SmD1, SmD2, SmD3, SmE, SmF and SmG (Meister et al, 2001a; Gubitz et al, 2004). The SMN complex facilitates the ATP-dependent transfer of these proteins onto the Sm site of a U snRNA. Thereby, a core snRNP is formed that, after hypermethylation of the RNA cap, is subject to nuclear import (Meister et al, 2001a; Pellizzoni et al, 2002). Recently, the SMN complex was shown to join forces with the PRMT5 complex (composed of PRMT5, pICln and WD45/Mep50), which modifies Sm proteins by symmetrical arginine methylation to increase the efficiency of snRNP assembly (Friesen et al, 2001; Meister et al, 2001b; Meister & Fischer, 2002).
Despite our knowledge about the role of the SMN/PRMT5 assembly unit, the regulation of its activity remains poorly understood. This study focuses on the identification of post-translational modifications in the SMN/PRMT5 complex and their functional relevance for U snRNP assembly.
Results
SMN/PRMT5 system factors are phosphorylated in vivo
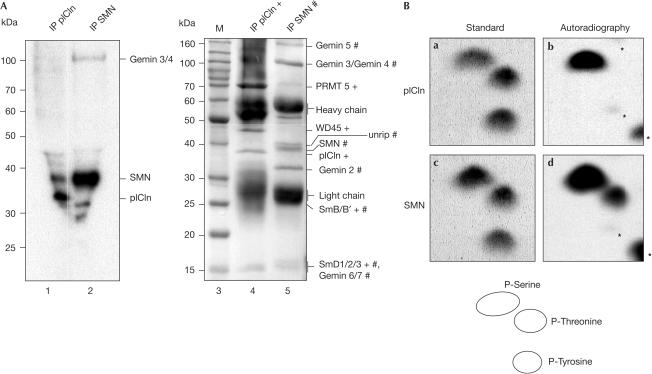
To analyse whether components of the assembly machinery of U snRNPs are phosphorylated in vivo, we performed immunoprecipitations on extracts derived from metabolically 32P-labelled HeLa cells. The antibodies applied were directed against SMN (monoclonal antibody 7B10) or pICln (polyclonal antiserum). Analysis of the immunoprecipitates by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and Coomassie staining indicated that 7B10 immunoprecipitated the SMN complex (Meister & Fischer, 2002), whereas the anti-pICln antibody co-isolated the PRMT5 complex along with sub-stoichiometric amounts of the SMN complex (Fig 1A, lanes 4 and 5). Autoradiographic analysis of the 7B10 immunoprecipitate showed phosphorylated components of the SMN complex with apparent molecular masses of 40 and 100 kDa (Fig 1A (lane 2) and supplementary information online). The 40 kDa component was identified as SMN by immunoblotting and MALDI-TOF (see below and not shown). On the basis of its mass, the 100 kDa phosphoprotein is likely to be Gemin 3 or 4; however, further studies are needed to verify this point. The anti-pICln precipitation identified pICln and SMN, which, in the first case, is consistent with data from Sanchez-Olea et al (1998).
Figure 1.
Phosphorylation of SMN and pICln in vivo. (A) Extracts from metabolically labelled HeLa cells were immunoprecipitated with anti-pICln and anti-SMN antibody, separated by SDS–PAGE and analysed by autoradiography (lanes 1 and 2). The arrows indicate phosphorylated SMN and pICln, and the asterisk indicates an unknown phosphorylated product. Coomassie-stained proteins of isolated complexes are shown on the right. Components of the SMN and the pICln complex are indicated (# and +, respectively). (B) Phosphoamino-acid analysis of immunoprecipitated and metabolically labelled SMN and pICln. In-vivo-labelled proteins shown in (A) were excised from the membrane and hydrolysed. The sample was mixed with nonlabelled phosphoamino acids and separated by 2D-TLC. In-vivo-labelled amino acids were detected by autoradiography (panels b and d) and compared with the ninhydrin-stained standard (panels a and c). The asterisks denote the partially hydrolysed protein.
To identify modified residues in SMN and pICln, we hydrolysed each phospho-labelled protein and separated the amino acids by thin-layer chromatography (TLC; see Methods for details). A comparison of the 32P-labelled spots with ninhydrin-stained phosphoamino-acid standard run on the same plate identified phosphorylation of SMN at serine and threonine residues, whereas pICln contained phosphoserine only (Fig 1B).
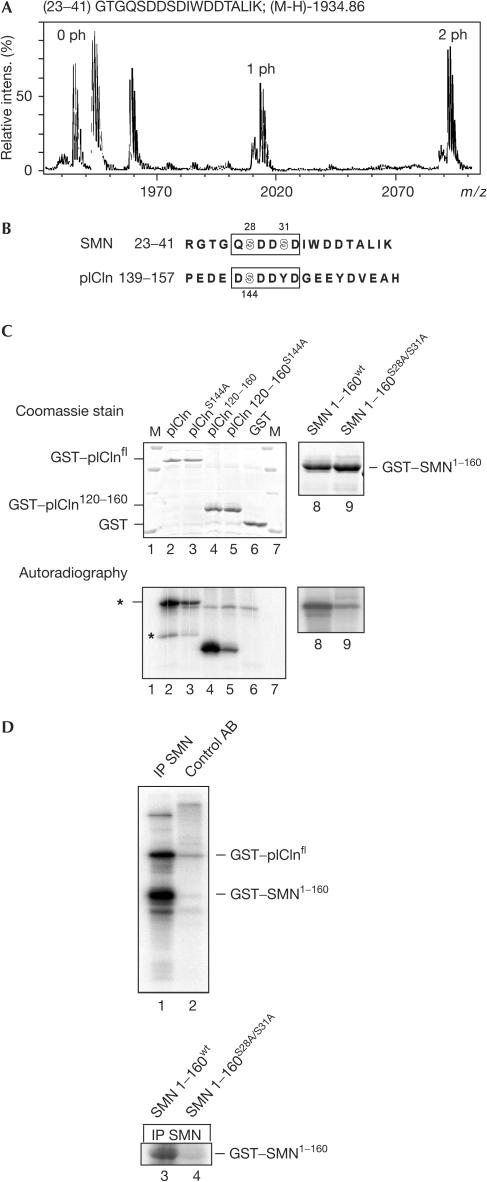
To characterize the sites of phosphorylation in SMN, we purified the protein from HeLa cells by immunoaffinity and analysed tryptic fragments by mass spectrometry. Initially, mass spectra were searched for peptides that differed by 80 mass units (the mass of a phosphate group). Using this approach, we identified an SMN peptide (position 23–41) containing two phosphoamino acids (Fig 2A, labelled 1 ph and 2 ph; 0 ph corresponds to the unphosphorylated peptide). Further fragmentation and nanospray Q-TOF analysis assigned phosphorylation to serines 28 and 31 (data not shown). These phosphoserines reside in a region conserved among SMN orthologues from higher eukaryotes that is implicated in binding to the SMN complex component Gemin 2 (Fig 2B and supplementary information online; Liu et al, 1997; Bühler et al, 1999). Intriguingly, a sequence similar to the above site in SMN also exists in pICln (Fig 2B, amino acids 143–148), raising the possibility that its embedded serine 144 could likewise be modified. Indeed, substitution of serine 144 by alanine reduced the extent of phosphorylation on pICln by cytosolic extract (Fig 2C, compare lanes 2 and 3). Consistently, a pICln fragment containing only two serine residues at positions 120 and 144 was phosphorylated, whereas a corresponding peptide containing an S144A mutation was about ten times less efficiently modified (lanes 4 and 5). Serine 144 is hence a bona fide phosphorylation target in pICln.
Figure 2.
Phosphoserines of SMN and pICln are embedded in a similar motif. (A) MALDI-TOF spectrum of a tryptic SMN fragment (positions 23–41). A selected mass range shows peaks originated from the peptide carrying no (0 ph), one (1 ph) or two (2 ph) phosphate groups. (B) Alignment of SMN and pICln carrying phosphorylated serines (highlighted). (C) Recombinant GST–pICln (lanes 2–7), GST–SMN(1–160) (lane 8) or GST–SMN(160)S28A/S31A (lane 9) protein was phosphorylated in cytoplasmic extract with [γ-32P]ATP. Immobilized proteins were subsequently washed, separated on an SDS gel and analysed by Coomassie staining (upper panel) and autoradiography (lower panel). Asterisks indicate unspecific 32P-incorporation (upper), and phosphorylation of a degradation product of pICln (lower). (D) Association of kinase activity with the SMN complex. Isolated SMN complex (lane 1) or a mock purification (lane 2) was incubated in the presence of [γ-32P]ATP with recombinant SMN(1–160) and pICln. In lanes 3 and 4, SMN(160) or SMN(1–160)S28A/S31A phosphorylated with purified SMN complex. Samples were fractionated by SDS–PAGE and analysed by autoradiography.
We next tested whether a kinase co-purifies with the SMN complex. To test this, the SMN complex was immunoprecipitated with 7B10 antibody and incubated with recombinant glutathione-S-transferase (GST)–SMN(1–160) (a fragment comprising amino acids 1–160) and GST–pICln in the presence of [γ-32P]ATP. Indeed, both substrates and none of the control proteins (GST, casein) were phosphorylated (Fig 2D, compare lanes 1 and 2; and data not shown). Furthermore, much weaker phosphorylation was observed with SMN in which the identified serines had been mutated to alanine (Fig 2D, compare lanes 3 and 4; see also Fig 2C, lanes 8 and 9, for phosphorylation in crude extract). Hence, these data indicate a stable, yet obviously sub-stoichiometric (Fig 1A, lane 5), binding of a specific kinase with the SMN complex.
SMN phosphorylation is crucial in U snRNP assembly
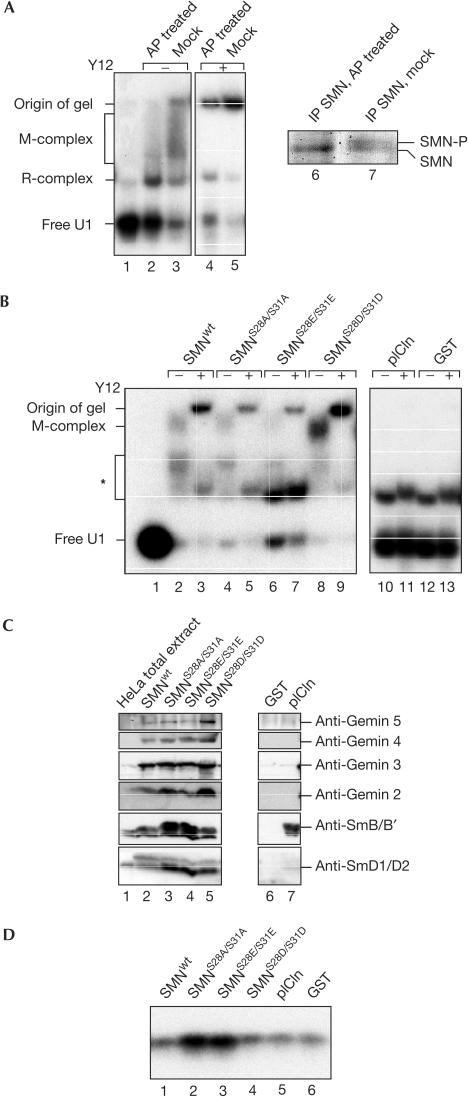
To analyse whether phosphorylation has a critical role in U snRNP assembly, the SMN complex isolated from the cytosol of HeLa cells was treated with alkaline phosphatase (AP; Fig 3A). AP treatment removes phosphate groups from SMN as evident by the faster migration of the protein on SDS–PAGE (Fig 3A, compare lanes 6 and 7). The ability of the dephosphorylated SMN complexes to assemble U1 snRNPs on 32P-labelled U1 snRNA was then assayed by native gel electrophoresis. In contrast to mock-treated SMN complex, the ability of dephosphorylated SMN complex to promote assembly of U1 snRNP was significantly reduced as indicated by much weaker formation of a previously reported ‘M-complex' that is supershifted by the anti-Sm antibody Y12 (Fig 3A, lanes 2–5). In contrast, complex ‘R', which is formed independently of SMN and consists of U1 snRNA and the U1-specific A protein only, was not affected on AP treatment. Having shown that AP treatment of the SMN complex reduces its activity, we next tested the functional relevance of phosphorylation at serines 28 and 31 in SMN. For this, the SMN complex was reconstituted by incubation of either immobilized wild-type or mutant GST–SMN with cytosolic extract. Dual mutations of serines 28 and 31 to either alanine or glutamic acid residues were applied to inhibit phosphorylation at these sites, whereas replacements by aspartic acid were used to mimic constitutive phosphorylation. The ability of reconstituted SMN complexes to assemble U1 snRNPs on 32P-labelled U1 snRNA was then assayed by native gel electrophoresis as above. Indeed, the reconstituted wild-type SMN complex promoted snRNP assembly, as indicated by the appearance of the M-complex (Fig 3B, lane 2) and the Y12 antibody-dependent supershift of this complex (lane 3). Note that, in this assay system, complexes of lower mass and unknown identity appear in addition, which are not identical to the R-complex seen when the assembly is analysed in extracts (Fig 3A, asterisk).
Figure 3.
U-rich small nuclear ribonucleoproteins (U snRNP) assembly requires phosphorylation of the SMN complex. (A) Isolated SMN complex was either AP treated (lanes 2 and 4) or mock treated (lanes 3 and 5) and subsequently incubated with 32P-labelled U1 snRNA. Assembly of U1 snRNP was analysed by native gel electrophoresis. In lanes 4 and 5, antibody Y12 was added to the assembly reaction to identify the Sm core domain (M). Lanes 6 and 7 show Coomassie-stained SMN protein of the Mock- and AP-treated SMN complex. SMN-P indicates the phosphorylated form of SMN. (B) SMN complexes reconstituted in vitro were tested in U1 snRNP assembly by native gel electrophoresis (lanes 2, 4, 6, 8, 10 and 12). In lanes 3, 5, 7, 9, 11 and 13, antibody Y12 was added to the assembly reaction to identify the Sm core domain (M). A faster migrating complex labelled with an asterisk can be detected in all assembly reactions. Although it co-migrates with the R-complex seen in assembly reactions with cytosolic extract, the identity of this complex is unknown. (C) Immobilized wild-type and mutant GST–SMN proteins were incubated with cytosolic HeLa extract and SMN-interacting proteins were identified by blotting. Recombinant GST or GST–pICln treated in the same way were used as controls (lanes 6 and 7). (D) Immobilized SMN complexes were incubated under assembly conditions with U1 snRNA and washed, and bound RNA was analysed by denaturing gel electrophoresis and autoradiography.
The serine-to-alanine substitutions at positions 28 and 31 in SMN significantly impaired the formation of the M-complex (lanes 4 and 5). Consistently, mutation of both serines to glutamic acid induced the same effect (lanes 6 and 7). By contrast, replacement of both serines to aspartic acid conferred an increase in assembly activity (lanes 8 and 9). Immunoblot analysis of the reconstituted complexes showed no loss of the SMN-binding factors Gemin 2–5, SmB/B′ and SmD1/D2 (Fig 3C, lanes 2–5). Instead, the hyperactive SMN complex contained reproducibly more Gemin 2, 4 and 5 and slightly more Sm proteins than its wild-type counterpart (compare lane 5 with 2), whereas the assembly-inefficient complexes showed strongly enhanced binding to SmB/B′ and SmD1/D2 (lanes 3 and 4). Therefore, the phospho-inactivating mutations in SMN seem to affect the release of Sm proteins or of assembled snRNPs, rather than the qualitative composition of the SMN complex. To follow up this issue, we asked whether the 32P-labelled U1 snRNA substrate was likewise retained on the immobilized SMN complex. Indeed, those SMN complexes harbouring phospho-inactivating mutations retained significantly more U1 snRNA than wild-type SMN or mock reconstitutions (Fig 3D, compare lanes 2 and 3 with lanes 1, 4, 5 and 6). In contrast, no binding was observed when labelled tRNA was used instead of U snRNA, indicating the specificity of the interaction (data not shown). Thus, these data suggest that phosphorylation of serines 28 and 31 is important for the kinetics of the assembly reaction and/or the subsequent release of U snRNP.
Compartment-specific modification of SMN
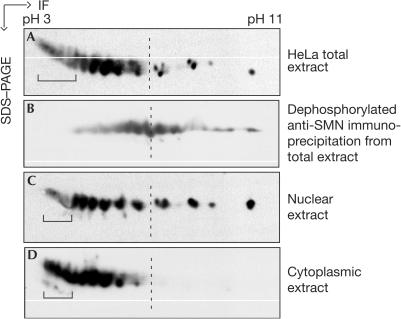
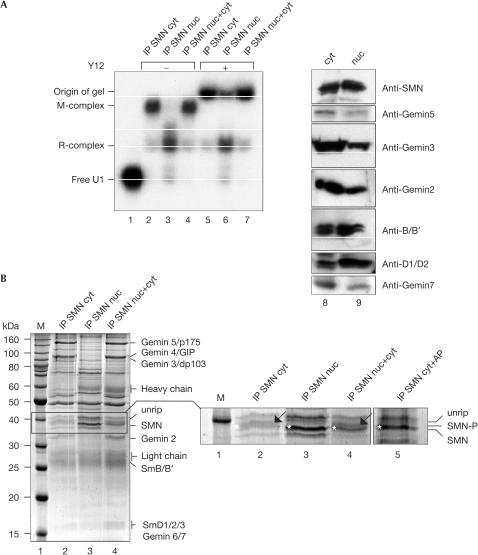
In view of the regulatory potential the phosphorylation of SMN has for snRNP assembly, we asked how the requirement for this modification relates to the subcellular localization of SMNs. We, therefore, compared the modification pattern of SMN from whole-cell extracts with that in cytoplasmic or nuclear extracts. In each case, the extracts were separated by two-dimensional PAGE (2D-PAGE), followed by anti-SMN immunoblot detection. As shown in Fig 4, the total cell extract contained several isoforms of SMN (Fig 4A). To test whether this pattern was, at least in part, caused by phosphorylation, we added AP to affinity-purified SMN complex from the total extract. Indeed, this treatment shifted the array of spots to higher pH (Fig 4B), demonstrating that many, but not all, of these isoforms had carried phosphate groups. Intriguingly, extracts obtained from either the nuclear (Fig 4C) or the cytoplasmic compartment (Fig 4D) showed clearly distinct modification patterns for SMN. Although nuclear SMN seems to contain all modifications observed in the total cell extract, only acidic isoforms of SMN are found in the cytoplasm (Fig 4). This encouraged us to test how these differences relate to SMN-mediated U snRNP assembly activity. SMN complexes that had been immunopurified from the nuclei or cytoplasm were tested for their ability to assemble U snRNPs on radiolabelled U1 snRNA. Strikingly, although both preparations contained identical amounts of SMN (Fig 5A, lanes 8 and 9), the cytoplasmic SMN complex promoted an efficient U snRNP assembly, whereas the nuclear complex was much less active (Fig 5A, compare lanes 2 and 3, and supplementary information online). Complementation of this nuclear SMN complex by SMN-depleted cytosolic extract fully restored its activity (Fig 5A (lane 4) and supplementary information online). In each case, the results were verified by supershift analysis using the Y12 anti-Sm antibody (Fig 5A, lanes 5–7). Taken together, these data indicate that cytosolic components are able to transform the SMN complex from an inactive nuclear state to its active cytosolic state.
Figure 4.
Compartment-specific modification of SMN. The total HeLa extract (A), nuclear extract (C) and cytoplasmic extract (D) were separated by 2D gel electrophoresis (isoelectric focusing, IF, followed by SDS–PAGE). The gel was subsequently blotted and SMN was detected using the monoclonal antibody 7B10. In (B), the SMN complex was isolated from HeLa total extract and dephosphorylated with AP. Analysis of the SMN isoforms was performed as above. Brackets indicate the hyperphosphorylated SMN forms.
Figure 5.
Reduced activity of the nuclear SMN complex in U snRNP assembly. (A) U1 snRNP assembly mediated by purified cytoplasmic (cyt; lane 2) and nuclear (nuc; lane 3) SMN complexes. Lane 4 shows the assembly reaction with nuclear complex that was reconstituted in the cytosolic extract depleted of SMN. In lanes 5–7, Y12 antibody was added to indicate Sm core assembly. Immunoblot analysis of the extracts with 7B10 and monospecific antibodies against Gemin 2, 3, 5, 7, SmB/B′ and SmD1/D2 is shown in lanes 8 and 9. (B) Composition of isolated cytoplasmic or nuclear SMN complex (lanes 2 and 3, respectively). Lane 4 shows immobilized nuclear SMN complex that had been incubated with SMN-depleted cytoplasmic extract. The enlarged part of the gel shows SMN in its phosphorylated or dephosphorylated form (indicated by an arrow and an asterisk, respectively). SMN complex, purified from the cytoplasm of HeLa cells and treated with AP, is shown in lane 5.
In view of these findings, we asked whether SMN complexes from either compartment contained the same set of proteins. In contrast to the active cytosolic SMN complex, which contained all known components (Fig 5B, lane 2), three proteins were under-represented in the nuclear complex, namely dp103 (Gemin 3), Gemin 4 and p175 (Gemin 5). Moreover, nuclear and cytoplasmic SMNs have different electrophoretic mobilities (see magnification in Fig 5B). One slower migrating band that appeared in the cytosolic SMN complex (lane 2, marked by an arrow) is absent from the purified nuclear SMN complex (lane 3) and reappeared on addition of SMN-depleted cytosolic extract (lane 4). Phosphatase treatment of cytosolic SMN complex removed this modification, changing the mobility of SMN to that observed in the nucleus (lane 5, marked by an asterisk). These data are consistent with the idea that the activity and composition of the SMN complex is regulated in a compartment-specific manner.
Discussion
Although isolated Sm proteins can assemble spontaneously onto U snRNA in vitro, this step in the formation of spliceosomal U snRNPs occurs as an active and ordered process in vivo (Meister et al, 2002; Yong et al, 2004). Two complexes, known as the SMN and the PRMT5, join activities to recruit Sm proteins and transfer them onto snRNA. However, little is known about their potential regulation in the cellular environment. In this study, we have shown that several proteins of these complexes, including SMN, pICln and at least one more protein (Gemin 3 and/or 4), undergo post-translational modifications. In the case of SMN, mutation of two phosphoserine residues (amino-acid positions 28 and 31) impairs snRNP assembly, indicating that phosphorylation of SMN is a crucial step in this process.
In the reconstitution assay applied here, snRNP assembly was impaired by mutations that inactivate phosphorylation at both serines, whereas mutations that mimic constitutive serine phosphorylation increased assembly activity. From these results, we conclude that the phosphorylated form is the active state of SMN. Isoelectric focusing of SMN (Fig 4) and the analysis of phosphoamino acids (Fig 1B) showed that, apart from serine phosphorylation, other modifications appear in addition. However, the functional relevance and the nature of these modifications are at present unclear.
The requirement for phosphorylation of the amino terminus of SMN prompted us to ask to which step in the assembly pathway serines 28 and 31 contribute. As mutation of both serines does not reduce the affinity of SMN for Gemin 2 (which interacts with the N-terminus of SMN, see supplementary information online), we favour the idea that phosphorylation affects the activity of the SMN complex rather than its composition. Indeed, the increased levels of Sm proteins or snRNA associated with complexes reconstituted on SMNS28A/S31A or SMNS28E/S31E (Fig 3) support this view. It remains to be uncovered, however, whether Sm protein transfer onto U snRNA is inhibited, as opposed to the release of the assembled particle from the SMN complex.
Although SMN localizes to both nucleus and cytoplasm, we have clearly shown that only the latter mediates efficient assembly of U snRNPs. As the SMN complex is believed to shuttle between both compartments (Massenet et al, 2002), there seems to be a need for activation of the SMN complex in the course of its subcellular transport. Consistent with this idea, the extent of post-translational modifications in nuclear or cytoplasmic SMN differs significantly (Fig 4). The finding that phosphatase treatment converts the mobility of cytoplasmic SMN to that of the nucleus (Fig 5) indeed suggests that the phosphorylation status of SMN changes on subcellular transport.
It is interesting to note that the SMN-containing complexes obtained from either compartment also differ with respect to their protein composition (Fig 5). In particular, Gemin 3, 4 and 5 are under-represented in the nuclear complex. As the activity of the nuclear complex can be restored on replenishment of these under-represented components (by addition of cytosolic extract), we believe that it is a functional intermediate rather than a dead end product. It remains to be shown what elicits the absence of the aforementioned proteins.
A potential regulator for the phosphorylation status of SMN is the serine/threonine protein phosphatase 4 (PPP4; Carnegie et al, 2003). This enzyme interacts with the SMN complex (presumably by direct contact with Gemin 3 and 4) and its overexpression enhances the temporal localization of U snRNPs in the nucleus. It will be interesting to analyse whether PPP4 dephosphorylates SMN in the nucleus to convert the SMN complex to an inactive state.
The intriguing similarity between the sequences surrounding the phosphoserines of SMN (DSDDSD) and pICln (QSDDYD) suggests that both proteins are similarly regulated. Although these sequences resemble the consensus site for casein kinase II (CKII), we failed to detect this protein by western blot analysis in the isolated SMN complex. Furthermore, the kinase that co-purifies with the SMN complex fails to phosphorylate casein, a principal substrate of CKII (data not shown). The identity of the enzyme that modifies SMN and pICln therefore remains elusive and its identification is in progress.
Methods
Analysis of phosphorylation in vivo. HeLa cells were labelled with [32P]ortho-phosphate and total cell lysates precipitated with anti-SMN (7B10) or anti-pICln antibodies as described by Meister & Fischer (2002). The precipitated proteins were separated by SDS–PAGE, transferred to a PVDF membrane and labelled components visualized by autoradiography. Labelled proteins were identified by western blotting and MALDI-TOF (see below). Phosphoproteins were excised from the PVDF membrane and proteins hydrolysed in 6 N HCl. Free amino acids were mixed with a phosphoamino acid standard (Sigma) and analysed by 2D-TLC (Boyle et al, 1991).
Preparation of HeLa cell extracts and isolation of the SMN complex. To obtain cytosolic extract, active in U snRNP assembly, 20 litres of HeLa suspension cells were pelleted and washed in PBS. The cell pellet (15 g) was resuspended in 2.5 volumes of PBS containing 0.01% Igepal. Cells were lysed by dounce homogenization. Nuclei and membranes were separated by centrifugation in a swing-out rotor (10,000 r.p.m., 10 min, 4°C). Nuclei were washed once in PBS containing 0.01% Igepal and pelleted again. The nuclei were resuspended in 2.5 volumes of PBS containing 0.01% Igepal and homogenized by sonication (Branson Sonifier 250, 90% duty cycle, output 4, three bursts for 20 s, interrupted by cooling on ice). The extract obtained was then centrifuged (10,000 r.p.m., 10 min, 4°C) to remove nuclear membranes. Both extracts were passed through a 0.45 μm low-protein-binding filter to clear the extracts completely. For total cell extracts, one pellet of HeLa cells was resuspended in 4 volumes of PBS containing 0.01% Igepal and broken up by sonication, centrifuged and filtered as above. The SMN complex was affinity-purified as described using the monoclonal anti-SMN antibody 7B10 (Meister et al, 2001b). Isolated complex immobilized on protein G–Sepharose was dephosphorylated by the addition of 100 U AP (MB-grade, 20 U/μl, Roche, Mannheim, Germany) for 2 h at 30°C in a thermo-mixer. The complex was subsequently washed with PBS to remove the phosphatase and tested for its activity in U snRNP assembly (see below).
Two-dimensional gel electrophoresis. For separation of proteins by 2D gel electrophoresis, cleared supernatants of cell extracts were precipitated with 5 volumes of acetone at −20°C. Precipitated proteins were solubilized in 8 M urea, 2% CHAPS, 18.5 mM DTT and 2% IPG buffer (pH 3–10; Amersham Biosciences, Germany), and 1 mg protein was separated in the first dimension by a nonlinear pH gradient (Immobiline DryStrip NL 3-10, Amersham-Pharmacia) at 90 kV/h. Separated proteins of the first dimension were resolved in the second dimension by standard SDS–PAGE. The gel was subsequently subjected to immunoblot analysis with the monoclonal anti-SMN antibody 7B10.
Mass spectrometry. Affinity-purified SMN was in-gel-digested by modified trypsin and desalted. MALDI-TOF mass spectra were acquired on a Reflex III instrument (Bruker Daltonik, Germany) in both positive and negative reflector modes using a 2,5-dihydroxybenzoic acid matrix. Peptides differing by >80 mass units were submitted to post-source decay fragment ion analysis. Peptides showing losses of 98 mass units (phosphoric acid) or 80 mass units (phosphate) were considered phosphopeptides. Tandem mass spectrometry was performed on a Q-TOF Ultima mass spectrometer (Micromass, England).
In vitro kinase assay. HeLa cytoplasmic extract or purified SMN complex (prepared as described by Meister et al, 2001a) was incubated with GST–SMN(1–160) and full-length GST–pICln for 30 min at 30°C with 10 μCi [γ-32P]ATP in 50 mM NaCl, 25 mM Tris (pH 7.5), 10 mM MgCl2 and 2 mM CaCl2. Samples were analysed by SDS–PAGE and autoradiography.
Assembly of U snRNPs in vitro. A 1 μg portion of wild-type or mutant GST–SMN protein was immobilized on glutathione beads and incubated with HeLa cell extract for 1 h at 4°C. Beads were washed three times with PBS/I to remove unbound components. For U snRNP assembly, incubations were carried out in the presence of 25 fmol [32P]U1 RNA, 2 μg tRNA, 1 mM ATP and 3 μl RNasin in a volume of 30 μl PBS/I (37°C, 45 min). The reactions were analysed by native gel electrophoresis. Assembly with isolated SMN complex was carried out as described by Meister et al (2001a).
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v6/n1/extref/6-7400301-s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We are indebted to B. Laggerbauer for stimulating discussions and expert assistance in the preparation of this manuscript. This work was supported by the Deutsche Forschunggemeinschaft (FOR426 and SFB581) and families of SMA (fsma). M.N. was supported by a European Community Marie Curie Fellowship.
References
- Boyle WJ, van der Geer P, Hunter T (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol 201: 110–149 [DOI] [PubMed] [Google Scholar]
- Bühler DV, Raker R, Lührmann R, Fischer U (1999) Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum Mol Genet 8: 2351–2357 [DOI] [PubMed] [Google Scholar]
- Carnegie GK, Sleeman JE, Morrice N, Hastie CJ, Peggie MW, Philp A, Lamond AI, Cohen PT (2003) Protein phosphatase 4 interacts with the Survival of Motor Neurons complex and enhances the temporal localisation of snRNPs. J Cell Sci 116: 1905–1913 [DOI] [PubMed] [Google Scholar]
- Friesen WJ, Massenet S, Paushkin S, Wyce A, Dreyfuss G (2001) SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol Cell 7: 1111–1117 [DOI] [PubMed] [Google Scholar]
- Gubitz AK, Feng W, Dreyfuss G (2004) The SMN complex. Exp Cell Res 296: 51–56 [DOI] [PubMed] [Google Scholar]
- Liu Q, Fischer U, Wang F, Dreyfuss G (1997) The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90: 1013–1021 [DOI] [PubMed] [Google Scholar]
- Massenet S, Pellizzoni L, Paushkin S, Mattaj IW, Dreyfuss G (2002) The SMN complex is associated with snRNPs throughout their cytoplasmic assembly pathway. Mol Cell Biol 22: 6533–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Fischer U (2002) Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J 21: 5853–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Bühler D, Pillai R, Lottspeich F, Fischer U (2001a) A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nat Cell Biol 3: 945–949 [DOI] [PubMed] [Google Scholar]
- Meister G, Eggert C, Buehler D, Brahms H, Kambach C, Fischer U (2001b) Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr Biol 11: 1990–1994 [DOI] [PubMed] [Google Scholar]
- Meister G, Eggert C, Fischer U (2002) SMN-mediated assembly of RNPs: a complex story. Trends Cell Biol 12: 472–478 [DOI] [PubMed] [Google Scholar]
- Pellizzoni L, Yong J, Dreyfuss G (2002) Essential role for the SMN complex in the specificity of snRNP assembly. Science 298: 1775–1779 [DOI] [PubMed] [Google Scholar]
- Raker VA, Plessel G, Lührmann R (1996) The snRNP core assembly pathway: identification of stable core protein heteromeric complexes and snRNP subcore particle in vitro. EMBO J 15: 2256–2269 [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Olea R, Emma F, Coghlan M, Strange K (1998) Characterization of pICln phosphorylation state and a pICln-associated protein kinase. Biochim Biophys Acta 1381: 49–60 [DOI] [PubMed] [Google Scholar]
- Yong J, Wan L, Dreyfuss G (2004) Why do cells need an assembly machine for RNA–protein complexes? Trends Cell Biol 14: 226–232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information