Abstract
It is well established that the mitogen-activated protein kinase (MAPK) signal is regulated through phosphorylation-dependent activation by the three-tiered MAPK cascade. However, our studies on the interaction of the MAPK ERK5 with the tyrosine kinase c-Abl and its oncogenic variants v-Abl and Bcr/Abl disclosed an alternative aspect of regulation. Independent of the MAPK cascade, Abl kinases were able to regulate the cellular amount of ERK5, at least in part, by stabilizing the protein. The resulting level of ERK5 and its intrinsic basal activity, but not necessarily its activation, were essential and sufficient to increase transformation by v-Abl and to mediate survival of Bcr/Abl-expressing leukaemia cells. These results suggest that the ability to regulate the cellular abundance of ERK5 contributes to the oncogenic potential of Abl kinases.
Keywords: MAPK, ERK5, Bcr/Abl, chronic myeloid leukaemia, survival
Introduction
Chronic myeloid leukaemia (CML) is a pluripotent haematopoietic stem cell disorder, which is characterized by the Philadelphia chromosome. This genomic abnormality gives rise to the Bcr/Abl fusion protein (Ben-Neriah et al, 1986). The strictly cytosolic Bcr/Abl dimerizes and autophosphorylates and thus mimics an activated receptor tyrosine kinase. The bcr/abl oncogene is sufficient to initiate CML-like disease in mice (Daley et al, 1990), and is responsible for leukaemia progression (Huettner et al, 2000). Treatment of patients with the Abl-specific kinase inhibitor imatinib (also termed Gleevec, Glivec and STI571) induced remission in the early chronic leukaemic phase as well as in the late blast crisis (Druker, 2002). Successful therapy was unfortunately hampered by the development of drug resistance, particularly in patients with advanced CML. Combinational therapy by additional targeting of survival pathways could be a strategy in the prevention of tumour progression that is caused by the expansion of resistant clones (O'Dwyer, 2002).
One principal survival signal is mediated by mitogen-activated protein kinases (MAPKs), which are involved in transcriptional regulation of prosurvival factors (Ballif & Blenis, 2001). MAPKs are evolutionarily conserved enzymes found in virtually all eukaryotes. They are activated by a broad variety of stimuli and regulate a large number of cellular processes, in part, by activating the transcriptional machinery. The activation of MAPKs is regulated through a three-tiered cascade composed of the MAPK itself, an activating MAPK kinase and an upstream MAPK kinase kinase (Chang & Karin, 2001). In Bcr/Abl-positive leukaemia cells, inhibition of the Raf–MEK1/2–ERK1/2 cascade by chemical compounds has been shown to enhance imatinib-induced apoptosis synergistically (Yu et al, 2002). Interestingly, the same inhibitors also inhibit the ERK5 pathway, but less effectively (Mody et al, 2001). This finding together with several recent observations that pointed towards an involvement of ERK5 in cell survival (Dong et al, 2001; Watson et al, 2001; Weldon et al, 2002; Shalizi et al, 2003; Cavanaugh, 2004) led us to investigate whether ERK5 might have a role in prosurvival signalling of Bcr/Abl-positive leukaemia cells. Here, we present results that support three main conclusions: (1) ERK5 levels are regulated by oncogenic Abl kinase activity; (2) nonactivated ERK5 possesses basal activity and (3) the level of ERK5 contributes to survival of Bcr/Abl-positive leukaemia cells.
Results And Discussion
ERK5 protein levels are sensitive to Abl kinase activity
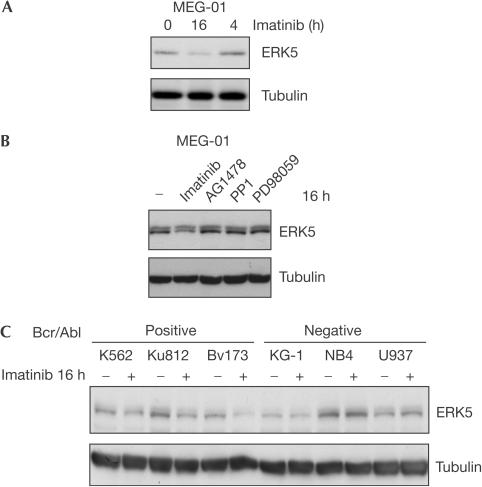
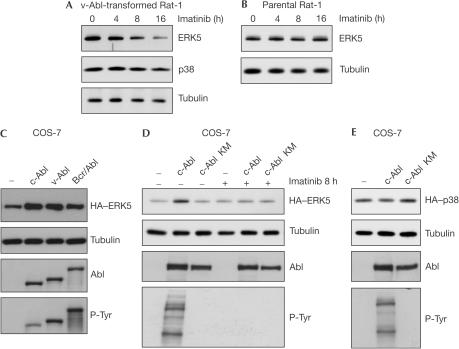
The signalling activity of the Bcr/Abl-expressing cell line MEG-01 is highly sensitive to Bcr/Abl inhibition, which is reflected by a drastic reduction of overall tyrosine phosphorylation in crude lysates on application of imatinib (supplementary Fig 1A online). Prolonged imatinib treatment was found to result in a reduction of ERK5 levels (Fig 1A), whereas other kinase inhibitors had no effect (Fig 1B). Comparing the levels of ERK5 in six different leukaemia cells, we found that the cellular amount of ERK5 did not correlate with the presence of the bcr/abl oncogene (Fig 1C). However, only in Bcr/Abl-positive cells, the protein levels of ERK5 were sensitive to imatinib (Fig 1C). Likewise, in v-Abl-transformed Rat-1 fibroblasts, the level of ERK5 but not of another MAPK p38 decreased with time of imatinib exposure, whereas in parental Rat-1 cells, ERK5 was not affected (Fig 2A,B). As inhibition of Abl kinase activity resulted in a decrease of ERK5 protein, we used overexpression in COS-7 cells to conduct the converse experiment. As shown in Fig 2C, increasing Abl kinase activity by overexpressing any Abl kinase enhanced the level of ERK5. Expression of Abl in the presence of imatinib or expression of the kinase-dead Abl KM construct had no effect (Fig 2D). The expression level of another MAPK p38 was not influenced by co-overexpression of Abl (Fig 2E).
Figure 1.
ERK5 expression levels in Bcr/Abl-expressing leukaemia cells are reduced on imatinib treatment. MEG-01, other Bcr/Abl-positive and Bcr/Abl-negative leukaemia cells were treated with 10 μM of the Abl kinase inhibitor imatinib or other kinase inhibitors.
Figure 2.
The protein level of ERK5 is sensitive to Abl kinase activity. (A,B) v-Abl-transformed Rat-1 fibroblasts (A) and parental Rat-1 cells (B) were treated with 5 μM imatinib as indicated. Immunoblot analyses of crude cell lysates using anti-ERK5, anti-p38 and anti-tubulin antibodies are shown. (C–E) Transfected COS-7 cells were treated with 10 μM imatinib for 8 h. Crude cell lysates were analysed by anti-HA, anti-Abl, anti-tubulin and antiphosphotyrosine (P-Tyr) immunoblot. The last was used to monitor activity of exogenous tyrosine kinases.
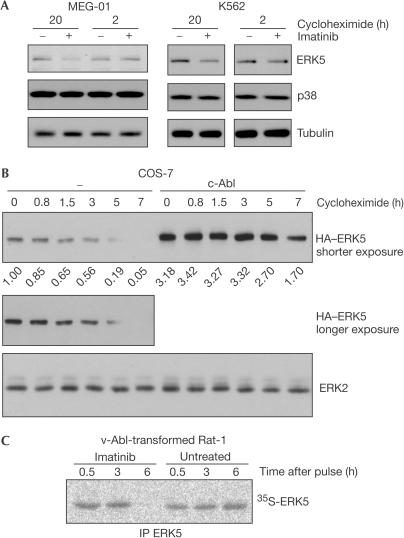
The fact that not only endogenous but also transiently transfected ERK5, which is expressed at a relatively constant rate, was sensitive to Abl kinase activity led us to speculate that the stability of the ERK5 protein was affected. To test this hypothesis, we blocked translation in MEG-01 and K562 cells by addition of cycloheximide and checked whether co-administration of imatinib would still be able to influence the cellular level of ERK5. As shown in Fig 3A, ERK5 but not p38 was reduced in cells treated with the inhibitor. Accordingly, the half-life of exogenous ERK5 was prolonged in COS-7 cells co-overexpressing Abl, whereas ERK2 protein levels were not affected (Fig 3B). As further shown in Fig 3C, the half-life of ERK5 was shortened in v-Abl-transformed Rat-1 fibroblasts when treated with imatinib (Fig 3C). These results suggest that the influence exerted by Abl kinases on ERK5 levels is, at least in part, post-translational and can thus be considered as the stabilization of the ERK5 protein.
Figure 3.
Abl kinases affect protein levels of ERK5 even in the presence of the translational inhibitor cycloheximide. (A) MEG-01 and K562 leukaemia cells were treated with 10 μM imatinib and 100 μM cycloheximide. Protein levels of ERK5, p38 and tubulin were analysed by immunoblot. (B) Transfected COS-7 cells expressing either HA-tagged ERK5 alone or together with exogenous c-Abl were treated with 100 μM cycloheximide for different times and analysed by anti-HA and anti-ERK2 immunoblot. Numbers below the top panel represent quantification of relative ERK5 levels. (C) Immediately after pulse labelling, v-Abl-transformed Rat-1 cells were treated with 5 μM imatinib or left untreated. ERK5 was immunoprecipitated at the time points indicated and analysed by autoradiography.
Stabilization of ERK5 is independent of its activation
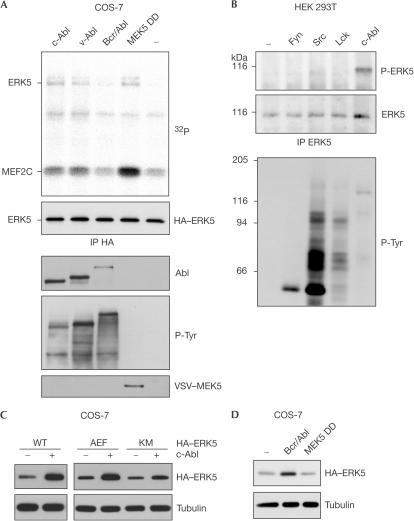
In analogy with other MAPKs, ERK5 is activated by MEK5-dependent dual phosphorylation of a threonine and a tyrosine residue in its activation motif. Although we were unable to detect activated ERK5 in MEG-01 and K562 cells by immunoblot using a phosphorylation-state-specific antibody (not shown), we tested whether Abl kinases would potentially be able to induce activation of ERK5 when overexpressed together in COS-7 cells. As shown in Fig 4A, c-Abl and v-Abl induced only moderate activation of ERK5 as indicated by its autophosphorylation as well as phosphorylation of its specific substrate MEF2C. Bcr/Abl, which was expressed to a lower extent, had no effect at all. Activated forms of ERK5 were also detectable in HEK 293T cells overexpressing c-Abl, whereas exogenous expression of Src family kinases had no effect (Fig 4B).
Figure 4.
Stabilization of ERK5 is independent of its activation. (A) COS-7 cells were transfected as indicated. An excess of cell lysate was used to precipitate comparable amounts of exogenous ERK5 using anti-HA antibodies. Immunoprecipitates (IP) were subjected to in vitro kinase assays containing GST–MEF2C and analysed by autoradiography and anti-HA immunoblot. Expression of effector proteins and tyrosine kinase activity was analysed by anti-Abl, anti-VSV and antiphosphotyrosine (P-Tyr) immunoblot of crude cell lysates. (B) Endogenous ERK5 was immunoprecipitated from transfected HEK 293T cells. Activated and total ERK5 were monitored by anti-phospho-ERK5 (P-ERK5) and anti-ERK5 immunoblot, respectively. Activity of overexpressed tyrosine kinases was again verified by anti-P-Tyr immunoblot. (C,D) Lysates from transfected COS-7 cells were analysed by anti-HA and anti-tubulin immunoblot.
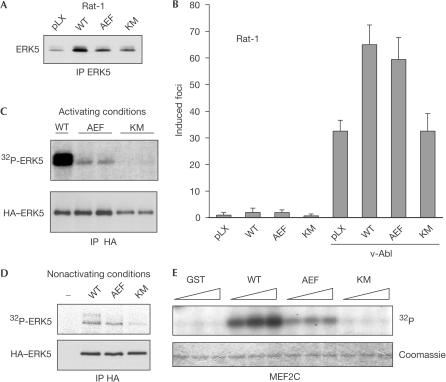
To address the question of whether activation and stabilization would be interdependent, we tested whether Abl kinases would affect the level of an ERK5 mutant (AEF) lacking both activating phosphorylation sites for MEK5. As shown in Fig 4C, overexpression of Abl elevated the amount of AEF mutant to a similar extent as that of wild-type ERK5. The level of a kinase-dead KM mutant of ERK5 was increased to a lesser extent. Moreover, constitutively active MEK5 DD, although potent in ERK5 activation (Fig 4A), did not enhance ERK5 protein levels (Fig 4D). Hence, Abl kinases can affect ERK5 by at least two independent mechanisms: by inducing its activation and by increasing its protein level. Under physiological conditions, the latter seems to be prevalent.
Basal activity of ERK5 enhances cell transformation
To get some preliminary information about the functional connection of ERK5 and oncogenic Abl signalling, we compared the efficacy of v-Abl in transforming Rat-1 fibroblasts stably overexpressing either wild-type ERK5, the AEF variant or the KM variant (Fig 5A). Overexpression of wild-type ERK5 increased the amount of foci induced by v-Abl, whereas kinase-dead ERK5 KM had no influence at all (Fig 5B). Strikingly, the AEF mutant of ERK5 was here as potent as the wild-type form.
Figure 5.
Basal activity of overexpressed ERK5 is sufficient to enhance v-Abl-induced transformation of Rat-1 fibroblasts. (A) An immunoblot of anti-ERK5 immunoprecipitates from polyclonal Rat-1 fibroblasts overexpressing either wild-type (WT) ERK5, nonactivatable AEF or kinase-dead KM mutant is shown. (B) Monolayers of these cells were infected with ecotrophic pLXSN-v-Abl retrovirus or empty control vector and were further cultivated for 14 days. The number of induced foci was determined. Data represent three independent experiments and s.d. (C,D) At 1 h before lysis, COS-7 cells were either treated with fresh serum (activating conditions (C)) or left untreated (nonactivating conditions (D)). Autophosphorylation of HA–ERK5 mutants was assessed by immunocomplex kinase assays and autoradiography (32P-ERK5). (E) Bacterially expressed fusion proteins of GST and the wild-type kinase domain of ERK5 (aa 1–409), or the AEF and KM mutant forms or GST alone were subjected to in vitro kinase reactions containing GST–MEF2C as substrate. Samples were analysed by autoradiography and Coomassie staining.
Although ERK5 AEF cannot be activated, it was found to harbour some activity, as indicated by its ability to autophosphorylate (Fig 5C). Although under activating conditions this activity is much lower than the activity of wild-type ERK5, under nonactivating conditions the activities of wild-type ERK5 and AEF were comparable (Fig 5C,D). As bacteria not only lack homologues of MEKs but are also completely devoid of tyrosine kinase activity, we next used a bacterial expression to generate unactivated ERK5. As shown in Fig 5E, a fusion protein of glutathione-S-transferase (GST) and wild-type ERK5 was able to phosphorylate the specific substrate MEF2C in vitro. GST–ERK5 fusion proteins carrying the AEF mutation, but not those bearing the KM mutation, were still able to phosphorylate MEF2C to some extent. In pathways regulating cellular functions that require the activation of ERK5 by MEK5, the AEF mutant should act as a dominant negative. It was shown to do so in MCF10A and HeLa cells by impairing growth-factor-induced proliferation (Kato et al, 1998). However, it is conceivable that, under conditions that do not require ERK5 activation, the AEF mutant could act as the wild-type form. Hence, our results show that activation of ERK5 was not required to enhance v-Abl-induced transformation, although kinase activity per se was involved as indicated by the loss of function of the KM mutant.
ERK5 contributes to MEG-01 cell survival
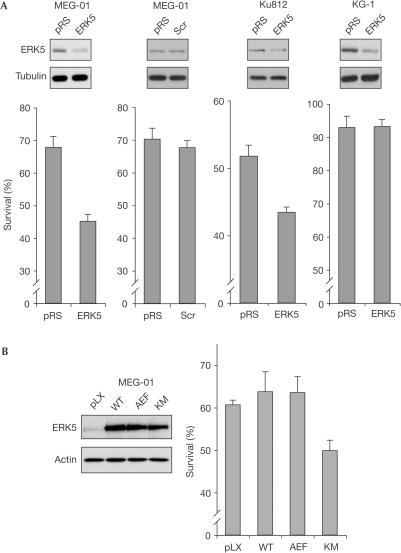
To address the question of ERK5 function in Bcr/Abl-positive leukaemia cells, we reduced endogenous ERK5 levels in MEG-01 and Ku812 cells by infection of an ERK5-specific small hairpin RNA-generating retroviral vector (Fig 6A). To study the potential influence of ERK5 on leukaemia cell survival, we transiently treated MEG-01 cells with various concentrations of imatinib for 2 days. Although higher concentrations of imatinib (10 μM) irreversibly triggered cell death even after drug removal, treating cells with a low dose of imatinib (0.2–0.3 μM) also significantly induced cell death but did allow cells to recover after removal (data not shown). We used the latter conditions to study the delicate balance between prosurvival and proapoptotic signals. As shown in Fig 6A, survival of MEG-01 cells was reduced by about one-third in cells with reduced ERK5 expression, whereas cells infected with a virus generating an unrelated small hairpin RNA (Scr) were not affected. Likewise, in Ku812 cells but not in Bcr/Abl-negative KG-1 cells, survival correlated with ERK5 expression levels. Under the same experimental conditions, overexpression of the kinase-dead KM mutant of ERK5 was able to interfere negatively with survival of MEG-01 cells. In contrast, wild-type ERK5 and the AEF mutant had virtually no effect (Fig 6B). Together, these results suggest that the cellular amount of ERK5 is one of the factors that determines the survival of Bcr/Abl-expressing leukaemia cells after treatment with the chemotherapeutic agent imatinib.
Figure 6.
ERK5 is involved in the survival of imatinib-treated Bcr/Abl-positive leukaemia cells. (A) Bcr/Abl-positive MEG-01 and Ku812 cells and Bcr/Abl-negative KG-1 cells were infected with a retroviral construct generating ERK5-specific small hairpin RNA, an unrelated hairpin (Scr) or the empty vector (pRS) and selected for 3 days with puromycin. Expression levels of ERK5 and tubulin are shown by immunoblot analysis of crude cell lysates (top panels). Polyclonal lines were treated with low concentrations of imatinib (0.2–0.3 μM) for 2 days and cell survival was assessed by flow cytometric analysis 24 h after drug removal. Data represent three independent experiments and s.d. (B) MEG-01 cells were infected with retroviral expression vectors for wild-type ERK5, KM, AEF or the empty vector (pLX) and selected. Cells were treated and analysed as described in (A).
Contrary to many cellular signalling pathways, which are transiently switched on by diverse stimuli, survival pathways should be constitutively active to attain their anticipated function. This includes maintaining transcription of prosurvival factors. In contrast to the KM mutant, ERK5 AEF was not able to interfere negatively with cell survival. This could indicate that basal activity of ERK5 was sufficient for its survival function. Controlling the amount of ERK5 protein could thus represent a way of regulation. The observation made in fibroblasts that ERK2 degradation but not its inactivation by phosphatases is a prerequisite for sorbitol-induced apoptosis (Lu et al, 2002) further substantiates the importance of regulating MAPK protein levels in the control of cell survival. It is noteworthy that the research of several other groups focusing on neurons and cancer cells also attributed survival functions to ERK5 (Watson et al, 2001; Weldon et al, 2002; Shalizi et al, 2003; Cavanaugh, 2004).
For the treatment of CML patients, inhibiting survival pathways could provide a strategy to support imatinib therapy. Our study illustrates that basal MAPK activity could be important, and hence an effective therapeutic agent should directly impede MAPK kinase activity rather than just interfere with the activating upstream kinase cascade like currently available compounds.
Methods
Antibodies and reagents. The following antibodies were purchased: tubulin (Sigma-Aldrich, Taufkirchen, Germany), haemagglutinin (HA) 12CA5 for immunoprecipitation and VSV P5D4 (Roche Diagnostics, Mannheim, Germany), ERK2, Abl and p38 (Santa Cruz Biotechnology, Heidelberg, Germany), Phospho-ERK5 (BioSource, Solingen, Germany), HA.11 for western blot (BAbCo) and phosphotyrosine 4G10 (Upstate Biotechnology Inc., Biomol, Hamburg, Germany). Rabbit anti-ERK5 antibodies were directed against a fusion protein of GST and the carboxy-terminal 100 amino acids (aa) of human ERK5. Imatinib was synthesized and provided by ViChem (Budapest, Hungary). Kinase inhibitors PD98059, PP1 and AG1478 were from Alexis, Grünberg, Germany and cycloheximide was from Sigma-Aldrich.
Plasmid construction. The following complementary DNAs used for transient transfection were in cytomegalovirus-promoter-driven expression plasmids: c-Abl, v-Abl, Bcr/Abl, c-Src, Fyn, Lck, HA–ERK2, HA–ERK5 and MEK5 DD (Buschbeck et al, 2002). Kinase-dead KM variants of ERK5 and c-Abl were generated by site-directed mutagenesis of ATP binding sites K83 and K271, respectively. Mutation of the activation motif TEY to AEF yielded the nonactivatable form of ERK5. cDNAs were subcloned into pLXSN (BD Biosciences Clontech, Heidelberg, Germany) and pGEX5X (AP Biotech, Cerdanyola, Spain). Primers containing 19 bases of ERK5 coding sequence (AGCTGCCCTGCTCAAGTCT) or unrelated sequence with similar GC content were ligated into the small hairpin RNA-generating pRetroSUPER vector (Brummelkamp et al, 2002). GST–MEF2C (aa 175–327) was kindly provided by S. Gutkind. GST–ERK5 (aa 1–409) and in analogy also its mutant forms were generated as described before (Buschbeck et al, 2002).
Cell culture, gene transfer, focus formation assay and pulse–chase labelling. Phoenix cells were kindly provided by G. Nolan (Stanford). All other cell lines were obtained from either the American or the German Tissue Culture Collection (ATCC or DSMZ, respectively). Transfections were carried out as described before (Buschbeck et al, 2002). Leukaemia cells were infected essentially as described elsewhere (Grignani et al, 1998). For stable expression, cells were selected with 1 mg/ml G418 for 12 days or 2 μg/ml puromycin for 3 days. For focus formation assays, polyclonal Rat-1 cells were infected with retroviral pLXSN v-Abl generated by transfected Phoenix cells. After infection, Rat-1 cells were further cultivated for 2 weeks and foci were counted. To generate the v-Abl-transformed Rat-1 cell line, cells able to form foci were enriched by partial trypsinization, re-plated and selected. For pulse–chase labelling, cells were starved in methionine-free medium for half an hour, incubated with medium containing 0.2 mCi/ml [35S]methionine for 20 min and chased with medium containing an excess of cold methionine (30 g/l). After lysis, immunoprecipitates were analysed by radiography.
Analysis of cell survival. Cells were treated for 48 h with imatinib (0.3, 0.2 and 1.0 μM for MEG-01, Ku812 and KG-1, respectively). After 24 h of further cultivation, cells were lysed in ice-cold hypotonic buffer containing 0.1% Triton X-100, 0.1% sodium citrate and 20 μg/ml propidium iodide. Nuclear DNA content of samples was analysed on a Becton Dickinson FACSCalibur flow cytometer. Apoptotic nuclei with less DNA stain than diploid nuclei were subtracted to get the relative rate of survival.
In vitro protein analysis. Immunoprecipitation, immunoblot and kinase assay were essentially carried out as described (Buschbeck et al, 2002). For the analysis of substrate phosphorylation, 3 μg of GST–MEF2C was included in kinase reactions.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v6/n1/extref/7400316s1.pdf).
Supplementary Material
Supplementary Data
Acknowledgments
We thank Dr I. Sures for discussions and her expert assistance in preparing the manuscript. We are further indebted to Dr S. Gutkind for providing invaluable reagents. Part of this work was supported by grants from the Spanish Ministry of Science and Education (EX2004-1144) to M.B., from the Hungarian National Science Foundation (OTKA 32415) to G.K. and from the Plan Nacional de I+D+I (BFU2004-03862/BMC) from the Ministry of Science and Education to L.D.C.
References
- Ballif BA, Blenis J (2001) Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ 12: 397–408 [PubMed] [Google Scholar]
- Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D (1986) The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science 233: 212–214 [DOI] [PubMed] [Google Scholar]
- Brummelkamp T, Bernards R, Agami R (2002) Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2: 243–247 [DOI] [PubMed] [Google Scholar]
- Buschbeck M, Eickhoff J, Sommer MN, Ullrich A (2002) Phosphotyrosine-specific phosphatase PTP-SL regulates the ERK5 signalling pathway. J Biol Chem 277: 29503–29509 [DOI] [PubMed] [Google Scholar]
- Cavanaugh JE (2004) Role of extracellular signal regulated kinase 5 in neuronal survival. Eur J Biochem 271: 2056–2059 [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410: 37–40 [DOI] [PubMed] [Google Scholar]
- Daley GQ, Van Etten RA, Baltimore D (1990) Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247: 824–830 [DOI] [PubMed] [Google Scholar]
- Dong F, Gutkind JS, Larner AC (2001) Granulocyte colony-stimulating factor induces ERK5 activation, which is differentially regulated by protein-tyrosine kinases and protein kinase C. Regulation of cell proliferation and survival. J Biol Chem 276: 10811–10816 [DOI] [PubMed] [Google Scholar]
- Druker BJ (2002) Inhibition of the Bcr–Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene 21: 8541–8546 [DOI] [PubMed] [Google Scholar]
- Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Lanfrancone L, Peschle C, Nolan GP, Pelicci PG (1998) High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res 58: 14–19 [PubMed] [Google Scholar]
- Huettner CS, Zhang P, Van Etten RA, Tenen DG (2000) Reversibility of acute B-cell leukaemia induced by BCR–ABL1. Nat Genet 24: 57–60 [DOI] [PubMed] [Google Scholar]
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD (1998) Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395: 713–716 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T (2002) The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell 9: 945–956 [DOI] [PubMed] [Google Scholar]
- Mody N, Leitch J, Armstrong C, Dixon J, Cohen P (2001) Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett 502: 21–24 [DOI] [PubMed] [Google Scholar]
- O'Dwyer M (2002) Multifaceted approach to the treatment of bcr–abl-positive leukemias. Oncologist 7: 30–38 [DOI] [PubMed] [Google Scholar]
- Shalizi A, Lehtinen M, Gaudilliere B, Donovan N, Han J, Konishi Y, Bonni A (2003) Characterization of a neurotrophin signaling mechanism that mediates neuron survival in a temporally specific pattern. J Neurosci 23: 7326–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA (2001) Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci 4: 981–988 [DOI] [PubMed] [Google Scholar]
- Weldon CB et al. (2002) Identification of mitogen-activated protein kinase kinase as a chemoresistant pathway in MCF-7 cells by using gene expression microarray. Surgery 132: 293–301 [DOI] [PubMed] [Google Scholar]
- Yu C, Krystal G, Varticovksi L, McKinstry R, Rahmani M, Dent P, Grant S (2002) Pharmacologic mitogen-activated protein/extracellular signal-regulated kinase kinase/mitogen-activated protein kinase inhibitors interact synergistically with STI571 to induce apoptosis in Bcr/Abl-expressing human leukemia cells. Cancer Res 62: 188–199 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data