Summary
The role of postsynaptic scaffold proteins in motor-protein–receptor complexes
Keywords: motor protein, neurotransmitter receptor, postsynaptic density, synapse, transport
Abstract
Synapse-associated proteins that are located at the postsynaptic density (PSD) have recently been shown to have a structural role at non-synaptic locations. Here, they act as adaptor proteins between neurotransmitter receptors and the microtubule- or microfilament-based motor-protein complexes that are responsible for transport to the PSD. The use of a common set of proteins that contain multiple domains for protein–protein interactions as both intracellular transport adaptors and synaptic scaffold proteins might contribute to the transport specificity and postsynaptic integration of receptors that underlie synapse formation and plasticity.
Introduction
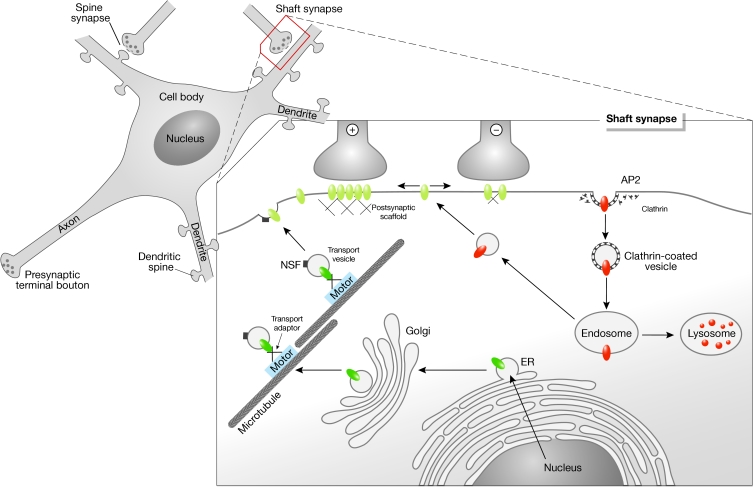
Neurons are highly polarized cells that receive, process and transmit information. The neuronal plasma membrane is heterogeneous and contains functional specializations such as presynaptic terminal boutons, which release neurotransmitters, and postsynaptic densities (PSDs), which contain neurotransmitter receptors and associated proteins. Most excitatory glutamate receptors (such as N-methyl D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors (AMPARs) and kainate receptors) are located at dendritic spines, whereas inhibitory glycine receptors (GlyRs) and γ-aminobutyric acid type A receptors (GABAARs) are mainly located at the shaft of dendrites (Fig 1). Both types of receptor are transported from the trans-Golgi network (TGN) to the cell surface by motor protein complexes. Receptors that reach a synaptic contact interact with submembrane components of the PSD, and thus become immobilized and participate in neuronal transmission and signal transduction. The structure and function of these protein scaffolds at PSDs are the subject of intense research (Kneussel & Betz, 2000; Sheng, 2001). However, individual components of PSDs, which mediate specific functions at postsynaptic sites, have also been shown to bind intracellular motor-protein complexes (Table 1). Consequently, they function as linker molecules between molecular motors and neurotransmitter receptors during the processes of cargo recruitment towards and/or from the postsynaptic specialization. Proteins that participate in scaffold reactions at the PSD usually contain multiple domains for protein–protein interactions, which might enable motors to interact with a large number of synaptic passengers. Understanding how a limited number of motors carries a relatively high number of synaptic components to their sites of action, and to what extent the same molecules participate in intracellular transport and synaptic scaffold reactions might help us to understand fundamental operational principles of synapse formation.
Figure 1.
Dynamic control of neurotransmitter receptor expression at the cell surface. Receptor-containing secretory vesicles are released from the trans-Golgi network and recruited by motor-protein-dependent transport complexes (shown in green). On expression at the cell surface, receptors are thought to diffuse laterally in the plasma membrane (depicted in yellow). Interactions with postsynaptic scaffold proteins immobilize receptors at sites of axo-dendritic contact and contribute to the number of receptors that are available for synaptic transmission (+ and −, respectively). Extrasynaptic receptors exchange with intracellular recycling pools (shown in orange). AP2, adaptor protein 2; ER, endoplasmic reticulum; NSF, N-ethylmaleimide-sensitive factor.
Table 1.
Known and putative transport complexes of neurotransmitter receptors and associated proteins
| Motor | Adaptor | Cargo | References |
|---|---|---|---|
| KIF17 | Lin2 (CASK), Lin7 (MALS/Velis), Lin10 (Mint/X11) | NMDAR2B | Setou et al, 2000 |
| KIF5A/B/C | GRIP1 | AMPAR GluR2 | Setou et al, 2002 |
| KIF1Bα | PSD-95(?), PSD-93(?), chapsyn 110(?), SAP-97(?), S-SCAM(?) | PSD-95(?), PSD-93(?), chapsyn 110(?), SAP-97(?), S-SCAM(?) | Mok et al, 2002 |
| Dynein | Gephyrin(?) | Gephyrin(?), GlyR(?) | Fuhrmann et al, 2002 Hanus et al, 2004 |
| Myosin V | GKAP(?), PSD-95(?) | GKAP(?), PSD-95(?) | Naisbitt et al, 2000 |
| Myosin VI | SAP-97 | AMPAR GluR1 | Wu et al, 2002 |
Motor-protein-dependent transport in neurons
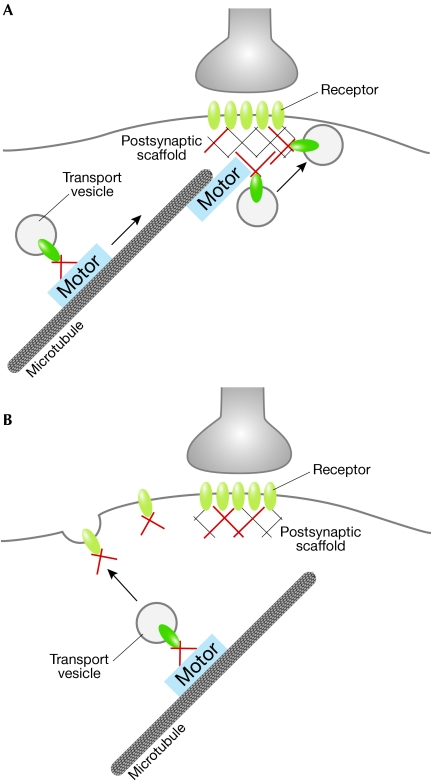
Neurons are polar cells with typically a single axon and many dendrites (Fig 1). Axons and dendrites carry out different functions: the dendritic surface receives and processes information from other neurons and carries nerve impulses to the cell body, whereas the axonal surface is specialized for the rapid transmission of electrical impulses towards the presynaptic terminal bouton that releases the neurotransmitter. In a primary sorting step, proteins of the presynaptic active zone and the PSD are directed to the respective compartment, which is either the axon or a dendrite. Within neuronal dendrites, neurotransmitter receptors must undergo another targeting step, which directs glutamate receptors, for instance, towards an excitatory spine synapse and GlyRs and GABAARs towards an inhibitory shaft synapse (Fig 1). Such delivery of neurotransmitter receptors to distinct postsynaptic specializations could be achieved by two possible mechanisms: selective and direct targeting towards the appropriate synapse (Fig 2A) or indirect targeting to the cell surface membrane combined with the selective retention of mobile receptors at synaptic sites (Fig 2B). In the latter context, it is unclear whether the surface membrane entry sites of receptors are random or specific. However, results that favour the second model for individual receptors include the observations that AMPARs and GlyRs display lateral mobility in the plane of the plasma membrane (Choquet & Triller, 2003). Moreover, connections that spontaneously form between neurons in isolated micro-island cultures contain mismatched appositions of pre- and postsynaptic components; for instance, GABAAR clusters localize inappropriately opposite non-GABAergic terminals (Rao et al, 2000), which suggests that selectivity mechanisms exist downstream of plasma membrane entry in vivo, but are lacking in this electrophysiologically artificial system.
Figure 2.
Proteins with dual functions in motor-protein-dependent transport and at postsynaptic densities (shown in red) contribute to the post-Golgi surface membrane delivery of neurotransmitter receptors. (A,B) Two possible transport mechanisms are shown. The available data favour the mechanism shown in (B), which suggests that the surface membrane entry of receptors occurs at extrasynaptic locations. It is unknown whether the interactions of the proteins (red) with receptors persist during the process of surface delivery and lateral diffusion into postsynaptic sites.
To reach the cell surface and postsynaptic membrane, excitatory NMDARs and AMPARs have been shown to depend on active, microtubule-dependent, transport mechanisms (Setou et al, 2000, 2002). In general, several mechanisms could act in combination to achieve postsynaptic delivery of individual neurotransmitter receptors towards either an excitatory or inhibitory synapse, located on spines or shafts, respectively: receptors might be sorted to different post-Golgi carriers at the level of the TGN; transport units might be directed to different subdomains within the dendrite; the fusion between individual transport vesicles and the plasma membrane might be selectively regulated, such as by the nature of membrane lipids; receptors might enter the surface membrane at non-synaptic but distinct positions and then subsequently reach the synapse through lateral diffusion or active intra-membrane transport; diffusion barriers within the plasma membrane might hinder individual receptors to enter or leave specific compartments; and finally, mechanisms that differentially regulate receptor endocytosis and receptor turnover might contribute to receptor densities at a given time and location.
In terms of motor-protein-dependent transport of components towards postsynaptic specializations, it is also important to consider that the number of cargo molecules to be transported within a given neuron is much higher than the number of motors available for the recruitment process. Therefore, adaptor proteins have been postulated and subsequently identified (Setou et al, 2000, 2002) that not only regulate binding affinities but also mediate transport specificity and cargo identity. The latter might be achieved through the combinatorial use of several polypeptides within the transport complex, many of which have different protein–protein interaction domains.
Microtubules: tracks for polarized transport
The nature of the tracks along which motors move is thought to be a crucial factor in dendritic transport, especially as cytoskeletal polymers display polarity and associate with several other proteins. Microtubules generally have a radial organization in many cell types, with their plus-ends typically orientated to the cell periphery and their minus-ends anchored in a microtubule-organizing centre (MTOC). With respect to neurons, this uniformity of microtubule polarity is found in axons but not in dendrites: axonal microtubules are directed with their plus-ends towards the growth cone, whereas dendritic microtubules show a mixed orientation. In proximal dendritic regions, about 75 μm from the cell body, roughly equal proportions of microtubules are orientated with their plus-ends directed towards both the growth cone and the cell body. However, in distal dendritic regions, within about 15 μm of the growth cone, microtubule polarity orientation is similar to that in axons (Baas et al, 1988). Microtubules often do not reach the cellular cortex at the cytoplasmic face of the plasma membrane; instead this region is rich in actin filaments, which are thought to represent the tracks for the final stages of delivery of many surface molecules. This morphological characteristic is also found in dendritic spines, which contain actin filaments but lack or contain few microtubules.
A recent study has suggested that microtubules themselves might provide directional information for polarized transport, as the kinesin-family motor KIF5 has a preference for microtubules in the initial segment of the axon and KIF5-driven post-Golgi axonal carriers move directly from the TGN towards the axonal compartment (Nakata & Hirokawa, 2003). According to this model, not only the polarity of the track, but also the microtubule-associated proteins (MAPs) could provide a cue for transport. Alternatively, regulation at the level of the interaction between the microtubule track and the individual motor head might account for such preferences. Regulatory mechanisms could in this context include phosphorylation, acetylation or methylation of target proteins within the system. It is therefore important that future research investigates to what extent directionality of polarized transport is encoded by the microtubule, the MAPs, the structure of the motor head, the different regulatory states of the respective components or by a combination of these different factors.
Motor–cargo interactions in excitatory synapse remodelling
All motors are enzymes that convert the chemical energy that is stored in ATP into molecular motion, thereby producing a force on the associated cytoskeletal polymer. The main binding characteristic of molecular motor complexes is their simultaneous affinity for cytoskeletal polymers and cargo elements. Typically, the ATPase function is mediated by the heavy chain of the respective motor protein complex, whereas accessory intermediate and light chains are specialized for self-assembly or interaction with molecular cargo. However, individual heavy chains have also been shown to interact directly with cargo molecules. Three families of motor-protein complexes—kinesin, dynein and myosin—are known, each of which consists of many domains or accessory subunits. Kinesins and dyneins use microtubules as transport tracks, whereas myosins represent actin-based motors that move along microfilaments.
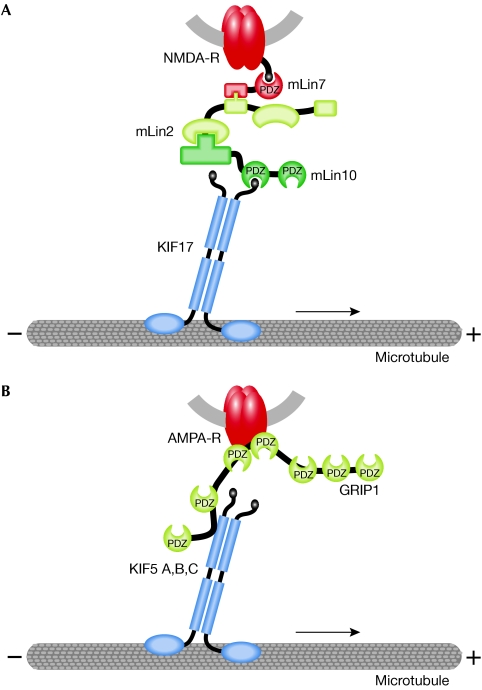
For the anterograde recruitment of glutamate receptors of both the NMDA- and AMPA-type, kinesin-family motors have been identified as the driving force of a cargo complex that consists of an individual receptor subunit, one or more adaptor proteins and the motor-protein components (Setou et al, 2000, 2002; Fig 3). For both receptor types, the same molecules that link the receptor complex to the motor complex have previously been shown to locate to and mediate specific functions within the PSD. The NMDAR NR2B subunit interacts with the kinesin-family motor Kif17 through a protein complex that consists of mouse Lin7 (also known as MALS/Velis), Lin2 (CASK) and Lin10 (Mint1/X11; Fig 3A). The microtubule-associated motor binds to the cytoplasmic Lin10 through a PDZ-domain-mediated interaction. The vesicular NR2B subunit interacts through its carboxy-terminal tail with cytoplasmic Lin7. To connect the cargo vesicle with its transport track, Lin2 then functions as a linker between the Lin10-motor complex and the Lin7-receptor-vesicle complex, thereby generating a large complex that transports NR2B-containing NMDARs towards postsynaptic sites (Setou et al, 2000). Notably, the protein Lin2, which represents a membrane-associated guanylate kinase (MAGUK), not only functions as a transport adaptor at intracellular transport complexes but also represents a component of the PSD. Transgenic overexpression of the motor protein Kif17 enhances NR2B-mediated spatial and working memory in mice, which confirms the physiological relevance of this form of microtubule-based NMDAR transport (Wong et al, 2002).
Figure 3.
Kinesin-mediated transport of NMDA receptors (A) and AMPA receptors (B). Schematic representation of microtubule-dependent transport complexes that consist of secretory vesicles and associated proteins. These contain receptor (shown in red) and adaptor proteins, which link receptors to kinesin motors (in blue) through PDZ-domain-mediated interactions. Note that proteins depicted in yellow represent polypeptides with dual functions at both the intracellular transport pathway and the postsynaptic membrane specialization. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; GRIP1, glutamate-receptor-interacting protein 1; KIF, kinesin family; NMDA, N-methyl D-aspartate; PDZ, postsynaptic density/discs large/zonula adherens.
With respect to the use of PSD components as transport adaptors, a similar observation has been made for an AMPAR transport complex. Here, the kinesin-family motors KIF5A, B and C bind to the AMPAR GluR2 subunit through glutamate-receptor-interacting protein 1 (GRIP1)-mediated interactions (Setou et al, 2002; Fig 3B). The expression patterns of KIF5 family members in different neuronal cell types (Kanai et al, 2000) are consistent with the distribution of the GRIP1 protein (Dong et al, 1999) and AMPAR GluR2 subunits in brain and spinal cord (Jakowec et al, 1995; Petralia & Wenthold, 1992). GRIP1 also represents a component of the postsynaptic scaffold, where it binds to synaptically localized AMPARs (Sheng, 2001). In the context of KIF5-mediated transport, not only does GRIP1 form a bridge between the motor and its cargo receptor, but also the minimal kinesin-binding domain of GRIP1 delocalized kinesin predominantly to the somatodendritic compartment and not significantly to axons. Thus, GRIP1 steers the KIF5 family heavy chains to dendritic compartments, despite the fact that these motors also mediate axonal transport reactions. These studies indicate that binding proteins can determine the direction of transport of a motor protein (Setou et al, 2002).
It is not clear whether the observed interactions represent the general anterograde microtubule motors for glutamate receptor transport or whether these motor–cargo interactions account for the specific delivery of certain receptor subtypes. Neurotransmitter receptors are known to be expressed and assembled in a spatio-temporal-dependent manner in the mammalian central nervous system. During development for instance, there is a shift in synthesis from NR2B-containing to NR2A-containing NMDARs. Moreover, NMDARs locate to and mediate specific functions at both synaptic and extrasynaptic locations (Tovar & Westbrook, 1999). Therefore, individual receptor subtypes could either use different molecular motors for postsynaptic delivery or, alternatively, use the same motors but different sets of adaptor and/or accessory proteins. This could account for the differing subunit composition or for transport towards a specific surface membrane microdomain. A candidate set of proteins for the regulated delivery of individual subunit compositions is known as the transmembrane AMPAR regulatory proteins (TARPs). The TARP stargazin/γ2 interacts with AMPARs in the dendritic cytoplasm before reaching the PSD (Greger et al, 2002; Tomita et al, 2003) and individual AMPAR complexes contain only one TARP isoform, which suggests that TARP–AMPAR complexes are strictly segregated (Tomita et al, 2003). Whether TARPs are components of KIF5–GRIP1–AMPAR complexes remains to be determined. However, although TARPs bind both AMPARs and the postsynaptic scaffold protein PSD-95, they precisely colocalize with AMPARs and are absent from excitatory PSD-95-positive synapses that lack AMPARs (Tomita et al, 2003). It is therefore likely that TARPs are fundamental to the control of AMPAR localization.
Further evidence for the view that different motors participate in the delivery of excitatory postsynaptic components has been provided by a yeast two-hybrid screen that used the C-terminal PDZ-domain-binding motif of the kinesin-family motor protein KIF1Bα, as bait. This screen identified several PSD components as direct KIF1Bα interactors, including PSD-95, PSD-93, chapsyn 110, synapse-associated protein 97 (SAP97) and the synaptic scaffolding molecule (S-SCAM; Mok et al, 2002). In addition, PSD-95 has been shown to form part of a protein complex containing guanylate-kinase-associated protein (GKAP), dynein light chain (Dlc) and the actin-based motor protein myosin V (Naisbitt et al, 2000). Although it remains unclear whether these interactions (Table 1) represent cargo, cargo adaptors or both, it is an appealing hypothesis that these factors, many of which contain several domains for protein–protein interactions, indicate the existence of larger transport complexes that include transmembrane components of the postsynaptic specialization.
With respect to the endocytosis of AMPARs, the actin-based motor myosin VI might be involved. Myosin VI has been implicated in endocytic mechanisms in different cell types (Hasson, 2003) and interacts with the postsynaptic scaffold protein SAP97 and the AMPAR GluR1 subunit (Wu et al, 2002; Table 1). Although there is as yet no evidence that these three binding partners colocalize at postsynaptic sites, the respective trimeric complex co-immunoprecipitates in light membrane fractions prepared from brain tissue (Wu et al, 2002). Moreover, AMPAR GluR2 subunits contain overlapping binding sites for the ATPase N-ethylmaleimide-sensitive factor (NSF) and the clathrin-binding adaptor protein 2 (AP2). NSF function is required to maintain AMPARs at postsynaptic sites, whereas AP2–clathrin interactions are involved in receptor internalization (Lee et al, 2002). Whether a molecular motor system contributes to the recruitment of AMPARs that are bound to NSF or AP2 is not known; however, both components are involved in transport and PSD-mediated reactions (Husi et al, 2000; Peng et al, 2004). Remarkably, as considered for the plasma membrane entry of receptors, the endocytosis of AMPARs through clathrin-coated pits also mainly occurs at extrasynaptic sites (Ashby et al, 2004; Petralia et al, 2003; Fig. 1), which suggests that surface delivery and removal might be an extrasynaptic phenomenon.
Motor–cargo interactions in inhibitory synapse remodelling
The intracellular transport of GlyRs and GABAARs to and from inhibitory postsynaptic sites is barely understood. However, the postsynaptic scaffold component gephyrin, which is essential for the clustering of GlyRs and individual GABAARs at synaptic sites, might also function as a motor–cargo adaptor at intracellular locations. Evidence that gephyrin binds to components of motor protein complexes has been obtained from a screen that identified Dlc1 and Dlc2 as direct binding partners of the gephyrin molecule (Fuhrmann et al, 2002). As Dlc proteins are components of both the microtubule-based dynein motor complex and the microfilament-based myosin Va motor complex, this interaction could lead to different transport pathways. In neurons derived from gephyrin-deficient mice, the expression of a gephyrin deletion mutant that can no longer interact with Dlc1 and -2 does not alter the synaptic localization of gephyrin. It is therefore likely that gephyrin–Dlc interactions contribute to retrograde neuronal transport, which is consistent with the dynein motor moving towards the minus-end of microtubules.
In a recent study, Hanus and coworkers analysed heterologously expressed GlyRs and gephyrin at intracellular locations of fibroblast cells (Hanus et al, 2004). A chimeric GlyR-α receptor subunit, which contains the gephyrin-binding motif of the GlyR-β subunit, localized gephyrin to intracellular structures. The movement of these putative GlyR–gephyrin aggregates was affected in the presence of nocodazole, a microtubule-depolymerizing drug, which suggests that recruitment of the colocalized structures is microtubule-dependent. Moreover, the presence of the gephyrin-binding motif in GlyR-α accelerated the accumulation of GlyR at the cell surface. Although this study was not performed in neuronal cells, it suggests that some GlyRs associate with gephyrin during their transport to the cell surface. Consistent with this model, gephyrin transport complexes are recruited within neuronal dendrites over time and enter or leave putative inhibitory postsynaptic scaffolds (M.K., unpublished data). Clearly, a transport complex remains to be identified that connects the vesicular GlyR with an anterograde dendritic motor for plasma membrane delivery. However, these observations suggest that the scaffold protein gephyrin also contributes to transport reactions at intracellular sites (Table 1). This view is further supported by the observation that gephyrin interacts with the GABAAR-associated protein (GABARAP; Kneussel et al, 2000), which is a putative post-Golgi transport factor that in turn binds to γ2-subunit-containing GABAAR transport complexes (Wang et al, 1999) and NSF (Kittler et al, 2001).
Common principles and future directions
Each motor-protein–cargo interaction encodes the identity of a certain transport complex and thereby contributes to the regulated and specific delivery of cargo to its cellular destination. It is becoming increasingly clear that molecular motors associate with their cargo through intermediate components, which include adaptor, scaffold and transmembrane proteins, as well as GTPases and other motors (Klopfenstein et al, 2000). With respect to the transport of neurotransmitter receptors in neurons, additional questions arise: what are the reasons and consequences for the use of certain proteins in both transport and membrane scaffold reactions? At which subcellular location do these interactions initially occur? Do the same interactions that regulate, for instance, NMDAR (Lin2/CASK) or AMPAR (GRIP1) transport, persist at postsynaptic sites and could the maintenance of such interactions beyond transport represent a general principle in synaptogenesis?
At present, it is not clear at which membrane positions neurotransmitter receptors enter the plasma membrane (Fig 2). Receptors could be incorporated into the plane of the plasma membrane at any position of the cell and final transport reactions could exclusively depend on lateral movements either through diffusion and/or in combination with actin-based motor systems (Fig 2B). A mode of active intramembrane transport is known for the endocytic receptor megalin in membranes of epithelial cells (Christensen & Birn, 2002). Alternatively, receptors could enter the surface membrane at distinct extrasynaptic sites, possibly encoded by microenvironments with defined lipid compositions, and reach the postsynaptic specialization as preformed extrasynaptic clusters. This mode of synaptic development has been reported for NMDAR clusters in cultured neurons (Rao et al, 1998). Another possible mechanism could position the transport complex near the PSD with direct incorporation of individual receptors into existing synapses (Fig 2A); however, a study performed in cultured neurons that contradicts this view for certain types of NMDAR has been reported (Guillaud et al, 2003). Here, NR2B subunit clusters either colocalize with the motor KIF17 or the pre- and postsynaptic markers synaptophysin and PSD-95, respectively. By contrast, no colocalization was observed between KIF17 and synaptic markers, which suggests that the motor complex does not reach the vicinity of the PSD.
Whether the surface expression of receptors depends on a single aforementioned mechanism or on a combination of different modes remains to be determined. For individual receptor subunits and associated proteins it is known that clusters appear at extrasynaptic locations before synaptogenesis (Meier et al, 2000; Rao et al, 1998). It is therefore important to understand whether receptor-binding proteins, some of which carry out a dual role in both transport and synaptic scaffolding reactions, contribute to surface delivery and lateral mobility of receptors. Polypeptides with such dual function might interact with receptors not only before, but also during their surface membrane entry; therefore, these interactions would persist until both binding partners reach the synapse. The incorporation of new receptors and their associated binding partners into extrasynaptic or synaptic sites might promote subsequent receptor clustering.
In summary, additional transport complexes need to be identified and characterized, particularly to understand whether specific subunits of the same neurotransmitter receptor type travel as passengers of distinct or identical motors. These questions require the combination of biochemical and imaging approaches and will highly benefit from loss-of-function experiments, including genetic ablation in mice.

Acknowledgments
M.K. is supported by the University of Hamburg and grants from the Deutsche Forschungsgemeinschaft (SFB 444/B7, Kn 556/1-1, Kn 556/1-2).
References
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM (2004) Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci 24: 5172–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA 85: 8335–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Triller A (2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4: 251–265 [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H (2002) Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266 [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang P, Liao D, Huganir RL (1999) Characterization, expression, and distribution of GRIP protein. Ann NY Acad Sci 868: 535–540 [DOI] [PubMed] [Google Scholar]
- Fuhrmann JC, Kins S, Rostaing P, El Far O, Kirsch J, Sheng M, Triller A, Betz H, Kneussel M (2002) Gephyrin interacts with dynein light chains 1 and 2, components of motor protein complexes. J Neurosci 22: 5393–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB (2002) RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 34: 759–772 [DOI] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N (2003) KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci 23: 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Vannier C, Triller A (2004) Intracellular association of glycine receptor with gephyrin increases its plasma membrane accumulation rate. J Neurosci 24: 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T (2003) Myosin VI: two distinct roles in endocytosis. J Cell Sci 116: 3453–3461 [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor–adhesion protein signaling complexes. Nat Neurosci 3: 661–669 [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Yen L, Kalb RG (1995) In situ hybridization analysis of AMPA receptor subunit gene expression in the developing rat spinal cord. Neuroscience 67: 909–920 [DOI] [PubMed] [Google Scholar]
- Kanai Y, Okada Y, Tanaka Y, Harada A, Terada S, Hirokawa N (2000) KIF5C, a novel neuronal kinesin enriched in motor neurons. J Neurosci 20: 6374–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ (2001) The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci 18: 13–25 [DOI] [PubMed] [Google Scholar]
- Klopfenstein DR, Vale RD, Rogers SL (2000) Motor protein receptors: moonlighting on other jobs. Cell 103: 537–540 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H (2000) Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci 23: 429–435 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wassle H, Olsen RW, Betz H (2000) The γ-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci USA 97: 8594–8599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M (2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36: 661–674 [DOI] [PubMed] [Google Scholar]
- Meier J, Meunier-Durmort C, Forest C, Triller A, Vannier C (2000) Formation of glycine receptor clusters and their accumulation at synapses. J Cell Sci 113: 2783–2795 [DOI] [PubMed] [Google Scholar]
- Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E (2002) Association of the kinesin superfamily motor protein KIF1Bα with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci 22: 5253–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M (2000) Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci 20: 4524–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N (2003) Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol 162: 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M (2004) Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem 279: 21003–21011 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ (1992) Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol 318: 329–354 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ (2003) Internalization at glutamatergic synapses during development. Eur J Neurosci 18: 3207–3217 [DOI] [PubMed] [Google Scholar]
- Rao A, Kim E, Sheng M, Craig AM (1998) Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci 18: 1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Cha EM, Craig AM (2000) Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci 20: 8344–8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N (2000) Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288: 1796–1802 [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N (2002) Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417: 83–87 [DOI] [PubMed] [Google Scholar]
- Sheng M (2001) Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA 98: 7058–7061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS (2003) Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol 161: 805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL (1999) The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci 19: 4180–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW (1999) GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 397: 69–72 [DOI] [PubMed] [Google Scholar]
- Wong RW, Setou M, Teng J, Takei Y, Hirokawa N (2002) Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci USA 99: 14500–14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC (2002) Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem 277: 30928–30934 [DOI] [PubMed] [Google Scholar]