Abstract
Five polychlorinated biphenyl (PCB)-degrading bacteria were tested for the ability to differentiate between the enantiomers of four atropisomeric PCB congeners (2,2′,3,6-tetra-CB; 2,2′,3,3′,6-penta-CB; 2,2′,3,4′,6-penta-CB; and 2,2′,3,5′,6-penta-CB) after growth in the presence of tryptone-soytone, biphenyl, carvone, or cymene. Enantioselectivity was shown to vary with respect to strain, congener, and cosubstrate.
Enantioselective biotransformation of polychlorinated biphenyls (PCBs) has been suggested from studies showing differential occurrence of PCB enantiomers in environmental samples, including river and riparian biota and sediments (19, 20). To date, however, there has been only a single study in the literature that has examined enantioselectivity by a PCB-degrading microorganism (7). The authors of that study concluded that despite a high degree of PCB biodegradation, the bacterial isolate, Jonibacter sp. strain MS3-02, did not demonstrate any evidence of enantioselectivity.
For this study, we tested five PCB-degrading bacteria, Ralstonia eutrophus H850 (2), Burkholderia cepacia LB400 (4), Rhodococcus globerulus P6 (6), Rhodococcus sp. strain ACS (15), and Arthrobacter sp. strain B1B (11), for the ability to differentiate between the enantiomers of the following four atropisomeric PCBs: PCB 45 (2,2′,3,6-tetra-CB), PCB 84 (2,2′,3,3′,6-penta-CB), PCB 91 (2,2′,3,4′,6-penta-CB), and PCB 95 (2,2′,3,5′,6-penta-CB). The strains were cultured in the presence of one of three cosubstrates as follows: biphenyl, (S)-(+)-carvone, or p-cymene at 1.0 g/liter, 0.25 g/liter, and 0.025 g/liter, respectively, in a 0.1% tryptic soy broth (TSB)-minimal salts medium (8, 13, 14). The concentration of carvone and cymene chosen was the maximum concentration tolerated by all isolates without negatively affecting growth. The study tested the hypothesis that growth of the PCB degraders in the presence of different cosubstrates can induce novel catabolic enzyme systems. Evidence of the activity of a novel enzymatic process may be observed by taking advantage of the unique chirality inherent in every enzyme which can thereby generate a shift in the enantioselective transformation patterns of each atropisomeric PCB. Conversely, if the enantioselective transformation pattern is unaltered by the presence of the cosubstrate and/or the PCB congener, the resulting enantioselective pattern should remain unchanged.
A resting cell assay was prepared (3) using a bacterial suspension at an optical density at 600 nm of 1.0. A 5-ml aliquot of the log-phase culture was transferred to a 40-ml vial containing a racemic mixture of the four PCBs (2 μg of PCB/liter; AccuStandard, New Haven, Conn.). The congeners were dissolved in acetone which was volatilized prior to the addition of the culture. The assay mixture was placed on an orbital shaker at 100 rpm at 25°C, and the reaction was terminated after 48 h by acidifying to pH 2 with HNO3. The culture was extracted into 5 ml of hexane containing 5 μg of pyrene per ml as an internal standard and was analyzed by achiral and chiral gas chromatography. Control cultures were prepared in 0.1% TSB-minimal salts medium as described above. Acid-killed controls were prepared for all strains, substrates, and cosubstrates to determine percent transformation and ensure that the extraction of PCBs was not enantioselective. All treatments were performed in quadruplicate and were subjected to pairwise t tests to determine if the observed PCB transformations were statistically significant (P < 0.05). Separate experiments were also performed on the five strains to assay enantioselectivity of more highly chlorinated chiral PCB congeners 132, 171, and 183; however, transformation of these congeners was much too limited to yield defensible comparisons. The presence of PCB metabolites was detected by gas chromatography-mass spectrometry on a DB-5 column (30 m by 0.25 mm by 0.25 μm; J&W, Folsom, Calif.), using the full-scan mode after concentration of extracts. Detection of pentachlorobiphenyl-ols and -diols was confirmed by the presence of the dihydroxylated parent ion, m/z 358, 360, 362, and the hydroxylated parent ion, m/z 344, 342, 340. Hydroxylated metabolites of tetrachlorobiphenyl were not observed.
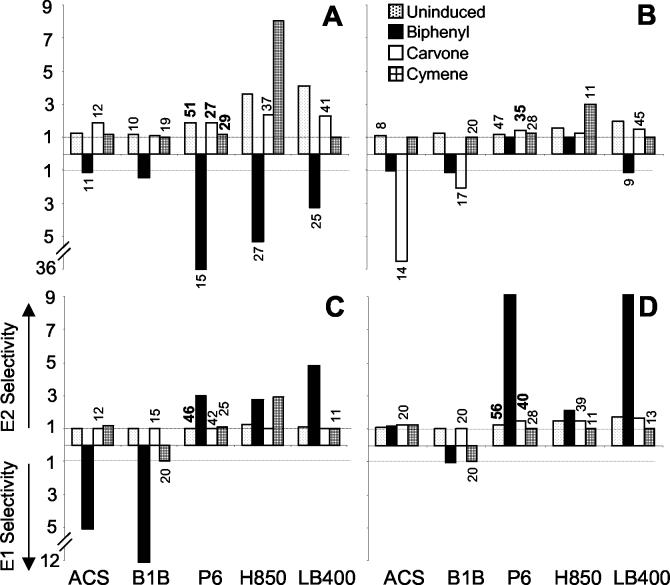
Chiral analysis of PCBs was performed by gas chromatography-mass spectrometry using three chiral columns: Cyclosil-B (30 m by 0.25 mm by 0.25 μm; J&W, Folsom, Calif.), separating the enantiomers of PCBs 45, 91, and 95 (18); Chirasil-Dex (25 m by 0.25 mm by 0.25 μm; Varian, Walnut Creek, Calif.), separating the enantiomers of PCBs 84, 91, and 95 (9); and BGB-172 (30 m by 0.25 mm by 0.18 μm; BGB Analytik AG, Adiswil, Switzerland), separating the enantiomers of PCBs 45, 84, and 91. Enantioselective transformation of each congener was assessed in terms of selectivity factors such that the data can be easily interpreted, providing the multiple by which one enantiomer is preferentially transformed over the other. For example, biphenyl-grown treatments of R. globerulus strain P6 exhibited preferential transformation of the first eluting enantiomer of PCB 45 over the second with a selectivity factor of 36.0; thus, there are 36 E1s transformed for every E2 transformed (Fig. 1A). E1 and E2 are the first and second eluting enantiomers, respectively, on Cyclosil-B (for PCB 45) and Chirasil-Dex (for PCBs 91 and 95) (18). E1 and E2 are the concentrations of the (+) and (−) enantiomers, respectively, for PCB 84, for which the elution order on Chirasil-Dex was previously determined (9). Enantiomer degradation was determined by averaging the peak areas from the appropriate two columns for that congener and calculating the percent change from the control treatments. Acid-killed controls and abiotic controls both displayed a selectivity factor of 1.00, demonstrating that the extraction procedure and abiotic processes in the microcosms were not stereoselective. Differences in measurements for congeners separated by both columns were of a <3% coefficient of variation (19).
FIG. 1.
Selectivity factors for each of the four PCBs, PCB 45 (A), PCB 84 (B), PCB 91(C), and PCB 95 (D), are shown for each of the five PCB degraders (x axis). Preferential transformation of the first eluting enantiomer (E1) is indicated by values greater than 1 below the x axis, while preferential transformation of E2 is indicated by values greater than 1 above the x axis. Treatments that demonstrated significant PCB degradation (P < 0.05) are noted with the percent degradation value above the corresponding bar; bold values indicate that P < 0.01. Note the break in the y axis shown left of panels A and C.
Seventy percent of all treatments exhibited significant (P < 0.05) biotransformation of PCB 45, followed by PCB 84 (55%), PCB 95 (45%), and lastly PCB 91 (35%). Such modest levels of PCB biotransformation are typical of recalcitrant, multiple ortho-chlorinated congeners (3, 6). PCB biotransformation was most commonly observed in carvone- and cymene-grown cultures (80 and 50%, respectively). In contrast, a low percentage of control and biphenyl-grown cultures (35 and 15%, respectively) demonstrated statistically significant PCB transformation, thereby preventing cross-comparisons between strains, congeners, and cosubstrates for the majority of available combinations of these experimental parameters.
Variations in enantioselectivity resulting from the activity of a single strain on a single congener grown on a different cosubstrate suggest the presence and activity of dissimilar PCB biotransformative pathways. This variation in enantioselectivity is presented in terms of selectivity factors (Fig. 1), thereby quantifying the degree of enantiomer preference. As mentioned earlier, biphenyl-stimulated strain P6 yielded a selectivity factor of 36, favoring E1 transformation of PCB 45; however, the control, carvone-grown, and cymene-grown cells generated selectivity factors of 1.9, 1.9, and 1.2, respectively, favoring transformation of E2. A similar shift in enantiomer preference was also observed for biphenyl-grown strains H850 and LB400 when transforming PCB 45. H850 and LB400 generated selectivity factors of 5.4 and 3.3, respectively; however, when grown on TSB, carvone, or cymene, these strains showed a consistent shift in enantioselectivity towards E2, with a maximum selectivity factor of 8.1 for cymene-grown H850 cells. Of note, the preference for E2 of cymene-grown H850 cells is not shared by the other isolates, for which largely racemic transformation was observed. Strains ACS and B1B exhibited strong selectivity towards E1 of PCB 84 when grown in the presence of carvone, with selectivity factors of 6.5 and 2.1, respectively, but shifted their enantiomer preference when grown on TSB, with selectivity factors of 1.1 and 1.2, respectively. Carvone-grown LB400 selectively transformed enantiomer E2 of PCB 84, with a selectivity factor of 1.5, while the biphenyl-grown culture selectively transformed E1, with a selectivity factor of 1.1. Racemic transformation of PCB 91 was consistently found for carvone-grown strains ACS, B1B, and P6. All strains demonstrated significant transformation of E2 of PCB 95 when grown in the presence of carvone, yielding selectivity factors ranging from near racemic for B1B to a maximum selectivity factor of 1.6 for strain LB400. Notably, no strain demonstrated significant enantioselective transformation of E1 for PCB 95. Strain H850 generated widely varying selectivity factors when grown on cymene, favoring transformation of E2 for PCB 45, 91, and 95, with selectivity factors of 8.1, 2.9, and 1.0, respectively, while a selectivity factor of 3.0 was observed for E1 of PCB 84. Carvone-grown cells of strain ACS also demonstrated a wide range in selectivity similar to that of H850, favoring transformation of E2 for PCB 45, 91, and 95, with selectivity factors of 1.9, 1.0, and 1.3, respectively, while a selectivity factor of 6.5 was observed for E1 of PCB 84.
The enantioselectivity of R. globerulus P6 is more similar to that of the two gram-negative strains than to that of the gram-positive strains. In particular, the selectivity factor shown for PCB 84 in the presence of carvone demonstrated preferential transformation of E2, which is very similar to the preference of the two gram-negative isolates, whereas the other two gram-positive isolates demonstrated enantioselectivity towards E1. The trend observed by R. globerulus P6 and the two gram-negative strains in the presence of TSB, biphenyl, and carvone resulted in strong enantioselectivity towards E2 of PCB 95, while ACS and B1B yielded largely racemic biotransformation. Differences displayed between gram-negative and gram-positive isolates may be the results of structural differences in the cell wall and membrane of gram-negative and gram-positive bacteria. Cell walls and membranes are composed of chiral compounds and therefore could interact preferentially with one of the two PCB enantiomers, thereby enabling preferential exposure of one enantiomer to the degradative enzyme. Enantioselective membrane transport has already been demonstrated for α-hexachlorocyclohexane across the blood-brain barrier in rats (16). However, this hypothesis does not explain why the gram-positive isolate R. globerulus P6 displays the gram-negative pattern of transformation. It also does not explain why enantioselectivity shifts as cosubstrates are changed. A partial answer may be found in the genetic similarity of the strains and the biphenyl dioxygenases. Asturias et al. (1) showed evidence that there is a 53 to 61% homology between the bphA1, -A2, -A3, and -A4 genes of R. globerulus P6 and B. cepacia LB400. The similarities in the bphA genes are consistent with the similarities found in the enantioselectivity between these two isolates. Williams et al. concluded that H850 and H850-like isolates contain distinctly different enzyme activities for certain PCB congeners from those of Arthrobacter sp. strain M5-like isolates (17). Future studies may benefit from comparisons between cell extracts and whole-cell assays to elucidate the potential influence of chiral membrane transporters that may influence enantioselective transformation.
In addition to enantioselective membrane transport, shifts in enantioselectivity of any one particular congener by a strain after growth in the presence of a cosubstrate may be the result of a number of other possible interactions. One possibility is that the cosubstrate acts as a substrate or nonsubstrate effector (10), potentially modifying the conformation of the PCB-degrading enzyme, thereby changing its enantiospecificity. Alternatively, the cosubstrate may induce an isoenzyme, shifting the enantioselectivity due to the unique chirality of the enzyme's active site. Buser et al. (5), in a study investigating enantioselective degradation of the pesticide metalaxyl, postulated that changes in enantioselectivity may be evidence of the involvement of two (or more) enzyme systems in metalaxyl degradation. Similar to the metalaxyl model, the shifts in enantioselectivity demonstrated by all the strains here suggest the involvement of multiple PCB-degrading enzyme systems. Additional support for this hypothesis is provided by the work of Master and Mohn (12), by which they demonstrated inhibitory effects of p-cymene and (S)-(+)-carvone on induction of PCB-degrading enzymes by biphenyl in the psychrotolerant PCB-degrader Pseudomonas sp. strain Cam-1.
The observed shifts in enantioselectivity for the same strain and cosubstrate, but for different congeners, can most easily be explained by the fact that each congener, by definition, has a different chlorination pattern. It is this unique chlorination pattern that predetermines the degree of interaction between the enzyme and the congener, making some congeners more suitable to attack, while others are more sterically hindered. Distinguishing strains not only by their congener transformation patterns but also by their atropisomeric PCB preference or enantioselectivity can potentially illuminate subtle differences in enzymes or the presence of isozymes. This technique may also assist efforts of bioremediation, for which microorganisms with complementary degradative patterns could be employed to more effectively remediate chiral pollutants (e.g., chlordane, DDT, α-hexachlorocyclohexane, linear alkylbenzenes, mecoprop, and toxaphene). The power of this enantioselection assay is that large numbers of microorganisms can be rapidly screened for their enantioselectivity, revealing subtle differences in a particular enzyme and its active site and the potential diversity of PCB-degradative oxygenases. Analysis of enantioselective degradation may also assist in screening for novel cosubstrates for xenobiotic degradation, while simultaneously lending insight into the mechanisms of biotransformation.
Acknowledgments
We thank A. K. Lilley, I. P. Thompson, T. Bidleman, and A. W. Garrison for their helpful suggestions, J. Jones for the hydroxy-PCB standard, and A. Peters for his assistance with identifying metabolites by gas chromatography-mass spectrometry.
This paper has been reviewed in accordance with the U.S. Environmental Protection Agency's peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
REFERENCES
- 1.Asturias, J. A., E. Diaz, and K. N. Timmis. 1995. The evolutionary relationship of biphenyl dioxygenase from gram-positive Rhodococcus globerulus P6 to multicomponent dioxygenases from gram-negative bacteria. Gene 156:11-18. [DOI] [PubMed] [Google Scholar]
- 2.Bedard, D. L., M. L. Haberl, R. J. May, and M. J. Brennan. 1987. Evidence for novel mechanisms for polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl. Environ. Microbiol. 53:1103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedard, D. L., R. Unterman, L. H. Bopp, M. J. Brennan, M. L. Haberl, and C. Johnson. 1986. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl. Environ. Microbiol. 51:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bopp, L. H. 1986. Degradation of highly chlorinated PCBs by Pseudomonas strain LB400. J. Ind. Microbiol. 1:23-29. [Google Scholar]
- 5.Buser, H.-R., M. D. Muller, T. Poiger, and M. E. Balmer. 2002. Environmental behavior of the chiral acetamide pesticide metalaxyl: enantioselective degradation and chiral stability in soil. Environ. Sci. Technol. 36:221-226. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa, K., N. Tomizuka, and A. Kamibayashi. 1979. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl. Environ. Microbiol. 38:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Ruiz, C., R. Andres, J. L. Valera, F. Laborda, and M. L. Marina. 2002. Monitoring the stereoselectivity of biodegradation of chiral polychlorinated biphenyls using electrokinetic chromatography. J. Sep. Sci. 25:17-22. [Google Scholar]
- 8.Gilbert, E. S., and D. E. Crowley. 1997. Plant compounds that induce polychlorinated biphenyl biodegradation by Arthrobacter sp. strain B1B. Appl. Environ. Microbiol. 63:1933-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haglund, P., and K. Wiberg. 1996. Determination of the gas chromatographic elution sequences of the (+)- and (−)-enantiomers of stable atropisomeric PCBs on Chirasil-Dex. J. High Resolut. Chromatogr. 19:373-376. [Google Scholar]
- 10.Hurtubise, Y., D. Barriault, J. Powlowski, and M. Sylvestre. 1995. Purification and characterization of the Comamonas testosteroni B-356 biphenyl dioxygenase components. J. Bacteriol. 177:6610-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler, H. P. E., D. Kohler-Staub, and D. D. Focht. 1988. Cometabolism of polychlorinated biphenyls: enhanced transformation of Aroclor 1254 by growing bacterial cells. Appl. Environ. Microbiol. 54:1940-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Master, E. R., and W. W. Mohn. 2001. Induction of bphA, encoding biphenyl dioxygenase, in two polychlorinated biphenyl-degrading bacteria, psychrotolerant Pseudomonas strain Cam-1 and mesophilic Burkholderia strain LB400. Appl. Environ. Microbiol. 67:2669-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park, Y.-I., J.-S. So, and S.-C. Koh. 1999. Induction by carvone of the polychlorinated biphenyl (PCB)-degradative pathway in Alcaligenes eutrophus H850 and its molecular monitoring. J. Microbiol. Biotechnol. 9:804-810. [Google Scholar]
- 14.Singer, A. C., E. S. Gilbert, E. Luepromchai, and D. E. Crowley. 2000. Bioremediation of polychlorinated biphenyl-contaminated soil using carvone and surfactant-grown bacteria. Appl. Microbiol. Biotechnol. 54:838-843. [DOI] [PubMed] [Google Scholar]
- 15.Singer, A. C., W. Jury, E. Luepromchai, C. S. Yahng, and D. E. Crowley. 2001. Contribution of earthworms to PCB bioremediation. Soil Biol. Biochem. 33:765-776. [Google Scholar]
- 16.Ulrich, E. M., and R. A. Hites. 1998. Enantiomeric ratios of chlordane-related compounds in air near the Great Lakes. Environ. Sci. Technol. 32:1870-1874. [Google Scholar]
- 17.Williams, W. A., H. H. Lobos, and W. E. Cheetham. 1997. A phylogenetic analysis of aerobic polychlorinated biphenyl-degrading bacteria. Int. J. Syst. Bacteriol. 47:207-210. [DOI] [PubMed] [Google Scholar]
- 18.Wong, C. S., and A. W. Garrison. 2000. Enantiomer separation of polychlorinated biphenyl atropisomers and polychlorinated biphenyl retention behavior on modified cyclodextrin capillary gas chromatography columns. J. Chromatogr. A 866:213-220. [DOI] [PubMed] [Google Scholar]
- 19.Wong, C. S., A. W. Garrison, and W. T. Foreman. 2001. Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic bed sediment. Environ. Sci. Technol. 35:33-39. [DOI] [PubMed] [Google Scholar]
- 20.Wong, C. S., A. W. Garrison, P. D. Smith, and W. T. Foreman. 2001. Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic and riparian biota. Environ. Sci. Technol. 35:2448-2454. [DOI] [PubMed] [Google Scholar]