Abstract
Human telomeres consist of tandem arrays of TTAGGG sequence repeats that are specifically bound by two proteins, TRF1 and TRF2. They bind to DNA as preformed homodimers and have the same architecture in which the DNA-binding domains (Dbds) form independent structural units. Despite these similarities, TRF1 and TRF2 have different functions at telomeres. The X-ray crystal structures of both TRF1- and TRF2-Dbds in complex with telomeric DNA (2.0 and 1.8 Å resolution, respectively) show that they recognize the same TAGGGTT binding site by means of homeodomains, as does the yeast telomeric protein Rap1p. Two of the three G-C base pairs that characterize telomeric repeats are recognized specifically and an unusually large number of water molecules mediate protein–DNA interactions. The binding of the TRF2-Dbd to the DNA double helix shows no distortions that would account for the promotion of t-loops in which TRF2 has been implicated.
Keywords: telomeric DNA recognition, TRF1, TRF2, telomeric DNA recognition, water-mediated contacts, X-ray crystallography
Introduction
Telomeres are specialized nucleoprotein complexes that protect the ends of linear eukaryotic chromosomes from degradation and from being recognized as double-stranded breaks (de Lange, 2002). Human telomeres contain 7–20 kilobase pairs (kbp) of tandem arrays of double-stranded TTAGGG repeats that are packaged into nucleosomes (Tommerup et al, 1994; Lejnine et al, 1995; Nikitina & Woodcock, 2004) and bound sequence specifically by two DNA-binding proteins, TRF1 and TRF2 (Broccoli et al, 1997). These two proteins are essential for the maintenance of functional telomeres. TRF2, in particular, has been implicated in the formation of a telomeric higher order structure, the t-loop (reviewed in de Lange, 2002; Smogorzewska & De Lange, 2004).
TRF1 and TRF2 bind to DNA as preformed homodimers (Bianchi et al, 1997, 1999; Broccoli et al, 1997). They have the same architecture defined by two conserved sequence motifs (Fig 1): (i) an amino-terminally located TRFH domain that mediates homodimerization in both proteins (Broccoli et al, 1997; Li et al, 2000), and has the same all α-helical structure (Fairall et al, 2001), and (ii) a carboxy-terminal DNA-binding domain (Dbd), originally identified through its sequence similarity to the Myb DNA-binding motif (Kanei-Ishii et al, 1990; Bilaud et al, 1996, 1997; Broccoli et al, 1997; Konig & Rhodes, 1997). This was later confirmed by the NMR structure of TRF1-Dbd (Nishikawa et al, 1998). Proteolysis studies on both proteins showed that the regions of sequence conservation between the two proteins (Fig 1) correspond to structured domains connected by highly protease-sensitive regions (Fairall et al, 2001; L.F. & M. O' Reilly, unpublished observations), precluding structural analysis of the full-length dimeric proteins. In both proteins, the Dbds are joined to their dimerization domains by means of very long linkers (about 100 amino acids in TRF1 and 150 amino acids in TRF2), which appear to have no inherent structure (Rhodes et al, 2002; Fig 1). This information, together with DNA-binding studies showing that full-length TRF1 can bind with an astonishing spatial flexibility (Bianchi et al, 1999), strongly suggests that in vitro the two Dbds bind to DNA essentially independently of the rest of the protein (Konig et al, 1998; Bianchi et al, 1999; Rhodes et al, 2002). However, in vivo, both Dbds seem to be required for the accumulation of the TRFs at telomeres (van Steensel & de Lange, 1997; van Steensel et al, 1998; reviewed in de Lange, 2002).
Figure 1.
Protein and DNA constructs used in the crystallization of the TRF1-Dbd and TRF2-Dbd complexes. (A) Schematic representation of the TRF1 and TRF2 proteins indicating the location of the two domains of sequence homology: the TRFH dimerization domain (yellow) and the Myb DNA-binding motif (red). The percentage sequence identity and homology are shown. (B) TRF1 and TRF2 protein constructs used in crystallization. The sequences of the two Dbds have been aligned, and identical (marked with an asterisk) and similar (marked with a dot) residues between TRF1 and TRF2 are denoted. The open rectangles indicate the position of the α-helices. In each case, the N-terminus of the protein is disordered, and the first residue for which there is electron density is residue R380 in TRF1 and K447 in TRF2. (C) The two DNA binding sites used in the crystals. The two sets of three G-C base pairs present in each binding site are highlighted in magenta.
Despite the similarities in sequence and architecture, the two TRFs have different functions at telomeres. TRF1 is a negative regulator of telomere length: overexpression of TRF1 leads to telomere shortening and that of a dominant-negative mutant to telomere elongation (van Steensel & de Lange, 1997). In contrast, although TRF2 is also involved in telomere length regulation (Smogorzewska et al, 2000), its crucial role is in capping and protection of chromosome ends (Ancelin et al, 1998; reviewed in de Lange, 2002). Expression of a dominant-negative mutant of TRF2 results in cells responding as if the telomeric ends are DNA breaks, leading to degradation of the single-stranded overhangs, ligation of chromosome ends and activation of the ATM/p53 damage response pathway (van Steensel et al, 1998; Karlseder et al, 1999). Despite their extensive homology, TRF1 and TRF2 do not heterodimerize (Broccoli et al, 1997; Fairall et al, 2001), and seem to form different complexes at telomeres. TRF1 is in a complex containing TIN2, Tankyrase 1 and 2, PINX1, Pot1 (reviewed in Smogorzewska & De Lange, 2004) and PTOP/PIP1 (Liu et al, 2004; Ye et al, 2004), whereas TRF2 interacts with RAP1, the Rad50/MRE11/NBS complex, the DNA-PK/Ku70/Ku80 complex (reviewed in de Lange, 2002) and also TIN2 (Kim et al, 2004).
Besides the role of TRF1 and TRF2 in binding directly to double-stranded telomeric DNA repeats and thereby mediating the recruitment of different factors to telomeres, TRF2 has an additional ‘structural' function at telomeres. From in vitro electron microscopy studies, it has been proposed that TRF2 itself has the ability to remodel telomeric DNA into t-loops (Stansel et al, 2001), a telomere fold-back structure first observed from in vivo crosslinking experiments (Griffith et al, 1999). In this structure, the 3′ telomeric end is buried through invasion of the double-stranded telomeric repeats, thus providing an elegant way of capping chromosomes (Griffith et al, 1999; reviewed in de Lange, 2004). We wanted to understand whether there are differences in the DNA-binding properties of TRF1 and TRF2 that could account for any differences in their function. Therefore, we determined the crystal structures of the Dbds of both TRF1 and TRF2 in complex with their double-stranded telomeric DNA sites.
Results And Discussion
TRF1 and TRF2 bind DNA by means of homeodomains
The crystal structures of the Dbds of both TRF1 and TRF2 in complex with a human telomeric DNA fragment were solved independently, to 2.0 and 1.8 Å resolution, respectively, using multiple isomorphous replacement methods (supplementary Tables 1 and 2 online). The oligonucleotides present in the two complexes contain two and a half TTAGGG repeats that constitute the binding site for two Dbds, as defined from comparison of footprints of full-length dimeric proteins and their isolated Dbds (Konig et al, 1998; Bianchi et al, 1999). So in the structure of each complex, two Dbds are bound to two adjacent TAGGGTT binding sites, mimicking the binding of the full-length dimeric proteins on telomeric DNA. Overall, the structures of the two complexes are very similar and differ only in detail. As illustrated by the TRF2-Dbd–DNA complex (Fig 2), the two Dbds are bound on the DNA with a 6 bp spacing, or 205° rotation, and hence are located on almost opposite faces of the DNA double helix.
Figure 2.
Global structure of two TRF2-Dbds bound to double-stranded telomeric DNA. The TRF1-Dbd–DNA complex has the same overall structure and hence is not shown. The protein molecules in the complex are shown as a ribbon representation. The two sets of three G-C base pairs present in the DNA binding site are highlighted in magenta. All figures of the structures were created using Pymol (DeLano, 2002).
The TRF1- and TRF2-Dbds consist of three-helix bundles, in which the second and third α-helices form a ‘helix–turn–helix' motif. Superposition of the two structures shows that they are very similar with an r.m.s.d. of 0.47 Å. Helix 3, the DNA-recognition helix, makes base-specific contacts in the DNA major groove. In addition, an N-terminal arm is inserted into the minor groove, where it makes specific contacts (Fig 2). The N-terminal arm classifies the Dbds as homeodomains, rather than Myb motifs, which are also three-helix bundles but lack the N-terminal arm (Ogata et al, 1992). The effect of the N-terminal arm is to extend the DNA-binding interface adding specificity and affinity. These structures confirm that telomeric proteins use homeodomains for double-stranded telomeric DNA recognition, as first observed in the crystal structure of the budding yeast ScRap1p in complex with telomeric DNA (Konig et al, 1996).
Protein–DNA recognition involves many water molecules
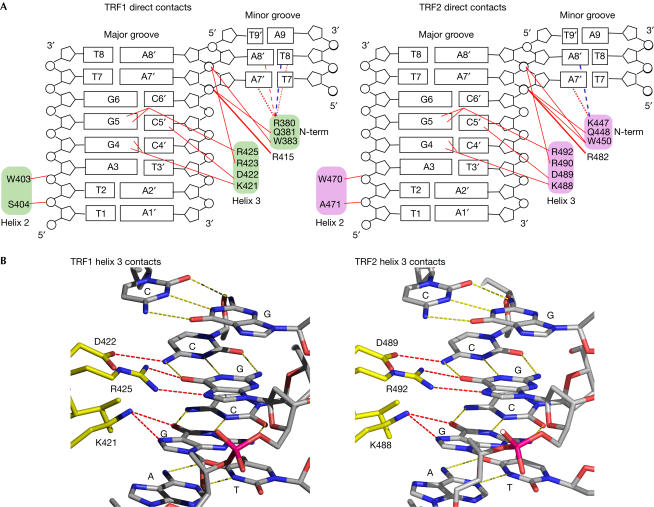
TRF1 and TRF2 bind to telomeric DNA by making essentially the same contacts (Figs 3, 4), consistent with the observation that the two Dbds bind with almost the same 10−9 M affinity to the DNA oligonucleotides used in the crystal structures (Konig et al, 1998; data not shown). Both TRF1 and TRF2 contact the same 7 bp TAGGGTT binding site (Fig 3A). Specificity in DNA recognition is achieved by several direct contacts from amino-acid side chains to the DNA and, interestingly, an extensive network of indirect water-mediated contacts. In each complex, the two Dbds dock onto the DNA through direct contacts to the ribose phosphate backbone on both sides of the major groove by residues from helix 2 and the N-terminal arm. These contacts orient the Dbd such that the DNA recognition helix enters the DNA major groove making sequence-specific hydrogen bonds to the G-C base-pair triplet of the binding site. The interactions of the DNA-recognition helix are identical for the two proteins (Fig 3B). In each case, a lysine side chain makes a bifurcated hydrogen bond and an arginine side chain makes two hydrogen bonds to two adjacent guanines (K421 and R425 in TRF1 and K488 and R492 in TRF2 to G4 and G5), and an aspartic acid makes a direct hydrogen bond to a cytosine on the opposite strand. Helix 2 contacts the DNA backbone slightly differently in the two complexes because the two proteins have different amino acids in equivalent positions: in TRF1, the side chain of S404 contacts O2 of the T2 phosphate in the G-rich strand, whereas in TRF2, the main chain of A471 contacts O1 of the equivalent phosphate group. In both TRF1 and TRF2, the N-terminal arm crosses the ribose phosphate backbone, allowing Q381 and W383 in TRF1 and Q448 and W450 in TRF2 to make conserved contacts to the C6′ and A7′ phosphates, respectively, thus fixing the orientation of the N-terminal arm. The basic side chain of the preceding residue is inserted into the minor groove contacting two A-T base pairs, but this interaction varies between TRF1 and TRF2 and also between different Dbds in the same complex, due to alternative side-chain orientations (Fig 3A).
Figure 3.
Summary of direct protein–DNA contacts in the TRF1-Dbd–DNA and TRF2-Dbd–DNA complexes. (A) Maps of protein–DNA contacts. The DNA is represented as an opened-out helix. Red lines indicate direct hydrogen bonds. These contacts are conserved between the two molecules in one complex. Direct contacts in the minor groove made by residues R380 of TRF1 and K447 of TRF2 that differ in the two protein molecules in one complex are indicated by dotted and dashed red lines. A dashed blue line depicts water-mediated contacts. (B) Views at atomic resolution of the hydrogen bonds between residues in the DNA-recognition helix and the bases in the major groove of DNA.
Figure 4.
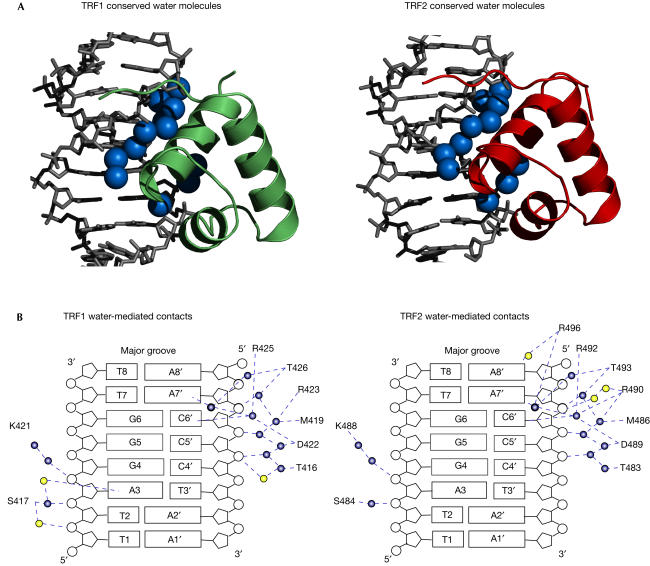
Summary of water-mediated protein–DNA contacts in the TRF1-Dbd–DNA and TRF2-Dbd–DNA complexes. (A) Conservation in the water structure at the protein–DNA interface. The two proteins are shown as ribbon representations and only half of each complex is shown. Conserved water molecules at the protein–DNA interface are represented by blue spheres. (B) Summary of water-mediated contacts in TRF1-Dbd–DNA and TRF2-Dbd–DNA complexes. Conserved water molecules are shown in blue and additional water molecules are shown in yellow. Blue dashed lines depict the network of water-mediated hydrogen bonds.
In addition to the direct contacts described above, water-mediated contacts have an important role in the recognition of telomeric DNA repeats by TRF1 and TRF2. The assignment of water molecules and their role at the protein–DNA interface was made possible by the high resolution of the two crystal structures. Although there are about twice as many ordered water molecules in the TRF2 than in the TRF1 complex, there is a set of 12 water molecules that occupy conserved positions (less than 1 Å apart after superposition of the DNA molecules) in the major groove of the DNA in the two structures. These water molecules form two complex spines of hydration on either side of the DNA-recognition helix, mediating additional hydrogen bond interactions between the protein and DNA. For instance, Arg 425 in TRF1 and Arg 492 in TRF2, both of which make two direct hydrogen bonds to G5, contact two other bases (C6′ and A7′) through water molecules, increasing both the specificity and affinity of DNA recognition. Other residues in the DNA-recognition helix make similar direct and indirect contacts, whereas some residues make only water-mediated contacts (Fig 4B). The conservation of the water structure in the two complexes suggests that the TRF proteins recognize the hydrated DNA structure rather than the DNA sequence per se.
Comparison between the NMR and crystal structures
Comparison of the crystal structure of the TRF1-Dbd–DNA complex with the NMR structure determined previously (Nishikawa et al, 2001) shows that overall they are very similar with an r.m.s.d. of 1.31 Å. However, there are several significant differences that are likely to arise from differences in the two structural methodologies used. The major and most significant difference is the observation in the crystal structure of a highly ordered network of water molecules at the protein–DNA interface, which contribute to the specificity and affinity of protein binding. This feature is totally absent from the NMR structure, preventing a full description of the protein–DNA interface and hence a full understanding of how the human telomeric proteins recognize DNA. There are also some differences in detail. Lys 421, which in both crystal structures is well ordered with a low temperature factor and makes an important bifurcated hydrogen bond to G4, has several conformations in the NMR structure. Furthermore, in the NMR structure, Ser 417 and Asp 422 make direct phosphate contacts, whereas in the crystal structure these contacts are water mediated.
DNA conformation in the TRF1 and TRF2 complexes
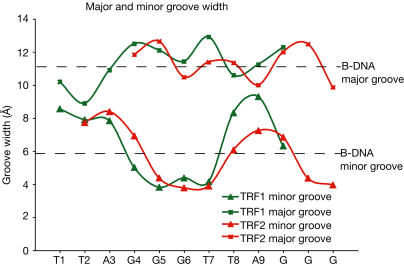
We analysed the DNA conformation in the TRF1- and TRF2-Dbd complexes to discover whether (i) there were any distortions caused by TRF2-Dbd binding that would give insight into t-loop formation (Stansel et al, 2001) and (ii) the TRFs could bind in a nucleosome context (Tommerup et al, 1994; Nikitina & Woodcock, 2004). The DNA double helix in the two complexes is essentially straight with an average helical twist very close to that of B-form DNA, and there are no obvious features in the binding of TRF2-Dbd that differentiate it from that of TRF1. However, in both complexes, there are subtle changes in the DNA groove widths: there is an opening of the major groove and a corresponding narrowing of the minor groove over the G-C base-pair triplet caused by protein binding. In contrast, the flanking A-T-rich sequences are distorted in the opposite direction, resulting in an unusually wide minor groove (Fig 5). First, this observation bears on the previous report that TRF2 alone can mediate t-loop formation in vitro (Stansel et al, 2001). In this study, using electron microscopy analysis, full-length TRF2 was observed to bind at the t-loop junction where it forms large aggregates, suggesting that TRF2 binding/oligomerization was sufficient for t-loop formation. However, the oligomerization state of the protein was not characterized biochemically (Stansel et al, 2001). T-loop formation requires both bending of the DNA and insertion of the single-stranded telomeric end into the double-stranded telomeric DNA (Griffith et al, 1999). The structure presented here shows that there are no DNA-binding characteristics of TRF2-Dbd that could account for t-loop formation, as the Dbd is a classical double-stranded binding domain and has no ability to untwist or bend the DNA. We believe that the lack of ‘remodelling' activity is likely to hold true for the full-length dimeric TRF2. This conclusion, in addition to the direct structural information on both the Dbd of TRF2 and its dimerization domain (Fairall et al, 2001), is on the basis of a comparison of the results of gel filtration, proteolysis and chemical crosslinking studies on full-length TRF2 and TRF1 (Fairall et al, 2001; L.F., M. O' Reilly & L.C., unpublished observations). Proteolysis studies show that in the case of both TRF1 and TRF2, the Dbds are joined to the dimerization domains through long unstructured linkers (Fig 1). Consistent with this observation, both proteins have gel filtration migrations much larger than their actual molecular mass. The TRF1 dimer has a molecular mass of 100 kDa and migrates as 220 kDa and TRF2 dimer has a molecular mass of 110 kDa and migrates as 270 kDa, but it has a longer linker than TRF1 (Fig 1). Crosslinking data show that both proteins are primarily dimeric in solution in the absence of DNA. Therefore, we conclude that TRF2 has the same flexible structure as its sister TRF1, in which the two Dbds in the full-length dimeric protein are known be unconstrained and bind to DNA in the same way as the isolated Dbds (Bianchi et al, 1999). The second conclusion from the structures is that the spatial arrangement of the two Dbds on opposite faces of the DNA double helix (Fig 2), as well as the observed changes in minor and major DNA groove width resulting from TRF binding (Fig 5), would exclude nucleosome formation (Satchwell et al, 1986). Instead, if TRF1 and TRF2 are bound within nucleosome-containing t-loops or linear telomeric chromatin (Nikitina & Woodcock, 2004), they are likely to do so on the naked telomeric DNA repeats located between nucleosomes.
Figure 5.
DNA structure in the TRF1 and TRF2 binding sites. The widths of the DNA major and minor grooves in the complexes were calculated using the program CURVES (Lavery & Sklenar, 1989). The average major and minor groove widths of B-form DNA are shown for reference.
Conclusions
The structures that we have presented here of the Dbds of TRF1 and TRF2 in complex with telomeric DNA repeats show that double-stranded telomeric DNA recognition occurs through homeodomains and is conserved between yeast and man. The specificity in recognition arises from direct contacts with the cluster of Gs, typical of telomeric repeats. TRF2-Dbd binds to double-stranded DNA without causing major distortions or untwisting of the DNA double helix, suggesting that its DNA-binding activity per se is unlikely to be involved in the remodelling of telomeric DNA into t-loops.
Methods
Protein expression and purification. TRF1-Dbd (residues 371–439) was cloned into pET13a vector, overexpressed in Rosetta (DE3) cells (Novagen, Merck Biosciences, Nottingham, UK) and purified by chromatography on a Source 30S Sepharose column (Pharmacia). TRF2-Dbd (residues 441–500) was expressed as a His6-tagged protein cloned into a modified pET30a vector with a TEV cleavage site, overexpressed in Rosetta (DE3) cells and purified using Ni-NTA resin (Qiagen Ltd, Crawley, UK). Following TEV cleavage, the tag was removed by reapplying the cleaved protein to Ni-NTA resin. The final chromatography step for both proteins was on a Superdex 200 column (Amersham Biosciences UK, Chalfont St Giles, UK).
Crystallization and data collection. Protein–DNA complexes were produced by estimating stoichiometries from gel mobility shift assays. Hanging-drop crystallization trials were performed with several different double-stranded oligonucleotides. The oligonucleotides producing crystals that diffracted to high resolution are shown in Fig 1. These oligonucleotides contained two binding sites for the Dbds and form pseudo-continuous DNA helices in the crystal lattice. The TRF1-Dbd–DNA crystals were grown in a buffer containing 50 mM MES pH 6.0, 0.1 M KCl, 2 mM MgCl2 and 10% PEG 400. The TRF2-Dbd–DNA crystals were grown in a buffer containing 50 mM MES pH 6.0, 5 mM MgOAc2, 1 mM spermine and 2 M NH4SO4. Derivatives were produced by substituting thymines in the DNA sites with 5-iodouridine (supplementary Table 1 online). X-ray data were collected at 21°C and 100 K. The crystals were cryo-protected using 1.1 × mother liquor and 20 and 15% glycerol for TRF1 and TRF2, respectively. Native and derivative data sets were collected in-house using standard X-ray generators, and the high-resolution native data sets were collected at station ID14.1 at ESRF, Grenoble.
Data processing, model building and refinement. All data were integrated using MOSFLM (Leslie, 1992) and scaled using SCALA (CCP4, 1994; supplementary Tables 1 and 2 online). Heavy atom positions were found using Solve (Terwilliger & Berendzen, 1999) and refined in autoSHARP (La Fortelle et al, 1997). Density modification using Solomon (Abrahams & Leslie, 1996) was implemented in autoSHARP. Maps were generated from autoSHARP output files using Mapman (Kleywegt & Jones, 1996) and visualized in the program O (Jones et al, 1991). Starting models were based on the solution structure of TRF1-Dbd–DNA complex (Nishikawa et al, 2001) and all refinements carried out with CNS (Brunger et al, 1998), using high-resolution synchrotron data from native crystals, with manual rebuilding in O (supplementary Table 2 online).
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v6/n1/extref/7400314s1.pdf).
Supplementary Material
Supplementary Tables
Acknowledgments
We thank D. Schlieper, A. Leslie, H. Powell and P. Evans for help with data collection and processing and B. Kligman for help with protein and DNA purifications. The atomic coordinates and structure factors have been deposited in the Protein Data Bank under the PDB ID codes 1W0T for TRF1 and 1W0U for TRF2.
References
- Abrahams JP, Leslie AGW (1996) Methods used in the structure determination of bovine mitochondrial F-1 ATPase. Acta Crystallogr D 52: 30–42 [DOI] [PubMed] [Google Scholar]
- Ancelin K, Brun C, Gilson E (1998) Role of the telomeric DNA-binding protein TRF2 in the stability of human chromosome ends. BioEssays 20: 879–883 [DOI] [PubMed] [Google Scholar]
- Bianchi A, Smith S, Chong L, Elias P, de Lange T (1997) TRF1 is a dimer and bends telomeric DNA. EMBO J 16: 1785–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Stansel RM, Fairall L, Griffith JD, Rhodes D, de Lange T (1999) TRF1 binds a bipartite telomeric site with extreme spatial flexibility. EMBO J 18: 5735–5744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilaud T, Koering CE, Binet-Brasselet E, Ancelin K, Pollice A, Gasser SM, Gilson E (1996) The telobox, a Myb-related telomeric DNA binding motif found in proteins from yeast, plants and human. Nucleic Acids Res 24: 1294–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilaud T, Brun C, Ancelin K, Koering CE, Laroche T, Gilson E (1997) Telomeric localization of TRF2, a novel human telobox protein. Nat Genet 17: 236–239 [DOI] [PubMed] [Google Scholar]
- Broccoli D, Smogorzewska A, Chong L, de Lange T (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet 17: 231–235 [DOI] [PubMed] [Google Scholar]
- Brunger AT et al. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D 54: 905–921 [DOI] [PubMed] [Google Scholar]
- CCP4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–763 [DOI] [PubMed] [Google Scholar]
- de Lange T (2002) Protection of mammalian telomeres. Oncogene 21: 532–540 [DOI] [PubMed] [Google Scholar]
- de Lange T (2004) T-loops and the origin of telomeres. Nat Rev Mol Cell Biol 5: 323–329 [DOI] [PubMed] [Google Scholar]
- DeLano WL (2002) The PyMOL molecular graphics system. http://pymol.sourceforge.net
- Fairall L, Chapman L, Moss H, de Lange T, Rhodes D (2001) Structure of the TRFH dimerization domain of the human telomeric proteins TRF1 and TRF2. Mol Cell 8: 351–361 [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97: 503–514 [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C, Sarai A, Sawazaki T, Nakagoshi H, He DN, Ogata K, Nishimura Y, Ishii S (1990) The tryptophan cluster: a hypothetical structure of the DNA-binding domain of the myb protooncogene product. J Biol Chem 265: 19990–19995 [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T (1999) p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283: 1321–1325 [DOI] [PubMed] [Google Scholar]
- Kim SH, Beausejour C, Davalos AR, Kaminker P, Heo SJ, Campisi J (2004) TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem 279: 43799–43804 [DOI] [PubMed] [Google Scholar]
- Kleywegt GJ, Jones TA (1996) xdlMAPMAN and xdlDATAMAN—programs for reformatting, analysis and manipulation of biomacromolecular electron-density maps and reflection data sets. Acta Crystallogr D 52: 826–828 [DOI] [PubMed] [Google Scholar]
- Konig P, Rhodes D (1997) Recognition of telomeric DNA. Trends Biochem Sci 22: 43–47 [DOI] [PubMed] [Google Scholar]
- Konig P, Giraldo R, Chapman L, Rhodes D (1996) The crystal structure of the DNA-binding domain of yeast RAP1 in complex with telomeric DNA. Cell 85: 125–136 [DOI] [PubMed] [Google Scholar]
- Konig P, Fairall L, Rhodes D (1998) Sequence-specific DNA recognition by the myb-like domain of the human telomere binding protein TRF1: a model for the protein–DNA complex. Nucleic Acids Res 26: 1731–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fortelle E, Irwin JJ, Bricogne G (1997) Advances in MIR and MAD phasing: maximum-likelihood refinement in a graphical environment, with SHARP. In Proceedings of the CCP4 Study Weekend, Wilson GDKS, Ashton AK, Bailey S (eds). Warrington, UK: Daresbury Laboratory [Google Scholar]
- Lavery R, Sklenar H (1989) Defining the structure of irregular nucleic acids: conventions and principles. J Biomol Struct Dyn 6: 655–667 [DOI] [PubMed] [Google Scholar]
- Lejnine S, Makarov VL, Langmore JP (1995) Conserved nucleoprotein structure at the ends of vertebrate and invertebrate chromosomes. Proc Natl Acad Sci USA 92: 2393–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AGW (1992) Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4+ESF-EAMCB Newslett Protein Crystallogr, issue 26 [Google Scholar]
- Li B, Oestreich S, de Lange T (2000) Identification of human Rap1: implications for telomere evolution. Cell 101: 471–483 [DOI] [PubMed] [Google Scholar]
- Liu D, Safari A, O'Connor MS, Chan DW, Laegeler A, Qin J, Songyang Z (2004) PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 6: 673–680 [DOI] [PubMed] [Google Scholar]
- Nikitina T, Woodcock CL (2004) Closed chromatin loops at the ends of chromosomes. J Cell Biol 166: 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Nagadoi A, Yoshimura S, Aimoto S, Nishimura Y (1998) Solution structure of the DNA-binding domain of human telomeric protein, hTRF1. Structure 6: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Okamura H, Nagadoi A, Konig P, Rhodes D, Nishimura Y (2001) Solution structure of a telomeric DNA complex of human TRF1. Structure (Camb) 9: 1237–1251 [DOI] [PubMed] [Google Scholar]
- Ogata K, Hojo H, Aimoto S, Nakai T, Nakamura H, Sarai A, Ishii S, Nishimura Y (1992) Solution structure of a DNA-binding unit of Myb: a helix–turn–helix-related motif with conserved tryptophans forming a hydrophobic core. Proc Natl Acad Sci USA 89: 6428–6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D, Fairall L, Simonsson T, Court R, Chapman L (2002) Telomere architecture. EMBO Rep 3: 1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchwell SC, Drew HR, Travers AA (1986) Sequence periodicities in chicken nucleosome core DNA. J Mol Biol 191: 659–675 [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, De Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73: 177–208 [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T (2000) Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansel RM, de Lange T, Griffith JD (2001) T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J 20: 5532–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J (1999) Automated MAD and MIR structure solution. Acta Crystallogr D 55: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerup H, Dousmanis A, de Lange T (1994) Unusual chromatin in human telomeres. Mol Cell Biol 14: 5777–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, de Lange T (1997) Control of telomere length by the human telomeric protein TRF1. Nature 385: 740–743 [DOI] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401–413 [DOI] [PubMed] [Google Scholar]
- Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T (2004) POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev 18: 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables