Abstract
The gasoline oxygenate methyl tert-butyl ether (MTBE) has become a widespread contaminant in groundwater throughout the United States. Bioaugmentation of aquifers with MTBE-degrading cultures may be necessary to enhance degradation of the oxygenate in some locations. However, poor cell transport has sometimes limited bioaugmentation efforts in the past. The objective of this study was to evaluate the transport characteristics of Hydrogenophaga flava ENV735, a pure culture capable of growth on MTBE, and to improve movement of the strain through aquifer solids. The wild-type culture moved only a few centimeters in columns of aquifer sediment. An adhesion-deficient variant (H. flava ENV735:24) of the wild-type strain that moved more readily through sediments was obtained by sequential passage of cells through columns of sterile sediment. Hydrophobic and electrostatic interaction chromatography revealed that the wild-type strain is much more hydrophobic than the adhesion-deficient variant. Electrophoretic mobility assays and transmission electron microscopy showed that the wild-type bacterium contains two distinct subpopulations, whereas the adhesion-deficient strain has only a single, homogeneous population. Both the wild-type strain and adhesion-deficient variant degraded MTBE, and both were identified by 16S rRNA analysis as pure cultures of H. flava. The effectiveness of surfactants for enhancing transport of the wild-type strain was also evaluated. Many of the surfactants tested were toxic to ENV735; however, one nonionic surfactant, Tween 20, enhanced cell transport in sand columns. Improving microbial transport may lead to a more effective bioaugmentation strategy for MTBE-contaminated sites where indigenous oxygenate degraders are absent.
The Clean Air Act Amendments of 1990 require that gasoline oxygenates, such as ethanol, ethyl tert-butyl ether, di-isopropyl ether, tert-amyl methyl ether, and methyl tert-butyl ether (MTBE), be used in fuels sold in some urban areas in the winter to reduce local emissions of carbon monoxide (37, 56). Gasoline reformulation with oxygenates is also required in regions with elevated ozone levels to reduce emissions of ozone and its precursory compounds. MTBE is the primary oxygenate used in both oxygenated and reformulated fuels, accounting for up to 15% by volume of these gasoline products (35), and is one of the most recalcitrant compounds in gasoline (43). The widespread use of MTBE in gasoline, most of which is stored in underground storage tanks, has resulted in the discharge of the oxygenate into soils and groundwater. More than 385,000 releases of gasoline from leaking underground fuel tanks have been confirmed by regulatory agencies as of December 1999, and it has been estimated that 250,000 of these spills involve MTBE (35). A recent report has suggested that, nationwide, between 5 and 10% of drinking water supply wells in areas where MTBE is used are contaminated with the oxygenate, with at least 1% of these wells containing more than 20 μg of MTBE/liter (4).
The biological degradation of MTBE has received increased attention during the past several years as its ubiquity as a groundwater contaminant has become apparent (17, 20, 22, 25, 26, 29, 33, 42, 47, 57). Recent data suggest that indigenous bacteria capable of aerobically degrading MTBE are present in some aquifers (10, 38, 49, 64). The injection of oxygen to these sites is often sufficient to promote MTBE biodegradation. However, at other locations, MTBE-degrading bacteria appear not to occur naturally or to occur at very low numbers, and the application of oxygen or other amendments at these sites does not appreciably enhance destruction of the oxygenate (36, 48, 51). The addition of exogenous cultures may be required for successful bioremediation of MTBE in these environments. This process, termed bioaugmentation, has been used with success to treat groundwater contaminated with various chlorinated solvents in situ (15, 58) and has recently been applied in field demonstrations for MTBE remediation at Port Hueneme Naval Station in California (31, 49).
One serious limitation to the widespread application of bioaugmentation for contaminant remediation is the inability to effectively transport and distribute inoculated bacteria throughout a contaminant plume. Bacterial cells often sorb strongly to solids and thus tend to concentrate near the point of injection, sometimes clogging injection wells (7, 28, 40, 53). For example, Harvey et al. (28) reported that more than 99% of bacteria injected into a sand and gravel aquifer in Cape Cod, Mass., in a field test did not move to a sampling well located 1.7 m from the injection site. Techniques used to improve microbial transport and distribution in aquifer sediments include the application of surfactants (6, 24, 40) and surfactant foams (34, 46), starvation of microorganisms to reduce cell size (39), and the development of adhesion-deficient variants with altered cell surface characteristics (12, 14).
A previous study reported the isolation and characterization of a pure culture (Hydrogenophaga flava ENV735) that is capable of completely mineralizing MTBE to CO2 (29, 59). Initial studies in our laboratory revealed that this organism does not move well through sediments. Therefore, experiments were undertaken to improve the transport of this bacterium. An adhesion-deficient variant of ENV735 with transport characteristics superior to those of the wild-type bacterium was isolated, and improved transport of this selected strain was confirmed in aquifer materials. Several characteristics of this variant, H. flava ENV735:24, including cell hydrophobicity, electrostatic interaction, electrophoretic mobility, cell size, and flagellation, were evaluated and compared with those of the wild-type strain to determine the reason for the altered adhesion. MTBE degradation by the adhesion-deficient variant and the wild-type strain were also evaluated. In addition, surfactants were tested for their ability to enhance the movement of the wild-type strain and for their influence on the degradation of MTBE by this organism.
MATERIALS AND METHODS
Chemicals.
MTBE, sodium pyrophosphate, Igepal CO-720, sodium dodecyl sulfate, Tween 80, and Brij 35 were purchased from Aldrich Chemical Co. (Milwaukee, Wis.). R2A agar was obtained from Becton Dickinson (Sparks, Md.). Octyl Sepharose CL-4B, Dowex 1X8-200, Dowex 50WX8-200, d-[U-14C]glucose, yeast extract, streptomycin, and Tween 20 were purchased from Sigma Chemical Co. (St. Louis, Mo.). Sodium bromide was obtained from Fisher Scientific (Fair Lawn, N.J.). Tergitol 15-S-12 was purchased from Union Carbide (Danbury, Conn.), and Steol CS-330 was obtained from Stepan Co. (Northfield, Ill). The biosurfactant JBR-425 was obtained from Jeneil Biosurfactant Co. (Saukville, Wis.).
Medium and culture conditions.
ENV735 and the adhesion-deficient variant ENV735:24 were grown in 125-ml shake flasks containing 50 ml of basal salts medium (BSM) (27) supplemented with 0.1% yeast extract. Flasks were shaken at room temperature (22 to 25°C) until cells reached the mid-logarithmic phase of growth. The cells were harvested by centrifugation for 15 min at 5,000 × g in a Beckman GS-6R centrifuge and then washed in BSM, Narrow Channel Artificial Groundwater (NCAGW; see below), or natural groundwater from a site in Dover, Del., prior to the experiments.
Wild-type strain and isolation of an adhesion-deficient variant.
The characteristics and isolation of the MTBE-degrading bacterium H. flava ENV735 have been reported previously (29, 59). Spontaneous adhesion-deficient variants of strain ENV735 were selected by passage of the wild-type strain through columns of sterile aquifer sediments from a site in Oyster, Va., or through sterile Ottawa sand by using the protocol of DeFlaun et al. (12). The characteristics of the Oyster sediments and Ottawa sand have been described elsewhere (9, 13). The columns were prepared by packing 12 g of autoclaved sediment or sand in sterile 20-ml syringe barrels. The length of each sediment column was 2.7 cm, and the width was 2.0 cm. Wild-type ENV735 was grown as described above and resuspended in artificial groundwater prepared to simulate the natural groundwater in Oyster, Va. (21). The groundwater, designated NCAGW, has a pH of 6.0 and contains the following constituents per liter of distilled water: MgSO4 · 7H2O, 60 mg; KNO3, 10 mg; NaHCO3, 60 mg; CaCl2 · 2H2O, 29 mg; Ca(NO3)2 · 4H2O, 70 mg; CaSO4 · 2H2O, 25 mg; and NaH2PO4, 0.4 mg.
A 3.5-ml volume of suspended cells was added to the top of each column to saturate the sediment or sand. After a 5-min equilibration period, excess liquid was allowed to drain from the columns. Following a 1-h incubation period, the columns were washed with 3.5 ml of sterile NCAGW. The effluent water passing through the columns was then collected in sterile test tubes. An aliquot of the effluent water, containing any cells that passed through the sand or sediment, was diluted and plated onto R2A agar. The number of cells passing through the columns in each successive trial was determined and compared with that in the initial inoculum to calculate the percentage of adherent cells. The remainder of the column effluent was then inoculated into fresh liquid medium (BSM supplemented with 0.1% yeast extract), and the medium was shaken at room temperature until logarithmic-phase growth of ENV735 was observed. The cells were then centrifuged, resuspended in NCAGW, and added to the surface of sterile columns of sand or sediment in duplicate, as described previously. The passage of cells through the columns was repeated in succession until a considerable decrease in the percentage of cells adhering to the sand or sediment was observed. Throughout the process, the purity of the cultures was continually checked on spread plates, and the ability of cells passing through the columns to degrade MTBE was tested (see below).
MTBE analysis and degradation assays.
Degradation of MTBE by ENV735 and ENV735:24 was measured in serum vials by gas chromatography (GC) (29, 57). Initially, 10 ml of a freshly grown culture was added to a 160-ml serum vial. The vial was then sealed with a Teflon-lined septum and amended with a solution of MTBE in distilled water to yield a final concentration of approximately 25 mg/liter. At the beginning of the incubation period and at a second point at least 3 h later, small aliquots (less than 0.5 ml) were removed from the vial and placed into microcentrifuge tubes. The tubes were centrifuged at 16,000 × g for 0.5 min to pellet the cells, and 1-μl aliquots of the supernatant were injected into a model 3400 GC (Varian Instrument Division, Walnut Creek, Calif.) equipped with a flame ionization detector (FID) and a VOCOL fused silica capillary column (30-m length, 0.53-mm inside diameter, 3.0-μm film thickness; Supelco, Bellefonte, Pa.). The column, injector, and detector were maintained at 45, 180, and 250°C, respectively. Centrifugation did not influence MTBE levels in samples (data not shown). Alternatively, microcosm samples were analyzed by Environmental Protection Agency method 8015, which uses a purge-and-trap GC coupled with an FID. MTBE degradation was confirmed by the disappearance of MTBE as well as the appearance of tert-butyl alcohol (TBA), an early product of MTBE degradation (57). The quantities of MTBE and TBA were determined by using a 3- to 5-point standard curve, with standards ranging from 1 to 50 mg of MTBE or TBA/liter. For direct comparisons between the two strains, both cell types were grown and harvested as described above and adjusted to an optical density at 550 nm of 0.2 in BSM plus 0.1% (wt/vol) yeast extract, and degradation assays were performed as described above. In these assays, samples were taken every hour for the first 6 h and then at 24, 28, and 30 h. Assays of MTBE degradation in the presence of Tween 20 were performed with ENV735 in the same manner, except that cells were resuspended in BSM with or without 0.01% (vol/vol) Tween 20.
Microbial transport in columns.
Experiments were conducted in model aquifers consisting of two glass columns, each measuring 7.6 cm in diameter and 20 cm in length (884-cm3 total volume). Each column was fitted with two aluminum end plates, which were attached to each other with three support rods. By evenly tightening nuts on the end of each support rod, the plates were tightly sealed to the glass column, creating a leak-free system. One column was packed with Ottawa sand, a uniform washed silica quartz sand with an average diameter of 0.45 to 0.50 mm (Fisher Scientific). Sediment from a coastal plain aquifer in Oyster, Va., which contains both iron and aluminum oxides, was used in the second column for evaluation of cell transport in a natural, heterogeneous matrix.
The two glass columns were initially filled approximately halfway with sterile NCAGW. Ottawa sand or Oyster sediment was then poured slowly through the groundwater while gently stirring with a sterile rod to avoid air bubbles and ensure even packing until the columns were full. The column with Ottawa sand received 1,290 g of sand and 320 ml of NCAGW, and the column prepared with Oyster sediment received 1,250 g of solids and 285 ml of NCAGW. Once the columns were packed, the end plates were evenly tightened by hand. Each end cap contained a central port for groundwater flow through the column. The port at the bottom of each column was attached with Tygon tubing (Cole-Parmer, Vernon Hills, Ill.) to a reservoir of sterile NCAGW. The water flowed through each column in an upward direction. A peristaltic pump (Masterflex; Cole-Parmer) with variable speed control was used to pump water through both columns simultaneously. Once a steady water flow was established, the flow rate through each column was adjusted to 0.7 to 0.8 ml/min. Water was then allowed to move through each column at the adjusted flow rate for over 1 h to equilibrate the columns and remove any residual air spaces that may have occurred during packing. The column experiments were conducted in a constant-temperature room at 15°C to simulate natural conditions.
ENV735 or ENV735:24 was grown to mid-logarithmic phase, washed with NCAGW, and then brought to a density of approximately 107 cells/ml in NCAGW. The cell suspension was amended with sodium bromide as a conservative tracer (50 mg/liter as bromide) and then split into two flasks for injection into the columns. Previous studies have demonstrated that 50 mg of bromide/liter (as sodium bromide) does not affect cell viability or transport characteristics (21). The inocula were simultaneously injected into the cores until each column had received approximately one-half pore volume of cell suspension (160 and 142 ml for the sand and sediment columns, respectively). The injection lasted 3.2 h, after which the influent tubing was immediately placed in the NCAGW reservoir. The central port at the top of each column was attached to a fraction collector (Pharmacia SuperFrac; Pharmacia, Piscataway, N.J.), and fractions were collected in sterilized, preweighed glass test tubes every 20 min for the duration of the experiment. The volume of effluent in each fraction was determined based on weight.
To quantify bacterial transport through the columns, every third effluent fraction was serially diluted in phosphate-buffered saline (PBS) (50) and plated on selective medium consisting of R2A agar and streptomycin (30 mg/liter) to inhibit contaminating bacteria. ENV735 and ENV735:24 are both naturally resistant to streptomycin at this concentration. After 7 days of incubation at 30°C, the number of CFU of ENV735 or ENV735:24 present on each plate was determined. The time of breakthrough, the number of pore volumes required for breakthrough, and the number of bacteria moving through each column were then calculated. After microbial analysis was complete, the bromide concentration in each fraction was measured using a pH/Ion Analyzer 455 (Corning, Inc., Corning, N.Y.) equipped with an electrode specific for bromide (Cole-Parmer). The meter was calibrated with standards in NCAGW of 1, 5, 10, 25, and 50 mg of bromide/liter. The minimum detection limit of the bromide electrode is approximately 1 mg/liter.
In order to assess the transport and viability of ENV735 or ENV735:24 within the sand and sediment matrices, a small subcore was taken vertically from each column at the conclusion of the transport experiment, and the number of test bacteria throughout each core was quantified. Initially, the sediment and sand columns were drained to remove excess groundwater. A core was then taken from each column by using a sterilized plastic sampling tube. The tube was inserted vertically into the top of one column and then pushed to the bottom and carefully extracted. A plunger was placed in the top of the tube to hold the sediments during removal of the core. After removal from the large column, each core was extruded into several sterile 50-ml screw-cap test tubes as 2-cm vertical sections. These core sections were vigorously mixed for 60 min with 0.1% (wt/vol) sodium pyrophosphate in sterile NCAGW to remove any sorbed cells, after which the samples were diluted and plated onto the selective medium (R2A plus streptomycin). After 7 days of incubation, the number of CFU of ENV735 or ENV735:24 present on each plate was determined.
Radiolabeling of bacteria.
Bacteria were radiolabeled for chromatography (see below) by a modification of the method of McEldowney and Fletcher (41). Colonies were removed from an R2A agar plate and incubated in BSM containing 0.1% yeast extract and 1.35 μCi of d-[U-14C]glucose/ml (specific activity, 248 mCi/mmol). Cells were incubated on a rotary shaker for 24 to 48 h at room temperature (mid- to late-logarithmic-phase growth) and then washed twice in NCAGW to remove any unincorporated radioactivity.
HIC and ESIC.
Hydrophobic interaction chromatography (HIC) and electrostatic interaction chromatography (ESIC) were performed as described by Dahlback et al. (11) and Pedersen (44), respectively. Briefly, the hydrophobic test resin Octyl Sepharose CL-4B, the anion exchange resin Dowex 1X8-200, and the cation exchange resin Dowex 50WX8-200 were mixed with NCAGW in a 1:1 (wt/vol) ratio and packed separately into glass Pasteur pipettes that had been plugged with glass wool and serially prerinsed with ethanol and NCAGW. One milliliter of 14C-labeled cells (average of 170,000 dpm/ml) at a density of 2 × 108 cells/ml in NCAGW was pipetted onto the top of each column and allowed to pass through by gravity. The columns were then washed four times with 3 ml of NCAGW. Each wash was collected into a separate scintillation vial. The column resin was removed from each column following the washing procedure and placed into a scintillation vial. Eight milliliters of scintillation cocktail (Liquiscint; National Diagnostics, Atlanta, Ga.) was added to each vial. The vials were then mixed, and the amount of radioactivity in each was quantified using a model 1209 Rackbeta scintillation counter (Pharmacia LKB Nuclear, Gaithersburg, Md.). HIC and ESIC results are expressed as the ratio of the number of cells adhering to the matrix to that of cells eluted based on radioactivity. Tests for leakage of radioactivity and for mass balance recovery were performed. All assays were performed in duplicate.
Electrophoretic mobility.
The electrophoretic mobility of ENV735 and ENV735:24 was determined using a cell concentration of 106 to 107 cells/ml. Electrophoretic mobility was measured in NCAGW (ionic strength = 0.003 M) to eliminate the effect of organic matter present in natural groundwater. The mobility measurements were made using a DELSA 440SX Doppler electrophoretic light scattering apparatus (Coulter Corporation, Miami, Fla.) under the following conditions: temperature, 25°C; measured conductivity of the artificial groundwater, 0.29 mS/cm; frequency range, 500 Hz; electric field strength, 6 V; on time, 2.5 s; off time, 0.5 s. The typical temperature drifts within a single experiment were less than 0.1°C, so the effect of convection arising from Joule heating was minimized. The effect of electroosmosis was eliminated by determination of the electrophoretic mobility at the station layers (16% channel thickness away from the side walls) according to the Komagata equation (32). Since these points are difficult to locate exactly, a series of measurements was made at 11 positions spanning the width of the cell. The resulting profile was fit to a parabola to determine the channel center, and then the empirical equation was used to find the electrophoretic mobility. Mobility measurements of standard carboxylate-modified polystyrene latex particles (nominal diameter = 300 nm) in 0.01 M sodium phosphate buffer at pH 7 were made periodically to check the stability of the DELSA instrument. Electrophoretic mobility measurements were performed twice on two separate cultures of ENV735 and ENV735:24 to ensure reproducibility.
TEM.
A negative-staining procedure was used to observe the cell morphology of ENV735 and ENV735:24. Briefly, a droplet of cell suspension was placed on Parafilm, and a copper transmission electron microscopy (TEM) grid was placed on the droplet for 2 to 5 min to allow cells to attach. The TEM grid was removed from the cell droplet and placed faceup on Parafilm. A droplet of uranyl acetate (1%, vol/vol) was placed on the grid for 1 to 2 min. TEM observations were made with a Zeiss 10C instrument operated at 80 kV and a beam current of 20 mA. Images were obtained at a magnification of ×10,000 to ×20,000.
Surfactant toxicity to ENV735.
H. flava ENV735 was grown in BSM with 0.1% yeast extract and then washed in NCAGW and resuspended to an optical density at 550 nm of 0.10, which corresponds to approximately 108 cells/ml. Ten milliliters of cell suspension was then pipetted into sterile 160-ml serum vials. A small amount of surfactant (10 or 100 μl of a 10% stock solution in PBS) was added to the appropriate vial, and vials were crimp sealed with sterile Teflon-lined rubber septa. Vials were placed horizontally on a rotary shaker operating at 150 rpm at room temperature. Aqueous samples were periodically collected through the septa using a sterile syringe and needle, and then the samples were serially diluted in PBS and plated onto R2A agar. The agar plates were incubated at 30°C for 5 to 7 days, at which time the CFU were counted.
Cell transport with surfactants.
One surfactant, Tween 20, was found to be relatively nontoxic to ENV735. The effect of this surfactant on the transport of ENV735 was examined. To evaluate cell adhesion, 20-ml syringe barrels were packed with 12 g of sterile solids, as described previously for the isolation of the adhesion-deficient variant. However, Ottawa sand rather than Oyster sediment was used in these studies so that the adsorption matrix was more uniform. In this case, variations in adhesion could be attributed to the presence or absence of surfactant rather than to variations in the matrix. Triplicate columns were prepared for each treatment. In the first set of experiments, ENV735 was grown to mid-logarithmic phase, washed, and then suspended in BSM, BSM plus 0.01% Tween 20, or BSM plus 0.1% Tween 20. The initial cell density was approximately 107 cells/ml. The suspended cells (3.0-ml volume) were then applied to the top of each column, and after a 1-h incubation period, the column was washed with 3.0 ml of BSM (i.e., surfactant-treated cells and nonsurfactant wash). In the second experiment, the cells were again suspended in the same three solutions to a density of 107 cells/ml. In this case, however, after the 1-h incubation, the columns were washed with the same solutions used to prepare the cells for injection (i.e., surfactant-treated cells plus surfactant wash). In the third set of experimental assays, sand in triplicate columns was pretreated with one of the three different solutions (3.0 ml each). After 5 min, the columns were inoculated with cells suspended in BSM, and after 1 h, the columns were washed with BSM (i.e., surfactant pretreatment of sand and nonsurfactant wash). The plating of column inocula, washes, and enumeration of CFU were performed as described previously for the isolation of ENV735:24.
Microcosm study with site sediments.
A microcosm study was performed to test MTBE degradation by strain ENV735:24 under the conditions found at an MTBE-contaminated site in Dover, Del. Sediments were collected from the site using a cone penetrometer, and groundwater was obtained from an existing monitoring well nearby. Sediments and water were shipped on ice to Envirogen Inc., Lawrenceville, N.J., after collection. For microcosm studies, the sediments were homogenized by hand and then 50-g portions were added to sterile 160-ml serum vials. Fifty milliliters of site water was then added to each vial. The pH of these groundwater-sediment slurries was low (∼3.8); therefore, some microcosms were amended with sodium carbonate (0.082 mg/liter) to bring the pH to neutrality prior to the addition of cells. The quantity of carbonate added was determined based on a titration performed with sediment slurries from the site (data not shown). In addition, some microcosms received inorganic nutrients (1.1 mg of N/liter and 0.8 mg of P/liter) from a commercial fertilizer (K-gro; K-Mart Corp., Troy, Mich.).
Cells of ENV735:24 were grown on 0.1% yeast extract and then washed and added to duplicate vials to a final concentration of approximately 108 cells/ml. Duplicate microcosms received no cells, and duplicates were amended with mercuric chloride (0.07%) to inhibit all microbial activity (killed controls). Vials were sealed with Teflon-lined septa and aluminum crimp seals. Five milliliters of headspace was removed from each vial and replaced with an equal volume of oxygen to ensure that aerobic conditions were maintained in the microcosms. Because the MTBE concentration in the samples was low, microcosms were amended with additional MTBE in distilled water to provide a final concentration of approximately 7 mg/liter. Vials were incubated with shaking at 15°C in the dark. Liquid samples (5 ml) were collected from each vial periodically and then passed through a 0.2-μm-pore-size syringe filter into serum vials with 50 μl of mercuric chloride solution (final concentration, 0.07%). The vials were crimp sealed and refrigerated until GC-FID analysis was performed. Duplicate vials were prepared and sampled for each treatment.
RESULTS
Isolation and degradation activity of an adhesion-deficient variant.
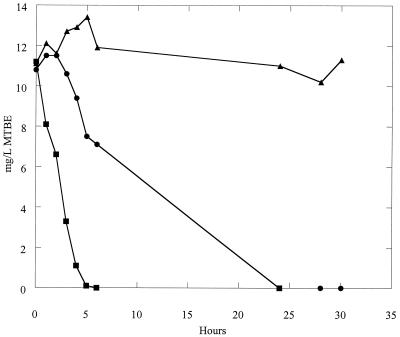
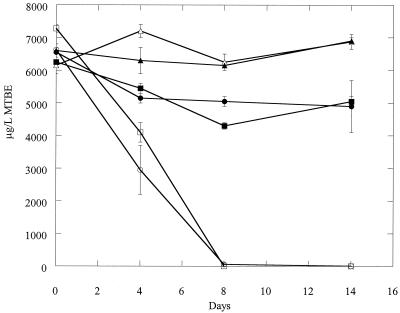
Several different stock cultures of strain ENV735 were initially used for selection of an adhesion-deficient variant. These included the streptomycin-resistant culture, a gentamicin-resistant variant, and several different laboratory stocks that were frozen (−80°C) at different times. The cultures were sequentially passed through sterile sand, and the percentage of adhesive cells was recorded. After several passages, many of the stock cultures lost the ability to degrade MTBE and were discarded. For some cultures, the percentage of cells transported through the column did not increase despite numerous passes, and further experiments using these cultures were also discontinued. One stock culture of ENV735 was sequentially passed through 27 columns of sterile Oyster sediment. In the initial set of duplicate columns, >99.5% of the ENV735 cells adhered to the sediment and were not eluted. After 27 sequential passes, however, the average percent of adhesion of cells to the sediment was reduced to 39 (n = 5; standard deviation = 13.9). This adhesion-deficient variant was designated ENV735:24. MTBE bottle assays confirmed that the adhesion-deficient variant ENV735:24 still actively degrades MTBE. A comparison of MTBE degradation rates is shown in Fig. 1. Although ENV735:24 degrades MTBE somewhat more slowly than the wild-type strain, all of the MTBE (12 mg/liter) was degraded to a level below detection (0.2 mg/liter) within 24 h.
FIG. 1.
Degradation of MTBE in BSM with no cells (▴), with wild-type ENV735 (▪), or with adhesion-deficient variant ENV735:24 (•). Values are the means from duplicate samples; standard deviations were 3.1 mg/liter or less.
Microbial transport in columns.
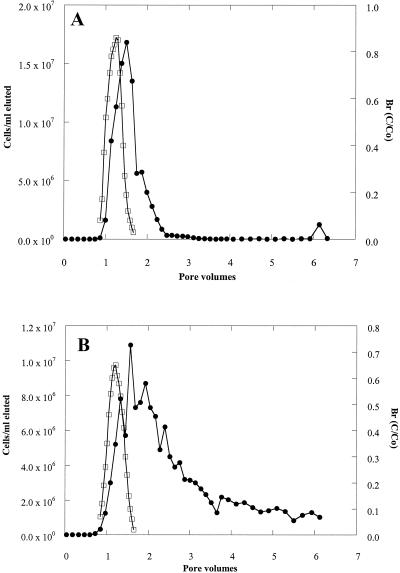
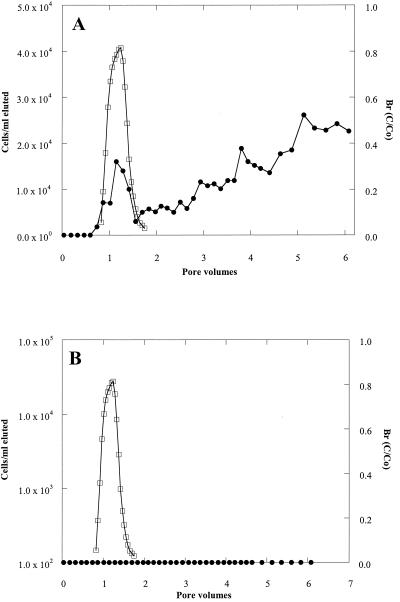
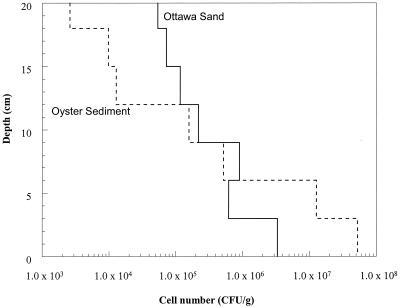
The transport of the adhesion-deficient strain (ENV735:24) was dramatically improved in both the Ottawa sand and in the Oyster sediment (Fig. 2) compared with that of the wild-type strain (Fig. 3). In the Ottawa sand (Fig. 2A), approximately 1.7 × 107 cells of ENV735:24/ml eluted from the column in a peak that was only slightly retarded compared with the bromide peak. This contrasts with a maximum concentration of only 2.5 × 104 cells/ml passing through the sand column for the wild-type strain (Fig. 3A). Similarly, in the sediment columns, the maximum concentration of ENV735:24 in effluent water reached approximately 107 cells/ml (Fig. 2B). By comparison, no cells of the wild-type strain (detection limit, 100 cells/ml) were observed in more than 6 pore volumes of flow through Oyster sediment (Fig. 3B). At the end of the experiment using wild-type ENV735, sediment was collected from different depths in each column. The sorbed cells were removed using a solution of pyrophosphate, and the numbers of ENV735 cells were determined using selective medium. In the Oyster sediment, most of the cells remained in the bottom 3 cm of the sediment column, and the cell numbers decreased by 5 orders of magnitude from the influent end to the effluent end of the core (Fig. 4). In the Ottawa sand, the majority of cells also remained in the first 3 cm of the column, although the cell numbers in this matrix decreased by only 2 orders of magnitude over the length of the core.
FIG. 2.
Transport of adhesion-deficient ENV735:24 (•) through Ottawa sand (A) and Oyster sediment (B) compared with a conservative bromide tracer (□).
FIG. 3.
Transport of wild-type ENV735 (•) through Ottawa sand (A) and Oyster sediment (B) compared with a conservative bromide tracer (□). The minimum detection limit for cells was 100 CFU/ml.
FIG. 4.
Distribution of wild-type ENV735 in sand and sediment columns. Solid line, Ottawa sand; dashed line, Oyster sediment.
HIC and ESIC.
Because bacterial transport is largely controlled by bacterial surface characteristics, the surface charge and the hydrophobicity of each bacterium were characterized, and the data for the wild-type strain and the adhesion-deficient variant were compared. Bacterial surface hydrophobicity was determined by HIC, and the surface charge was determined by ESIC and electrophoretic mobility. HIC measures the affinity of the bacteria to a hydrophobic resin. A higher affinity, measured as the ratio of cells retained in the gel matrix to cells eluted (g/e ratio), suggests a greater tendency towards hydrophobic interaction. HIC assays of wild-type ENV735 yielded an affinity value (i.e., g/e ratio) of 21.20 ± 1.35 (all values are the means and standard deviations from duplicate samples). The log of this average affinity is 1.33, a positive value, indicating that this strain is hydrophobic. In contrast, the g/e ratio of the adhesion-deficient variant ENV735:24 was 0.44 ± 0.02. The log of this value is −0.36, denoting that the variant is hydrophilic. This was the most notable difference between the chromatography results for the two organisms.
ESIC measures the affinity of bacteria to a negatively charged cation exchange resin or positively charged anion exchange resin. As with HIC, the results are expressed as the ratio of cells retained in the resin to cells eluted (r/e ratio). The adhesion-deficient variant had an affinity for the cation exchange resin of 0.37 ± 0.09, which was over 18 times higher than that of the wild-type bacterium (0.02 ± 0.01); however, both of these affinity values are considered extremely low, suggesting that the organisms are not positively charged. Conversely, both bacteria demonstrated notably high affinities for the anion exchange resin. The affinity values of the adhesion-deficient variant and the wild-type strain for this resin were 44.74 ± 19.47 and 57.72 ± 2.62, respectively, indicating that both strains exhibit a very high net negative charge. These values were not significantly different according to a t test. Only the results of chromatography assays meeting the quality assurance and quality control standards set by Dahlback et al. (11) were used.
Electrophoretic mobility and TEM.
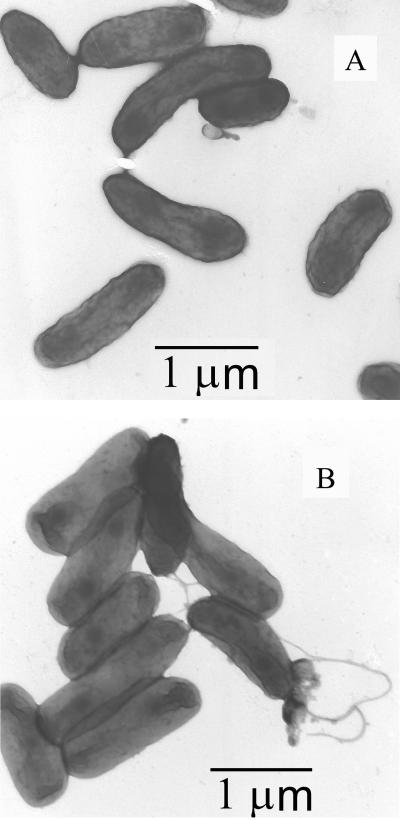
Zeta potential is another method used to determine bacterial surface charge. This value is derived from electrophoretic mobility measurements by either microelectrophoresis (61) or capillary electrophoresis (23). The electrophoretic mobility results indicate that wild-type ENV735 contains two distinct subpopulations having mobility values of (−1.8 ± 0.1) × 10−8 and (−1.3 ± 0.1) × 10−8 m2 V−1 s−1, with 67% of the culture exhibiting the more negative mobility value. In contrast, the adhesion-deficient strain has only a single population with the less negative mobility value of −1.3 × 10−8 m2 V−1 s−1. The mobility assay was performed on two separate occasions with two freshly grown cultures of ENV735 and ENV735:24. The electrophoretic mobility results from the first and second trial showed the same trend but a somewhat different relative proportion of the two subpopulations for the wild-type strain. Approximately 40% of cells exhibited the more negative mobility value in the second trial compared with 67% of cells in the first trial. TEM observations supported the results from the electrophoretic mobility studies. Micrographs showed that the wild-type strain and the adhesion-deficient variant were similar in size (1.32 ± 0.26 by 0.53 ± 0.04 μm and 1.18 ± 0.26 by 0.48 ± 0.05 μm, respectively). However, the wild-type bacterium has both flagellated and nonflagellated populations, whereas the entire population of the adhesion-deficient variant appears to be nonflagellated (Fig. 5). This conclusion was supported by the viewing of more than 100 TEM images of each organism on two separate occasions. In addition, when the second mobility assay was conducted, both cultures were analyzed by 16S rRNA sequencing and were determined to be identical.
FIG. 5.
TEM micrographs of adhesion-deficient ENV735:24 (A) and wild-type ENV735 (B).
Surfactant toxicity to ENV735.
The surfactants tested for toxicity to strain ENV735 and the results of these tests are presented in Table 1. All of the surfactants were toxic to the bacterium at a 0.1% (vol/vol) concentration, and with the exception of Tween 20, all were also toxic at a 0.01% concentration. The lower detection limit for the cell survival assay was 10 CFU/ml, and in most cases, no cells were observed at this density. Interestingly, ENV735 appears to be capable of growth on 0.01% Tween 20. After 24 h, cells incubated with 0.01% Tween 20 were observed to be present at 102% of the number in the control without surfactant. This increased to 478% after 3 days of incubation and then to 5,630% after 7 days (data not shown).
TABLE 1.
Toxicity of surfactants to wild-type ENV735
| Surfactant | % Survival after 24 h with:
|
|
|---|---|---|
| 0.1% (vol/vol) Surfactant | 0.01% (vol/vol) Surfactant | |
| Control (no surfactant) | 100 | 100 |
| Tergitol 15-S-12 | <0.00002 | <0.00002 |
| Steol CS-330 | <0.00002 | 14 |
| Igepal CO-720 | <0.00002 | <0.00002 |
| Sodium dodecyl sulfate | NDa | <0.00002 |
| Tween 20 | 2 | 102 |
| Tween 80 | <0.00002 | <0.00002 |
| JBR-425 | <0.00002 | <0.00002 |
| Brij 35 | <0.00002 | <0.00002 |
ND, not determined.
Influence of Tween 20 on cell transport and MTBE degradation.
The results of column studies to evaluate the influence of Tween 20 on the transport of ENV735 are presented in Table 2. In each study, the presence of surfactant appears to have facilitated bacterial transport through the sand matrix. In the first experiment, in which the cells were added to columns in surfactant solution followed by a wash in BSM, the use of 0.01% Tween 20 caused an 18% increase in the number of cells passing through the Ottawa sand matrix. Although 0.1% Tween 20 was toxic to ENV735 over a 24-h incubation period, the number of cells did not decline appreciably over the short period required to conduct the transport study. An experiment was therefore conducted to determine the influence of increased surfactant concentration on cell transport. When cells were passed through columns with 0.1% Tween 20, there was a 28% increase in the number of cells in the effluent.
TABLE 2.
Influence of Tween 20 on the adhesion of wild-type ENV735 to Ottawa sand
| Surfactant | % of cells adhering to sand aftera:
|
||
|---|---|---|---|
| Surfactant treatment of cells and BSM washb | Surfactant treatment of cells and surfactant washc | Surfactant treatment of sand and BSM washd | |
| None | 83 (5) | 75 (2) | 76 (1) |
| 0.01% Tween 20 | 65 (9) | 52 (5) | 46 (6) |
| 0.1% Tween 20 | 55 (11) | 55 (2) | 45 (6) |
Values in parentheses indicate standard deviations for triplicate columns.
Cells were suspended in surfactant solution for injection, and the column was subsequently washed with BSM.
Cells were suspended in and the column was washed with the surfactant solution.
The sand was prewashed with the surfactant solution, and the cells were added and washed with BSM without surfactant.
In a separate experiment, cells were added to columns in surfactant solution and then the columns were washed with the same solution (rather than with BSM only). The numbers of cells passing through the columns with this procedure were 20 and 23% (two separate trials) higher than that in controls without surfactant. The lowest overall adhesion of cells in the columns was observed when the Ottawa sand was pretreated with surfactant prior to cell inoculation. The transport of ENV735 through the sand matrix was 30% greater in the columns that were pretreated with Tween 20 compared with that in the untreated columns. As in the previous studies, there was no significant difference between the results with the 0.01 and 0.1% surfactant treatments.
An experiment was conducted to evaluate the influence of Tween 20 on MTBE biodegradation by ENV735. Cells of ENV735 (2 × 108 cells/ml) were added to serum vials containing BSM or BSM plus 0.01% Tween 20 with 18 mg of MTBE/liter. The data showed that Tween 20 did not inhibit MTBE degradation by the strain. In fact, MTBE degradation in the presence of Tween 20 was more rapid than that without the surfactant. In samples receiving Tween 20, ENV735 degraded MTBE from 18 mg/liter to a level below detection within 2 h. The bacterium took more than 6 h to completely degrade the same amount of MTBE in the absence of Tween 20 (data not shown).
Microcosm study with site samples.
The pH of the groundwater-sediment slurries used for microcosm studies (Dover, Del.) was approximately 3.8. Previous studies with ENV735 showed that the rate of MTBE degradation by this strain is highest at a pH of approximately 7.1 and decreases as the pH increases or decreases from this value (unpublished data). Therefore, in microcosm studies, some samples were amended with sodium carbonate to increase the pH to neutral. Complete degradation of MTBE and its breakdown product TBA was observed in pH-adjusted microcosms amended with ENV735:24 after about 8 days of incubation (Fig. 6). Conversely, no appreciable degradation of MTBE occurred in bioaugmented microcosms that remained at low pH. The addition of nutrients did not influence MTBE degradation. There was no appreciable loss of MTBE in microcosms that were not amended with ENV735:24 or in microcosms that were treated with mercuric chloride to inhibit microbial activity. The data suggest that bioaugmentation with ENV735:24 will be a viable option for MTBE remediation at this site but only if the pH of the treatment zone can be buffered.
FIG. 6.
Degradation of MTBE by ENV735:24 in groundwater-sediment microcosms under the following conditions: pH adjusted to 7 (○), pH adjusted to 7 and additional nutrients added (□), no pH adjustment (pH 3.8) (•), no pH adjustment (pH 3.8) and additional nutrients added (▪), killed control (▴), and live, uninoculated control (▵).
DISCUSSION
The application of exogenous bacteria for pollutant remediation may be an effective strategy at sites where appropriate degradative bacteria are not present or where they are present in very low numbers. Bioaugmentation has been applied for remediation of chlorinated solvents, including trichloroethene, dichloroethene, and vinyl chloride (58), and recently for MTBE (31, 49). For this strategy to be widely successful, however, the augmented bacteria must be biologically active against the contaminant of interest, they must survive and compete successfully in the environment to which they are added, and they must disperse adequately throughout the zone of contamination to cause significant reduction of contaminant levels. Even in instances where bacteria are injected into barrier walls (i.e., biobarriers) and contaminants are allowed to move through these walls, microbial dispersion must be sufficient to provide high numbers of cells between injection points. Otherwise, gaps in the barrier wall will allow contaminant to pass through untreated. The data presented here suggest that the wild-type strain of ENV735, like most wild-type bacteria, will not move or disperse well through site sediments. Most cells of this bacterium moved less than 6 cm in natural sediments during column studies. Conversely, subsurface transport and distribution of the adhesion-deficient strain ENV735:24 may be much more extensive than that for the wild-type bacterium. The improved transport, and thus the greater potential zone of dispersion for ENV735:24 compared with that for the wild-type strain, is likely to improve the effectiveness of MTBE bioaugmentation efforts with this bacterium.
There has been great interest in microbial transport through porous media in the past two decades. Research during this period has resulted in improved understanding of the microbial and geochemical factors that control cell transport and, subsequently, of possible mechanisms to improve bacterial transport and distribution (8, 16, 19, 52). Measurements of cell hydrophobicity and electrostatic charge have been widely used to predict bacterial attachment to solid surfaces, including phagocytic cells (18), epithelial cells (63), polystyrene (41, 45), and subsurface sediments (14, 60, 62). Our results indicate that the adhesion-deficient variant ENV735:24 is much less hydrophobic than the wild-type strain. The correlation between reduced hydrophobicity and enhanced cell transport was also observed recently by DeFlaun et al. (14). In their report, a nonadhesive variant of Burkholderia cepacia G4 had considerably lower hydrophobicity than the wild-type strain. Similarly, Stenstrom (60) observed that bacterial cells displaying high hydrophobicity values tend to exhibit enhanced adsorption to mineral particles.
Electrophoretic mobility is a measure of the bacterial surface charge density, which has been used as a predictor of bacterial transport (24, 62). Our data indicate that wild-type ENV735 contains two distinct subpopulations with different electrophoretic mobilities. In contrast, the adhesion-deficient variant has only a single population whose electrophoretic mobility corresponds to that of the less prevalent subpopulation in the wild-type strain. Recent data suggest that wide variations in cell characteristics can influence cell transport (3, 5, 54, 55). This variability can include the existence of distinct subpopulations within a pure culture (1). For example, Baygents et al. (3) quantified two subpopulations with differing electrophoretic mobilities within each of two pure cultures. These findings have led researchers to propose a dual-alpha model for cell transport and deposition. In this model, two subpopulations with different sticking efficiencies (i.e., adhesion) are described within a singular microbial culture (5, 54). The ENV735 data presented here supports this approach to describing microbial transport. It appears that sequential adhesion assays selected the less adhesive bacterial subpopulation (ENV735:24) from wild-type ENV735.
TEM was used to examine the characteristics of ENV735 and ENV735:24. The microscopic assessment appears to confirm the electrophoretic mobility data. TEM observations showed that the wild-type strain has two subpopulations which differ in flagellation. One subpopulation has abundant flagella, and the second is nonflagellated. The adhesion-deficient variant is similar in size to the wild-type culture and has no flagella. It is apparent from the HIC and ESIC data that the cell surface characteristics of the adhesion-deficient variant are also substantially different from those of the wild-type strain. Based on the length of time and the high number of passages required to obtain the adhesion-deficient strain (which should have been relatively simple if it were just the selection of one subpopulation of ENV735), it is likely that these surface characteristics are critical to the enhanced transport of this organism.
Surfactants have been studied for their effects on the solubilization, transport, and biological degradation of aquifer contaminants (30, 46, 65). Surfactants can also enhance bacterial transport (2, 6, 24, 40). Several column assays demonstrated that the adhesion of ENV735 to sand was considerably reduced in the presence of Tween 20. The data support the results of Gross and Logan (24) and Li and Logan (40), who observed reduced adhesion of the bacterial strain Alcaligenes paradoxus to borosilicate beads in the presence of Tween 20. Our results also agree with those of Brown and Jaffe (6) and Bai et al. (2), who also observed increased bacterial transport in sands in the presence of surfactants, although the surfactants they used were Brij 30, Brij 35 (6), and a monorhamnolipid isolated from Pseudomonas aeruginosa ATCC 9027 (2). The data presented here indicate that the surfactant Tween 20 may be an effective tool for facilitating bacterial transport of ENV735 in the subsurface. MTBE degradation assays in which 0.01% Tween 20 was added to the matrix indicate that the presence of the surfactant may also increase the rate of MTBE degradation, although the reason for this is unclear.
Selection of an adhesion-deficient variant of ENV735 took several months, as the strain often lost activity after five or six passes through the Oyster sediment matrix. Once an adhesion-deficient variant with stable degradative activity (ENV735:24) was obtained, the rates of MTBE metabolism by the variant and wild-type strain were compared. The rate of MTBE degradation by the adhesion-deficient variant was lower than that of the wild-type culture, although both strains completely degraded added MTBE within 24 h. During field applications, the improved transport of the adhesion-deficient variant, as demonstrated in column studies, is anticipated to outweigh the slightly reduced rate of MTBE metabolism exhibited by this variant compared with that of the wild-type strain. We expect this variant to readily disperse in a field aquifer and to quickly degrade MTBE and TBA. The improved transport of this organism, combined with its capacity to biodegrade MTBE, suggests that it will be useful for the cleanup of MTBE-contaminated aquifers.
Acknowledgments
This work was supported by the National Science Foundation Small Business Innovative Research (SBIR) program (award no. DMI-0091432).
We thank Tim McHale of the National Environmental Technology Testing Site in Dover, Del., for providing site samples. We also thank Mary DeFlaun and Robert Steffan for reviewing the manuscript and providing helpful comments.
REFERENCES
- 1.Albinger, O., B. K. Biesemeyer, R. G. Arnold, and B. E. Logan. 1994. Effect of bacterial heterogeneity on adhesion to uniform collectors by monoclonal populations. FEMS Microbiol. Lett. 124:321-326. [Google Scholar]
- 2.Bai, G., M. L. Brusseau, and R. M. Miller. 1997. Influence of a rhamnolipid biosurfactant on the transport of bacteria through a sandy soil. Appl. Environ. Microbiol. 63:1866-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baygents, J. C., J. R. Glynn, Jr., O. Albinger, B. K. Biesemeyer, K. L. Ogden, and R. G. Arnold. 1998. Variation of surface charge density in monoclonal bacterial populations: implications for transport through porous media. Environ. Sci. Technol. 32:1596-1603. [Google Scholar]
- 4.Blue Ribbon Panel on Oxygenates in Gasoline. 1999. Achieving clean air and clean water: the report of the blue ribbon panel on oxygenates in gasoline. U.S. Environmental Protection Agency (EPA) report 420-R-99-021. U.S. Government Printing Office, Washington, D.C.
- 5.Bolster, C. H., A. L. Mills, G. Hornberger, and J. Herman. 2000. Effect of intra-population variability on the long-distance transport of bacteria. Ground Water 38:370-375. [Google Scholar]
- 6.Brown, D. G., and P. R. Jaffe. 2001. Effects of nonionic surfactants on bacterial transport through porous media. Environ. Sci. Technol. 35:3877-3883. [DOI] [PubMed] [Google Scholar]
- 7.Caccavo, F., Jr., N. B. Ramsing, and J. W. Costerton. 1996. Morphological and metabolic responses to starvation by the dissimilatory metal-reducing bacterium Shewanella alga BrY. Appl. Environ. Microbiol. 62:4678-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camesano, T. A., and B. E. Logan. 1998. Influence of fluid velocity and cell concentration on the transport of motile and nonmotile bacteria in porous media. Environ. Sci. Technol. 32:1699-1708. [Google Scholar]
- 9.Chen, J., Y. Rubin, and S. Hubbard. 2001. Estimating the hydraulic conductivity at the South Oyster Site from geophysical tomographic data using Bayesian techniques on the normal linear regression model. Water Resour. Res. 37:1603-1613. [Google Scholar]
- 10.Church, C. D., P. G. Tratnyek, J. F. Pankow, J. E. Landmeyer, A. L. Baehr, M. A. Thomas, and M. Schirmer. 1999. Effects of environmental conditions on MTBE degradation in model column aquifers, p. 93-101. In U.S. Geological Survey, Water Resources Investigations Report 99-4018C, vol. 3. U.S. Geological Survey, West Trenton, N.J.
- 11.Dahlback, B., M. Hermansson, S. Kjelleberg, and B. Norkrans. 1981. The hydrophobicity of bacteria—an important factor in their initial adhesion at the air-water interface. Arch. Microbiol. 128:267-270. [DOI] [PubMed] [Google Scholar]
- 12.DeFlaun, M. F., A. S. Tanzer, A. L. McAteer, B. Marshall, and S. B. Levy. 1990. Development of an adhesion assay and characterization of an adhesion-deficient mutant of Pseudomonas fluorescens. Appl. Environ. Microbiol. 56:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFlaun, M. F., C. J. Murray, W. Holben, T. Scheibe, A. Mills, T. Ginn, T. Griffin, E. Majer, and J. L. Wilson. 1997. Preliminary observations on bacterial transport in a coastal plain aquifer. FEMS Microbiol. Rev. 20:473-487. [Google Scholar]
- 14.DeFlaun, M. F., S. R. Oppenheimer, S. Streger, C. W. Condee, and M. Fletcher. 1999. Alterations in adhesion, transport, and membrane characteristics in an adhesion-deficient pseudomonad. Appl. Environ. Microbiol. 65:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFlaun, M. F., and R. J. Steffan. 2002. Bioaugmentation, p. 434-442. In G. Bitton (ed.), Encyclopedia of environmental microbiology. John Wiley & Sons, New York, N.Y.
- 16.Dong, H., T. C. Onstott, M. F. DeFlaun, M. E. Fuller, T. D. Scheibe, S. H. Streger, R. K. Rothmel, and B. J. Mailloux. 2002. Relative dominance of physical versus chemical effects on the transport of adhesion-deficient bacteria in intact cores from South Oyster, Virginia. Environ. Sci. Technol. 36:891-900. [DOI] [PubMed] [Google Scholar]
- 17.Eweis, J. B., D. P. Y. Chang, and E. D. Schroeder. 1997. Meeting the challenge of MTBE biodegradation. In Proceedings of the Air and Waste Management Association's 90th Annual Meeting and Exhibition. Toronto, Ontario, Canada.
- 18.Ferreiros, C. M., M. T. Criado, V. Sainz, B. Suarez, J. Carballo, and M. C. del Rio. 1989. Changes in surface hydrophobicity and charge in Neisseria meningitidis and their correlation with the association to phagocytic cells. Rev. Esp. Fisiol. 45:105-110. [PubMed] [Google Scholar]
- 19.Fontes, D. E., A. L. Mills, G. M. Hornberger, and J. S. Herman. 1991. Physical and chemical factors influencing transport of microorganisms through porous media. Appl. Environ. Microbiol. 57:2473-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortin, N. Y., M. Morales, Y. Nakagawa, D. D. Focht, and M. A. Deshusses. 2001. Methyl tert-butyl ether (MTBE) degradation by a microbial consortium. Environ. Microbiol. 3:407-416. [DOI] [PubMed] [Google Scholar]
- 21.Fuller, M. E., B. J. Mailloux, P. Zhang, S. H. Streger, J. A. Hall, S. N. Vainberg, A. J. Beavis, W. P. Johnson, T. C. Onstott, and M. F. DeFlaun. 2001. Field-scale evaluation of CFDA/SE staining coupled with multiple detection methods for assessing the transport of bacteria in situ. FEMS Microbiol. Ecol. 37:55-66. [Google Scholar]
- 22.Garnier, P. M., R. Auria, C. Augur, and S. Revah. 1999. Cometabolic biodegradation of methyl t-butyl ether by Pseudomonas aeruginosa grown on pentane. Appl. Microbiol. Biotechnol. 51:498-503. [DOI] [PubMed] [Google Scholar]
- 23.Glynn, J. R., Jr., B. M. Belongia, R. G. Arnold, K. L. Ogden, and J. C. Baygents. 1998. Capillary electrophoresis measurements of electrophoretic mobility for colloidal particles of biological interest. Appl. Environ. Microbiol. 64:2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross, M. J., and B. E. Logan. 1995. Influence of different chemical treatments on transport of Alcaligenes paradoxus in porous media. Appl. Environ. Microbiol. 61:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson, J. R., C. E. Ackerman, and K. M. Scow. 1999. Biodegradation of methyl tert-butyl ether by a bacterial pure culture. Appl. Environ. Microbiol. 65:4788-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardison, L. K., S. S. Curry, L. M. Ciuffetti, and M. R. Hyman. 1997. Metabolism of diethyl ether and cometabolism of methyl tert-butyl ether by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 63:3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hareland, W. A., R. L. Crawford, P. J. Chapman, and S. Dagley. 1975. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J. Bacteriol. 121:272-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey, R. W., L. H. George, R. L. Smith, and D. R. LeBlanc. 1989. Transport of microspheres and indigenous bacteria through a sandy aquifer: results of natural- and forced-gradient tracer experiments. Environ. Sci. Technol. 23:51-56. [Google Scholar]
- 29.Hatzinger, P. B., K. McClay, S. Vainberg, M. Tugusheva, C. W. Condee, and R. J. Steffan. 2001. Biodegradation of methyl tert-butyl ether by a pure bacterial culture. Appl. Environ. Microbiol. 67:5601-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman, D. C., Y. Zhang, and R. M. Miller. 1997. Rhamnolipid (biosurfactant) effects on cell aggregation and biodegradation of residual hexadecane under saturated flow conditions. Appl. Environ. Micrbiol. 63:3622-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hristova, K. R., C. M. Lutenegger, and K. M. Scow. 2001. Detection and quantification of methyl tert-butyl ether-degrading strain PM1 by real-time TaqMan PCR. Appl. Environ. Microbiol. 67:5154-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter, R. J. 1981. Zeta potential in colloid science: principles and applications. Academic Press, New York, N.Y.
- 33.Hyman, M., P. Kwon, K. Williamson, and K. O'Reilly. 1998. Cometabolism of MTBE by alkane-utilizing microorganisms. p. 321-326. In G. B. Wickramanayake and R. E. Hinchee (ed.), Natural attenuation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, Ohio.
- 34.Jackson, A., D. Roy and G. Breitenbeck. 1994. Transport of a bacterial suspension through a sediment matrix using water and an anionic surfactant. Water Res. 28:943-949. [Google Scholar]
- 35.Johnson, R., J. Pankow, D. Bender, C. Price, and J. Zogorski. 2000. MTBE: to what extent will past releases contaminate community water supply wells? Environ. Sci. Technol. 34:2A-9A. [DOI] [PubMed] [Google Scholar]
- 36.Kane, S. R., H. R. Beller, T. C. Legler, C. J. Koester, H. C. Pinkart, R. U. Halden, and A. M. Happel. 2001. Aerobic biodegradation of methyl tert-butyl ether by aquifer bacteria from leaking underground storage sites. Appl. Environ. Microbiol. 67:5824-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchstetter, T. W., B. C. Singer, R. A. Harley, G. R. Kendall, and M. Traverse. 1999. Impact of California reformulated gasoline on motor vehicle emissions. 1. Mass emission rates. Environ. Sci. Technol. 33:318-328. [Google Scholar]
- 38.Landmeyer, J. E., F. H. Chapelle, H. H. Herlong, and P. M. Bradley. 2001. Methyl tert-butyl ether biodegradation by indigenous aquifer microorganisms under natural and artificial oxic conditions. Environ. Sci. Technol. 35:1118-1126. [DOI] [PubMed] [Google Scholar]
- 39.Lappin-Scott, H. M., and J. W. Costerton. 1992. Ultramicrobacteria and their biotechnological applications. Curr. Opin. Biotechnol. 3:283-285. [Google Scholar]
- 40.Li, Q., and B. E. Logan. 1999. Enhancing bacteria transport for bioaugmentation of aquifers using low ionic strength solutions and surfactants. Water Res. 33:1090-1100. [Google Scholar]
- 41.McEldowney, S., and M. Fletcher. 1986. Effect of growth conditions and surface characteristics of aquatic bacteria on their attachment to solid surfaces. J. Gen. Microbiol. 132:513-523. [Google Scholar]
- 42.Park, K., and R. M. Cowan. 1997. Effects of oxygen and temperature on the biodegradation of MTBE, p. 421-423. In Proceedings of the 213th American Chemical Society National Meeting. American Chemical Society, Washington, D.C.
- 43.Pasteris, G., D. Werner, K. Kaufmann, and P. Hohener. 2002. Vapor phase transport and biodegradation of volatile fuel compounds in the unsaturated zone: a large-scale lysimeter experiment. Environ. Sci. Technol. 36:30-39. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen, K. 1980. Electrostatic interaction chromatography, a method for assaying the relative surface charges of bacteria. FEMS Microbiol. Lett. 12:365-367. [Google Scholar]
- 45.Rosenberg, M. 1981. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl. Environ. Microbiol. 42:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothmel, R. K., R. W. Peters, E. St. Martin, and M. F. DeFlaun. 1998. Surfactant foam/bioaugmentation technology for in situ treatment of TCE-DNAPLs. Environ. Sci. Technol. 32:1667-1675. [Google Scholar]
- 47.Salanitro, J. P., L. A. Diaz, M. P. Williams, and H. L. Wisniewski. 1994. Isolation of a bacterial culture that degrades methyl t-butyl ether. Appl. Environ. Microbiol. 60:2593-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salanitro, J. P., C. S. Chou, H. L. Wisniewski, and T. E. Vipond. 1998. Perspectives on MTBE biodegradation and the potential for in situ aquifer bioremediation. p. 40-54. In Proceedings of the National Groundwater Association Southwest Focused Groundwater Conference, Anaheim, Calif.
- 49.Salanitro, J. P., P. C. Johnson, G. E. Spinnler, P. M. Maner, H. L. Wisniewski, and C. Bruce. 2000. Field-scale demonstration of enhanced MTBE bioremediation through aquifer bioaugmentation and oxygenation. Environ. Sci. Technol. 34:4152-4162. [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Schirmer, M., and J. F. Barker. 1998. A study of long-term MTBE attenuation in the Borden Aquifer, Ontario, Canada. Ground Water Monit. Remediation 18:113-122. [Google Scholar]
- 52.Scholl, M. A., and R. W. Harvey. 1992. Laboratory investigations on the role of sediment surface and groundwater chemistry in transport of bacteria through a contaminated sandy aquifer. Environ. Sci. Technol. 26:1410-1417. [Google Scholar]
- 53.Shaw, J. C., B. Bramhill, N. C. Wardlaw, and J. C. Costerton. 1985. Bacterial biofouling in a model core system. Appl. Environ. Microbiol. 49:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simoni, S. F., H. Harms, T. N. P. Bosma, and A. J. B. Zehnder. 1998. Population heterogeneity affects transport of bacteria through sand columns at low flow rates. Environ. Sci. Technol. 32:2100-2105. [Google Scholar]
- 55.Simoni, S. F., T. N. P. Bosma, H. Harms, and A. J. B. Zehnder. 2000. Bivalent cations increase both the subpopulation of adhering bacteria and their adhesion efficiency in sand columns. Environ. Sci. Technol. 34:1011-1017. [Google Scholar]
- 56.Squillace, P. J., J. S. Zogorski, W. G. Wilber, and C. V. Price. 1996. Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993-1994. Environ. Sci. Technol. 30:1721-1730. [Google Scholar]
- 57.Steffan, R. J., K. McClay, S. Vainberg, C. W. Condee, and D. Zhang. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steffan, R. J., K. L. Sperry, M. T. Walsh, S. Vainberg, and C. W. Condee. 1999. Field-scale evaluation of in situ bioaugmentation for remediation of chlorinated solvents in groundwater. Environ. Sci. Technol. 33:2771-2781. [Google Scholar]
- 59.Steffan, R. J., S. Vainberg, C. Condee, K. McClay, and P. Hatzinger. 2000. Biotreatment of MTBE with a new bacterial isolate, p. 165-173. In G. B. Wickramanayake, A. R. Gavaskar, B. C. Alleman, and V. S. Magar (ed.), Bioremediation and phytoremediation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, Ohio.
- 60.Stenstrom, T. A. 1989. Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl. Environ. Microbiol. 55:142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Mei, H. C., and H. J. Busscher. 2001. Electrophoretic mobility distribution of single-strain microbial populations. Appl. Environ. Microbiol. 67:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walan, A., and E. Kihlstrom. 1988. Surface charge and hydrophobicity of Campylobacter jejuni strains in relation to adhesion to epithelial HT-29 cells. APMIS 96:1089-1096. [DOI] [PubMed] [Google Scholar]
- 64.Wilson, R. D., D. M. Mackay, and K. M. Scow. 2002. In situ MTBE biodegradation supported by diffusive oxygen release. Environ. Sci. Technol. 36:190-199. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, Y., and R. M. Miller. 1995. Effect of rhamnolipid (biosurfactant) structure on solubilization and biodegradation of n-alkanes. Appl. Environ. Microbiol. 61:2247-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]