Abstract
The δ2 glutamate receptor (GluRδ2) has a crucial role in cerebellar functions; disruption of GluRδ2 alleles in mice (δ2−/−) impairs synapse formation and long-term depression, which is thought to underlie motor learning in the cerebellum, and consequently leads to motor discoordination. However, it has been unclear whether GluRδ2 is activated by glutamate analogues. Here we introduced a GluRδ2 transgene, which had a mutation (Arg514Lys) in the putative ligand-binding motif conserved in all mammalian ionotropic glutamate receptors (iGluRs) and their ancestral bacterial periplasmic amino-acid-binding proteins, into δ2−/− mice. Surprisingly, a mutant GluRδ2 transgene, as well as a wild-type GluRδ2 transgene, rescued all abnormal phenotypes of δ2−/− mice. Therefore, these results indicate that the conserved arginine residue, which is crucial for the binding of iGluRs to glutamate analogues, is not essential for the restoration of GluRδ2 functions in δ2−/− mice.
Keywords: glutamate receptor, Purkinje cell, cerebellum, LTD, mouse
Introduction
Fast excitatory neurotransmission in the mammalian central nervous system is mainly mediated by L-glutamate, which activates postsynaptic ionotropic glutamate receptors (iGluRs): α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), kainate and N-methyl-D-aspartate (NMDA) receptors. On the basis of amino-acid sequence, the δ2 glutamate receptor (GluRδ2) family is positioned at equal distances in a phylogenetic tree from these three receptor families (Araki et al, 1993; Lomeli et al, 1993). Although GluRδ2 is predominantly expressed at parallel fibre (PF)–Purkinje cell synapses in the cerebellum, it does not seem to mediate normal synaptic transmission, which is completely blocked by the AMPA receptor-specific antagonist (Kano & Kato, 1987). Instead, GluRδ2 has a crucial role in forming PF–Purkinje cell and climbing fibre (CF)–Purkinje cell synapses during development, and impaired synaptogenesis is associated with motor discoordination in δ2−/− mice (Kurihara et al, 1997; Morando et al, 2001; Ichikawa et al, 2002). In addition to its role during development, GluRδ2 signalling has a unique role in modulating existing synapses in adult mice (Hirai et al, 2003).
Despite their importance, the mechanisms by which GluRδ2 participates in cerebellar functions have been elusive: GluRδ2 does not form functional glutamate-gated ion channels when they are expressed, either alone or with other iGluRs, in heterologous cells (Araki et al, 1993; Lomeli et al, 1993), and radio-ligand-binding assays failed to detect binding of GluRδ2 to glutamate analogues (Lomeli et al, 1993). Interestingly, the extracellular amino-terminal region of GluRδ2 contains a putative ligand-binding motif conserved in all mammalian iGluRs but not in metabotropic glutamate receptors (Fig 1). An arginine residue in this domain is especially conserved from the ancestral bacterial periplasmic amino-acid-binding proteins to mammalian iGluRs; X-ray crystallographic analyses demonstrated its essential interaction with the α-carboxyl moieties of amino-acid ligands (Oh et al, 1993; Hsiao et al, 1996; Armstrong et al, 1998; Mayer et al, 2001; Furukawa & Gouaux, 2003). Indeed, a substitution of this arginine residue with lysine completely abolishes the ligand-binding or channel activities of iGluRs (Hirai et al, 1996; Kawamoto et al, 1997; Laube et al, 1997; Jouppila et al, 2002). Therefore, to address the question whether GluRδ2 is activated by glutamate analogues, we did not rely on in vitro binding or functional assays, but instead we generated mice that express a mutant GluRδ2 transgene in which lysine replaced the conserved arginine at position 514 (TgR/K) onto a δ2−/− background. We hypothesized that the function of GluRδ2 is abrogated by the mutation if GluRδ2 is activated by glutamate or related amino acids in a conventional manner similar to that of other iGluRs.
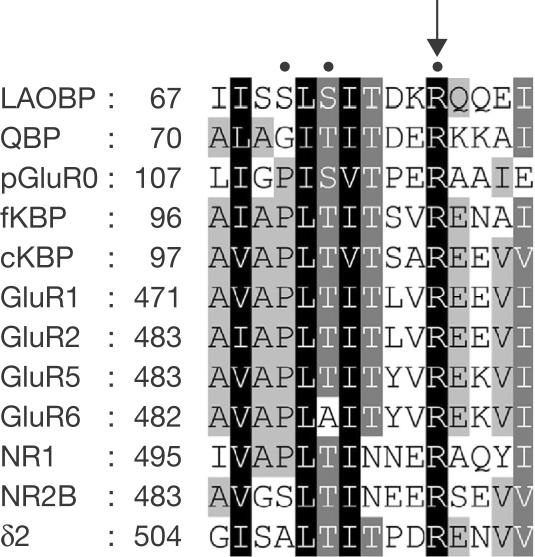
Figure 1.
Amino-acid alignment involving the conserved arginine of iGluRs and bacterial periplasmic binding proteins. The conserved arginine residue of GluRδ2 aligns with that of the following: bacterial periplasmic binding proteins leucine-arginine-ornithine-binding protein (LAOBP) and glutamine-binding protein (QBP), prokaryotic glutamate receptor (pGluR0), chick and frog kainate-binding proteins (cKBP and fKBP, respectively), AMPA receptor subunits GluR1 and GluR2, kainate receptor subunits GluR5 and GluR6 and NMDA receptor subunits NR1 and NR2B. Letters are shaded according to the percentage of conserved similar amino acids at each position (100%, black shading; 80%, mid-grey; 60%, light grey). The amino-acid position of the first residue of each sequence is indicated. Critical residues in LAOBP, QBP, pGluR0, GluR2 and NR1 that interact directly with ligands are indicated by dots above the sequences (Oh et al, 1993; Hsiao et al, 1996; Armstrong et al, 1998; Mayer et al, 2001; Furukawa & Gouaux, 2003). The arrow indicates the position of the highly conserved arginine residue.
Results
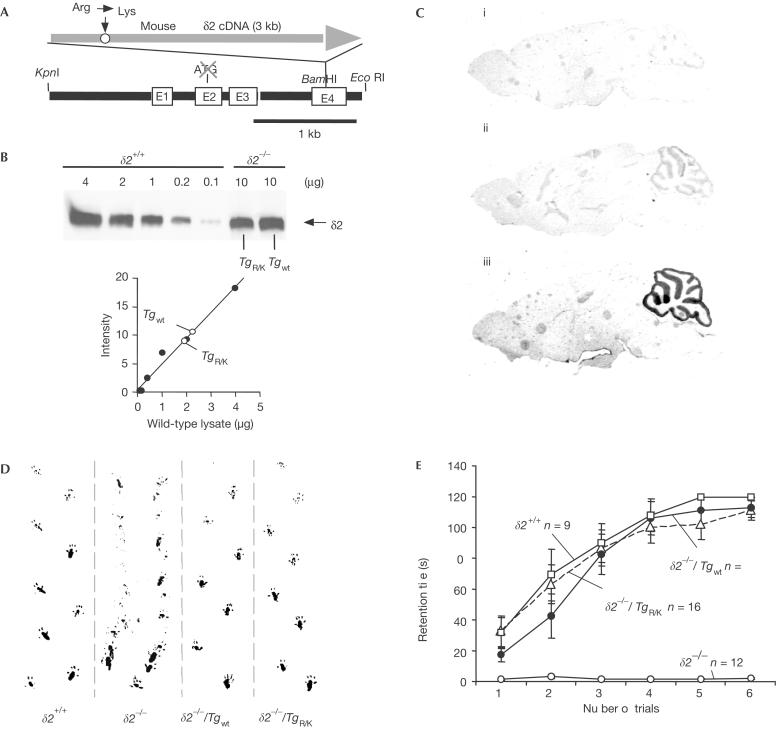
We used the Purkinje-cell-specific L7 promoter (Tomomura et al, 2001; Fig 2A) to drive the expression of a wild-type GluRδ2 transgene (Tgwt) and TgR/K. By breeding transgenic mice onto a δ2−/− background, we obtained transgenic ‘rescue' lines called δ2−/−/Tgwt and δ2−/−/TgR/K. Proper insertion of transgenes into the genome was confirmed by sequencing the genomic DNA from each transgenic line (supplementary Fig S1 online). We further analysed lines that expressed equivalent levels of construct-encoded proteins (Fig 2B). Immunohistochemical staining showed cerebellum-specific expression of TgR/K (Fig 2C) and Tgwt (data not shown). Although the levels of expression of transgenic GluRδ2 protein were about 20% of those of endogenous GluRδ2 protein in wild-type cerebellum (Fig 2B), δ2−/−/Tgwt mice showed no ataxic gait and could walk along a straight line (Fig 2D). Surprisingly, the ataxic gait was not observed in δ2−/−/TgR/K mice (Fig 2D). Results of the rotarod test confirmed the complete restoration of the motor performance of δ2−/−/TgR/K mice (Fig 2E). These results indicated that TgR/K as well as Tgwt could rescue the motor discoordination of δ2−/− mice.
Figure 2.
Rescue of the ataxic phenotype of δ2−/− mice by GluRδ2 transgene expression. (A) Structure of the transgene construct. Mouse wild-type or mutant GluRδ2 cDNA was inserted into the BamHI site of the L7 gene in which the translational start codon was disrupted (Tomomura et al, 2001). E1–E4, exons. (B) Western blot of δ2+/+, δ2−/−/Tgwt and δ2−/−/TgR/K cerebellar cells. Total lysates were blotted, and the blots were incubated with a polyclonal antibody against GluRδ2. The total amount of protein is indicated above each lane. To quantify the expression level of transgenes (lower graph), band intensities of the GluRδ2 protein in 10 μg of δ2−/−/Tgwt or δ2−/−/TgR/K cerebellar cell lysates were compared with those in various amounts of wild-type cerebellar cell lysates. (C) Immunohistochemical analysis of parasagittal sections of brains from (i) δ2−/−, (ii) δ2−/−/TgR/K and (iii) δ2+/+ (wild-type) mice. These sections were stained with anti-GluRδ2 antibody. (D) Footprint patterns. Tottering steps were made by the δ2−/− mice, and their feet tended to sweep along as they moved. In contrast, δ2−/−/TgR/K and δ2−/−/Tgwt mice walked along a straight line as did δ2+/+ mice. (E) Results of the rotarod task. Mice were allowed a maximum retention time of 120 s per trial. The number of mice in each group is indicated in the graph. Error bars indicate s.e.m.
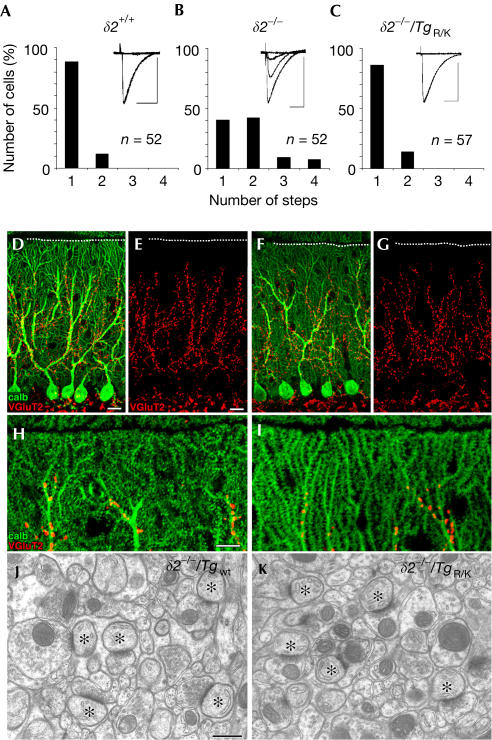
Immature Purkinje cells are innervated by multiple CFs that originate from the inferior olive of the medulla (Crepel et al, 1976). As animals grow, redundant CFs are gradually eliminated, and a relationship of one CF to one Purkinje cell is established by the end of the third postnatal week. Previous studies showed that GluRδ2 has an essential role in this elimination process (Kashiwabuchi et al, 1995; Hashimoto et al, 2001). To examine whether each Purkinje cell was innervated by a single CF in the δ2−/−/TgR/K cerebellum, we recorded the CF-evoked excitatory postsynaptic currents (EPSCs) from postnatal day 21 (P21) to P30 Purkinje cells in slice preparations. Because a single CF has a single threshold for excitation, increasing the stimulus intensity normally elicits CF-evoked EPSCs in an all-or-none manner. Single EPSCs were elicited in about 90% of wild-type P30 Purkinje cells (Fig 3A), whereas only 40% of δ2−/− Purkinje cells attained a one-to-one relationship with CFs (Hashimoto et al, 2001; Fig 3B). In contrast, δ2−/−/TgR/K mice had almost the same percentage (86%; Fig 3C) of single Purkinje cell–single CF innervation as the wild-type mice, which indicates that TgR/K restored the normal process of CF synapse elimination.
Figure 3.
Restoration of impaired synapse formation of δ2−/− Purkinje cells by TgR/K expression. (A–C) Electrophysiological estimation of the number of CFs innervating single Purkinje cells in δ2+/+ (A), δ2−/− (B) and δ2−/−/TgR/K (C) cerebella. EPSCs were elicited by stimulation of CFs in the granule cell layer. The number of EPSCs induced by different stimulus thresholds was counted (inset traces). The number of Purkinje cells tested is indicated. Horizontal scale bars, 20 ms; vertical scale bars, 500 pA. (D–I) Normal distribution of CF terminals in δ2−/−/Tgwt (D,E,H) and δ2−/−/TgR/K (F,G,I) cerebella. Cerebellar sections were stained with antibodies to VGluT2 (red; a marker of CF terminals) and calbindin (green; a marker of Purkinje cells). The pial surface is indicated by the dotted line. (J,K) Electron micrographs of PF–Purkinje cell synapses in the molecular layer. The asterisks indicate Purkinje cell spines in contact with PF terminals. Scale bars: (D,E) 20 μm; (H) 10 μm; (J) 500 nm.
Purkinje cell dendrites have two separate domains (Bravin et al, 1999): proximal regions innervated by CFs and distal regions innervated by PFs. CFs abnormally invade the ‘PF domain' of distal dendrites of δ2−/− Purkinje cells (Ichikawa et al, 2002). To examine the pattern of CF innervation of Purkinje cells, sections were stained with antibody to vesicular glutamate transporter 2 (VGluT2), which is predominantly expressed in CF terminals. The most distal terminal of CFs penetrated 95.1±0.4% of the molecular layer thickness in δ2−/− cerebella (Ichikawa et al, 2002), 81±1% in δ2−/−/Tgwt cerebella (Fig 3D,E) and 80±2% in δ2−/−/TgR/K cerebella (Fig 3F,G; mean±s.e.m. from three mice). In high-power micrographs, CF terminals were mainly associated with shaft dendrites in δ2−/−/TgR/K cerebella (Fig 3H,I), whereas they terminate at distal spiny branchlets in δ2−/− cerebella (Ichikawa et al, 2002). Similarly, although CFs innervating the distal dendrites of δ2−/− Purkinje cells are associated with EPSCs with a slow rise time (Hashimoto et al, 2001), CF-evoked EPSCs in δ2−/−/TgR/K Purkinje cells had a fast rise time (0.6±0.1 ms, n=12) similar to those in wild-type cells (0.6±0.1 ms, n=13). Therefore, we concluded that the abnormal distal CF innervation of δ2−/− Purkinje cells was also rescued by TgR/K.
Previous studies revealed that about 40% of spines on δ2−/− Purkinje cells are ‘naked' ones that lack presynaptic contact but have postsynaptic density (PSD)-like condensations (Kurihara et al, 1997; Lalouette et al, 2001). Furthermore, the remaining PF–Purkinje cell synapses in δ2−/− mice frequently show another specific abnormality—the length of the PSD does not equal that of the opposing presynaptic active zone (Lalouette et al, 2001). However, serial electron microscopic analysis (Fig 3J,K) revealed few free spines in both strains of transgenic mice (0.7±0.7% in δ2−/−/Tgwt cerebella and 0.3±0.3% in δ2−/−/TgR/K cerebella; 300 total spines counted in three representative mice of each line). Similarly, mismatching between the PSD and the active zone at PF synapses was rare (0.3±0.3% in δ2−/−/Tgwt cerebella and 0.3±0.3% in δ2−/−/TgR/K cerebella; 300 spines counted in three representative mice of each line). These results indicate that abnormal PF–Purkinje cell synaptogenesis in δ2−/− cerebella was rescued by TgR/K as well as by Tgwt.
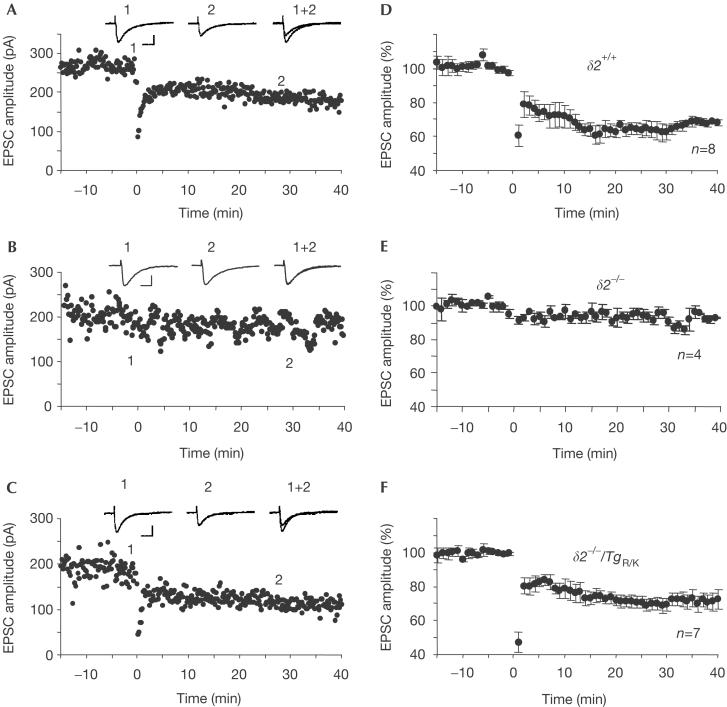
Simultaneous activation of PFs and CFs induces long-term depression (LTD) of PF–Purkinje cell transmission (Ito, 1989). CF stimulation can be replaced by direct depolarization of Purkinje cells to rule out any effect due to the CF innervation pattern. This protocol robustly induced LTD of PF-EPSCs in wild-type (Fig 4A,D) but not in δ2−/− (Fig 4B,E) Purkinje cells. The amplitudes of PF-EPSCs 40 min after conjunctive stimulation (eight Purkinje cells isolated from seven wild-type mice) were 68±2% of those before the start of conjunctive stimulation. Similarly, the same protocol induced LTD of PF-EPSCs in δ2−/−/TgR/K Purkinje cells (Fig 4C,F). The amplitudes of such EPSCs 40 min after conjunctive stimulation were 71±5% (seven Purkinje cells isolated from six mice) and were not significantly different from those in wild-type Purkinje cells (P>0.5; as determined by Student's t-test). Cerebellar LTD is believed to occur solely in postsynaptic Purkinje cells, probably by increased endocytosis of postsynaptic iGluRs (Matsuda et al, 2000; Wang & Linden, 2000). Therefore, TgR/K probably has a role similar to that of wild-type GluRδ2 in postsynaptic signalling pathways involved in synaptic plasticity at PF–Purkinje cell synapses.
Figure 4.
Expression of long-term depression in δ2−/−/TgR/K Purkinje cells. Representative EPSC amplitudes over time are shown. (A) Results for eight Purkinje cells isolated from seven wild-type mice. (B) Results for four Purkinje cells isolated from two δ2−/− mice. (C) Results for seven Purkinje cells isolated from six δ2−/−/TgR/K mice. The means (±s.e.m.) for each (wild type (D), δ2−/− (E) and δ2−/−/TgR/K (F)) are shown. The amplitude of the PF-EPSC was normalized to the baseline value, which was the average of 5-min responses that occurred just before conjunctive stimulation. Inset traces are EPSCs just before (1) and 30 min after (2) the conjunctive stimulation.
Discussion
Here we demonstrated that TgR/K expression effectively rescued all principal abnormal phenotypes of δ2−/− mice: motor discoordination, multiple CF innervation, impaired PF synaptogenesis and abrogated LTD. In contrast, a substitution of the corresponding arginine residue with lysine completely abolishes the ligand-binding or channel activities of other iGluRs (Hirai et al, 1996; Kawamoto et al, 1997; Laube et al, 1997; Jouppila et al, 2002). In addition, the arginine residue is essential for the binding of bacterial periplasmic proteins to amino-acid ligands, including glutamate, aspartate, glutamine, glycine, lysine, serine, arginine, ornithine and histidine (Oh et al, 1993; Hsiao et al, 1996; Armstrong et al, 1998; Mayer et al, 2001; Furukawa & Gouaux, 2003). Therefore, we speculate that GluRδ2 does not require glutamate-like amino acids to function in Purkinje cells.
However, we do not have direct evidence that TgR/K does not bind any glutamate analogues; it may still bind them in an unconventional way. It is also theoretically possible that, although TgR/K does not bind glutamate analogues, wild-type GluRδ2 does. For example, if TgR/K makes an abnormal heteromeric channel with other iGluRs, glutamate binding to these subunits may substitute for glutamate binding to wild-type GluRδ2 and restore the altered phenotypes of δ2−/− mice. However, the binding of three or more glutamate molecules to each receptor-channel complex is required for full activation of iGluRs (Rosenmund et al, 1998; supplementary Fig S3 online). Thus, it seems unlikely that a heteromeric channel composed of TgR/K and iGluR subunits could substitute for a channel formed by wild-type GluRδ2. In any case, GluRδ2 is unique in that its conserved arginine is not essential for the rescue of the altered phenotypes of δ2−/− mice.
Recently, we found that the application of an antibody specific for GluRδ2's extracellular N-terminal region to Purkinje cells specifically abrogated LTD by disrupting the endocytosis of AMPA receptors and caused transient cerebellar ataxia (Hirai et al, 2003). This finding indicates that GluRδ2 signalling could be controlled by the binding of a ligand to the extracellular domain. In addition, the role in controlling endocytosis suggests that GluRδ2 acts as a metabotropic receptor (Yuzaki, 2004). Interestingly, the AMPA receptor GluR2 subunit also activates a metabotropic pathway to induce dendritic spine formation in cultured hippocampal neurons (Passafaro et al, 2003), and this activity is mediated by the extracellular N-terminal domain of GluR2, which is distinct from the conventional glutamate-binding domain. Therefore, further studies using δ2−/−/TgR/K mice to explore such mechanisms exploited by GluRδ2 are warranted.
Methods
Generation of transgenic mice. Mouse δ2 complementary DNA was inserted into the BamHI site of pL7ΔAUG (Fig 2A). The resulting plasmid was digested with KpnI and EcoRI, and the linearized L7-δ2 construct was injected into fertilized eggs (Tomomura et al, 2001). Seven Tgwt and eight TgR/K founders were bred onto a δ2−/− background. Homozygous transgenic lines were established and confirmed by backcrossing with wild-type mice. All procedures relating to the care and treatment of animals were carried out according to NIH guidelines, and the experimental protocol was approved by the Animal Resource Committee of St Jude Children's Research Hospital.
Immunoblotting and microscopic analysis. Whole cerebella of wild-type and transgenic mice were homogenized, and the homogenates were used for immunoblotting as described previously (Hirai et al, 2003). Adult mice were anaesthetized and perfused transcardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) for light microscopy or with 2% paraformaldehyde plus 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for electron microscopy. Sagittal cryosections (20 μm in thickness) were immunostained with anti-GluRδ2 antibody as described previously (Hirai et al, 2003). To visualize CF terminals under a confocal laser scanning microscope (Fluoview FV1000, Olympus, Tokyo, Japan), microslicer sections (50 μm in thickness; VT1000S, Leica, Wien, Austria) were treated overnight with a mixture of calbindin antiserum (dilution, 1:10,000) and anti-VGluT2 antibody (0.5 μg/ml) as described previously (Ichikawa et al, 2002). For electron microscopy (H-7100, Hitachi, Tokyo, Japan), serial ultrathin sections (70 nm in thickness; UCT ultramicrotome, Leica) were prepared as described previously (Kurihara et al, 1997). When the edge of the PSD was more than 100 nm from the corresponding active zone in any serial sections, the spine was defined as a free spine.
Electrophysiology. Parasagittal cerebellar slices (200 μm) were prepared from wild-type, δ2−/− and δ2−/−/TgR/K mice. Whole-cell voltage-clamp recordings were made of Purkinje cells identified visually at 24°C as described (Hirai et al, 2003). Patch pipettes were pulled from borosilicate glass capillaries to achieve a resistance of 4–5 MΩ when filled with a solution containing 140 mM cesium methanesulphonate, 2 mM Na2ATP, 0.3 mM Na2GTP, 10 mM HEPES and 0.4 mM EGTA (pH 7.3, 290 mOsm/kg). Square pulses were applied every 10 s using an isolation unit (Axon Instruments, Foster City, CA, USA) for focal stimulation.
After stable PF-EPSCs had been observed for at least 15 min, LTD was induced by conjunctive stimulation that consisted of 30 single PF stimuli together with a 50 ms depolarizing pulse from a holding potential of −70 to +20 mV. A hyperpolarizing pulse (−10 mV, 50 ms) was applied 420 ms before each PF stimulus to monitor the access resistance. If the resistance differed from the original value by more than 20%, the record was discarded. PF-EPSCs were evoked every 10 s by a glass electrode placed in the molecular layer (pulse width, 10 μs; strength, 20–100 μA) about 100 μm away from the pial surface.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v6/n1/extref/7400312s1.pdf).
Supplementary Material
Online Supplemental Material
Acknowledgments
We thank T. Torashima for technical assistance and J.C. Jones for editing the manuscript. This work was supported in part by the Uehara Memorial Foundation (H.H. and M.Y.), the National Institutes of Health grant NS36925, Cancer Center Support Core grant CA21765, the American Lebanese Syrian Associated Charities, the Naito Memorial Foundation and a Grant-in-Aid for Scientific Research provided by the Japan Society for the Promotion of Science (M.Y.).
References
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M (1993) Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun 197: 1267–1276 [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, Gouaux E (1998) Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 395: 913–917 [DOI] [PubMed] [Google Scholar]
- Bravin M, Morando L, Vercelli A, Rossi F, Strata P (1999) Control of spine formation by electrical activity in the adult rat cerebellum. Proc Natl Acad Sci USA 96: 1704–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N (1976) Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol 7: 567–578 [DOI] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E (2003) Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J 22: 2873–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K et al. (2001) Roles of glutamate receptor δ2 subunit (GluRδ2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci 21: 9701–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Kirsch J, Laube B, Betz H, Kuhse J (1996) The glycine binding site of the N-methyl-D-aspartate receptor subunit NR1: identification of novel determinants of co-agonist potentiation in the extracellular M3–M4 loop region. Proc Natl Acad Sci USA 93: 6031–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Launey T, Mikawa S, Torashima T, Yanagihara D, Kasaura T, Miyamoto A, Yuzaki M (2003) New role of δ2-glutamate receptors in AMPA receptor trafficking and cerebellar function. Nat Neurosci 6: 869–876 [DOI] [PubMed] [Google Scholar]
- Hsiao CD, Sun YJ, Rose J, Wang BC (1996) The crystal structure of glutamine-binding protein from Escherichia coli. J Mol Biol 262: 225–242 [DOI] [PubMed] [Google Scholar]
- Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, Mishina M, Inoue Y, Watanabe M (2002) Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor d2. J Neurosci 22: 8487–8503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M (1989) Long-term depression. Annu Rev Neurosci 12: 85–102 [DOI] [PubMed] [Google Scholar]
- Jouppila A, Pentikainen OT, Settimo L, Nyronen T, Haapalahti JP, Lampinen M, Mottershead DG, Johnson MS, Keinanen K (2002) Determinants of antagonist binding at the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit, GluR-D. Role of the conserved arginine 507 and glutamate 727 residues. Eur J Biochem 269: 6261–6270 [DOI] [PubMed] [Google Scholar]
- Kano M, Kato M (1987) Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature 325: 276–279 [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N et al. (1995) Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR δ2 mutant mice. Cell 81: 245–252 [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Uchino S, Xin KQ, Hattori S, Hamajima K, Fukushima J, Mishina M, Okuda K (1997) Arginine-481 mutation abolishes ligand-binding of the AMPA-selective glutamate receptor channel α1-subunit. Brain Res Mol Brain Res 47: 339–344 [DOI] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M (1997) Impaired parallel fiber–Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor δ2 subunit. J Neurosci 17: 9613–9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J (2001) Neurobiological effects of a null mutation depend on genetic context: comparison between two hotfoot alleles of the δ-2 ionotropic glutamate receptor. Neuroscience 105: 443–455 [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J (1997) Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron 18: 493–503 [DOI] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, Wisden W (1993) The rat δ-1 and δ-2 subunits extend the excitatory amino acid receptor family. FEBS Lett 315: 318–322 [DOI] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H (2000) Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J 19: 2765–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Olson R, Gouaux E (2001) Mechanisms for ligand binding to GluR0 ion channels: crystal structures of the glutamate and serine complexes and a closed apo state. J Mol Biol 311: 815–836 [DOI] [PubMed] [Google Scholar]
- Morando L, Cesa R, Rasetti R, Harvey R, Strata P (2001) Role of glutamate δ-2 receptors in activity-dependent competition between heterologous afferent fibers. Proc Natl Acad Sci USA 98: 9954–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh BH, Pandit J, Kang CH, Nikaido K, Gokcen S, Ames GF, Kim SH (1993) Three-dimensional structures of the periplasmic lysine/arginine/ornithine-binding protein with and without a ligand. J Biol Chem 268: 11348–11355 [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M (2003) Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature 424: 677–681 [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF (1998) The tetrameric structure of a glutamate receptor channel. Science 280: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tomomura M, Rice DS, Morgan JI, Yuzaki M (2001) Purification of Purkinje cells by fluorescence-activated cell sorting from transgenic mice that express green fluorescent protein. Eur J Neurosci 14: 57–63 [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ (2000) Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25: 635–647 [DOI] [PubMed] [Google Scholar]
- Yuzaki M (2004) The δ2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cells. Cerebellum 3: 89–93 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplemental Material