Abstract
At all stages of its life (from transcription to translation), an RNA transcript interacts with many different RNA-binding proteins. The composition of this supramolecular assembly, known as a ribonucleoprotein particle, is diverse and highly dynamic. RNA-binding proteins control the generation, maturation and lifespan of the RNA transcript and thus regulate and influence the cellular function of the encoded gene. Here, we review our current understanding of protein–RNA recognition mediated by the two most abundant RNA-binding domains (the RNA-recognition motif and the double-stranded RNA-binding motif) plus the zinc-finger motif, the most abundant nucleic-acid-binding domain. In addition, we discuss how not only the sequence but also the shape of the RNA are recognized by these three classes of RNA-binding protein.
Keywords: double-stranded RNA-binding motif, RNA-binding proteins, RNA recognition, RNA-recognition motif, zinc-finger motif
Introduction
The association of RNA-binding proteins (RBPs) with RNA transcripts begins during transcription. Some of these early-binding RBPs remain bound to the RNA until it is degraded, whereas others recognize and transiently bind to RNA at later stages for specific processes such as splicing, processing, transport and localization (Dreyfuss et al, 2002). The RBPs cover the RNA transcripts and control their fate. Some RBPs function as RNA chaperones (Lorsch, 2002) by helping the RNA, which is initially single-stranded, to form various secondary or tertiary structures. When folded, these structured RNAs, together with specific RNA sequences, act as a signal for other RBPs that mediate gene regulation. Here, we review our current structural understanding of protein–RNA recognition mediated by the two most abundant RNA-binding domains, the RNA-recognition motif (RRM) and the double-stranded RNA-binding motif (dsRBM), and by the most abundant nucleic-acid-binding motif, the CCHH-type zinc-finger domain. We discuss how these three small domains recognize RNA: some bind single-stranded RNA by direct readout of the primary sequence, whereas others recognize primarily the shape of the RNA or both the sequence and the shape. Other types of RNA-binding domains, such as the K-homology (KH) domain or the oligonucleotide/oligosaccharide-binding (OB) fold, have recently been reviewed and are not discussed here (Messias & Sattler, 2004).
RNA shape-dependent recognition by double-stranded RBM
The dsRBM is a 70–75 amino-acid domain with a conserved αβββα protein topology in which the two α-helices are packed along one face of a three-stranded anti-parallel β-sheet (Fig 1; Fierro-Monti & Mathews, 2000; St Johnston et al, 1992). These domains occur mostly in multiple copies (up to five) and have so far been found in 388 eukaryotic proteins, 72 of which are human (data taken from the SMART database; Letunic et al, 2004). These proteins have an essential role in RNA interference, RNA processing, RNA localization, RNA editing and translational repression (Doyle & Jantsch, 2002; Saunders & Barber, 2003).
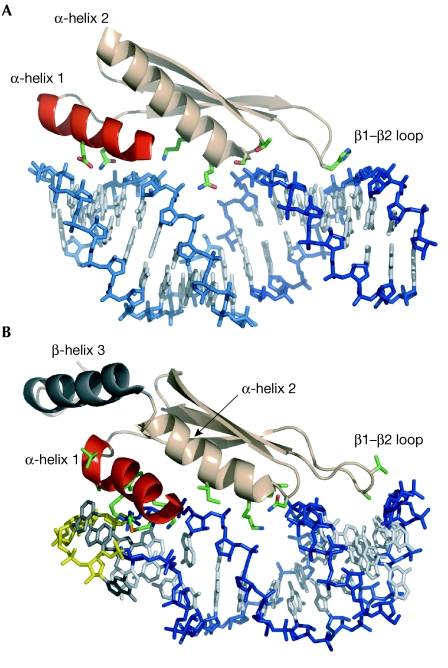
Figure 1.
Double-stranded RNA recognition by double-stranded RNA-binding motifs. (A) The double-stranded RNA-binding motif (dsRBM) of Xlrbpa2 bound to dsRNA (Ryter & Schultz, 1998). The α-helix 1 (in red), amino-terminal part of α-helix 2, and β1–β2 loop recognize non-sequence-specifically the shape of dsRNA. Backbones are coloured blue and light blue for two co-axially stacked duplexes. (B) The dsRBM of Rnt1p bound to the stem–loop closed by the AGNN tetraloop (backbone in yellow and bases in black; Wu et al, 2004). The α-helix 1, a key element for shape-specific recognition by dsRBMs, is highlighted in red. The dsRBM of Rnt1p has an additional carboxy-terminal α-helix 3 (in black), that modulates the conformation of α-helix 1 (Leulliot et al, 2004). Side chains involved in intermolecular interactions are shown.
So far, only three structures of dsRBMs in complex with dsRNA have been determined (Table 1): a 1.9 Å crystal structure of the second dsRBM of Xenopus laevis RNA-binding protein A (Xlrbpa2) bound to two coaxially stacked dsRNA molecules, each 10 bp long (Ryter & Schultz, 1998); a nuclear magnetic resonance (NMR) structure of the third dsRBM from the Drosophila Staufen protein in complex with a symmetrical GC-rich 12-bp duplex capped by a UUCG tetraloop (Ramos et al, 2000); and an NMR structure of the dsRBM of Rnt1p (an RNase III homologue from budding yeast) bound to a 14-bp RNA duplex capped by an AGAA tetraloop (Wu et al, 2004). All three structures have several common features that reveal how a dsRBM is able to bind to any dsRNA but not to dsDNA, regardless of its base composition. The dsRBMs interact along one face of the RNA duplex through both α-helices and their β1–β2 loop (Fig 1). The contacts with the RNA cover 15 bp that span two consecutive minor grooves separated by a major groove. In all three structures, the contacts to the sugar-phosphate backbone of the major groove and of one minor groove (Fig 1) are mediated by the β1–β2 loop and the amino-terminal part of α-helix 2. These interactions are non-sequence-specific as they involve 2′-hydroxyls and phosphate oxygens and are perfectly adapted to the shape of an RNA double helix. By contrast, the interactions mediated by α-helix 1 are different in all three complexes. In the dsRBM of Xlrbpa2, α-helix 1 interacts nonspecifically with the other minor groove of the RNA (Fig 1A), with a few contacts to the bases. In the dsRBM of Staufen, α-helix 1 interacts with a UUCG tetraloop that caps the RNA double helix. Although the UUCG tetraloop is not a natural substrate of Staufen, this finding led to the proposal that α-helix 1 modulates the specificity of individual dsRBMs (Ramos et al, 2000). Indeed, this was recently confirmed by the structure of the dsRBM of Rnt1p bound to its natural RNA substrate (Fig 1B), in which α-helix 1 recognizes the specific shape of the minor groove created by the conserved AGNN tetraloop (Wu et al, 2004). The α-helix 1, the conformation of which is stabilized by an additional carboxy-terminal α-helix 3 (Fig 1B; Leulliot et al, 2004), is tightly inserted into the RNA minor groove and contacts the sugar-phosphate backbone and the two non-conserved tetraloop bases, whereas the conserved A and G bases are not involved in the interactions (Wu et al, 2004). This structure illustrates how this dsRBM recognizes the specific shape of its RNA target but not its sequence. dsRBMs are highly conserved and have the same structural framework, but are chemically distinct through variations in key residues. The structure of the dsRBM of Rnt1p in complex with RNA highlights the essential role of the α-helix 1 in the recognition of structured elements that deviate from regular dsRNA. The α-helix 1 is the least-conserved secondary structure element among various dsRBMs and seems to have a different spatial arrangement relative to the rest of the domain in different dsRBMs. This variability may be an important factor as many biochemical experiments have shown that dsRBM-containing proteins have binding specificity for a variety of RNA structures, such as stem–loops, internal loops, bulges or helices with mismatches (Doyle & Jantsch, 2002; Fierro-Monti & Mathews, 2000; Ohman et al, 2000; Stephens et al, 2004). Clearly, further structures are needed to decipher the extent of RNA shape-dependent recognition by dsRBMs.
Table 1.
Various structures of RNA-binding proteins bound to RNA
| Complex | RNA secondary structure | No. of RBDs in structures vs in full-length protein | RNA sequence recognized specifically by RRM β-sheet | Function | Reference; Protein Data Bank (PDB) ID |
|---|---|---|---|---|---|
| dsRBM type | |||||
| Second dsRBM of Xlrbpa2 | Duplexa | 1/3 | – | hnRNP association, translation repression | Ryter & Schultz, 1998; 1DI2 |
| Third dsRBM of Staufen | Stem–loopb | 1/5 | – | mRNA localization, translation control | Ramos et al, 2000; 1EKZ |
| dsRBM of Rnt1p | Stem–loop | 1/1 | – | RNA processing | Wu et al, 2004; 1T4L |
| RRM type | |||||
| N-terminal RRM of U2B″ (in U2B″– U2A′–RNA complex) | Stem–loop | 1/2 | CAG | Pre-mRNA splicing | Price et al, 1998; 1A9N |
| N-terminal RRM of U1A | Stem–loop | 1/2 | CAC | Pre-mRNA splicing | Oubridge et al, 1994; 1URN |
| N-terminal RRM of U1A | Internal loop | 1/2 | CAC | Pre-mRNA splicing | Allain et al, 1997; 1AUD, Varani et al, 2000; 1DZ5 |
| Two N-terminal RRMs of nucleolin | Stem–loop | 2/4 | RRM1-CG RRM2-UC | Ribosome biogenesis | Allain et al, 2000; 1FJE, Johansson et al, 2004; 1RKJ |
| Two N-terminal RRM of PABP | Single strand | 2/4 | RRM1-AAA RRM2-AAA | Translation initiation | Deo et al, 1999; 1CVJ |
| Two RRMs of sex-lethal | Single strand | 2/2 | RRM1-UUU RRM2-UGU | Alternative splicing | Handa et al, 1999; 1H2T |
| Two N-terminal RRMs of HuD | Single strand | 2/3 | RRM1-UUU RRM2-UU | mRNA stability, translation regulation | Wang & Hall, 2001; 1FXL, 1G2E |
| RRM of CBP20 | Single strand | 1/1 | m7GpppG | Maturation of pre-mRNA and U-rich snRNA | Mazza et al, 2002; 1H2V |
| Zinc finger | |||||
| Fourth, fifth and sixth zinc fingers (CCHH- type) of TFIIIA | Truncated 5S RNAc | 3/9 | – | Transcription regulation | Lu et al, 2003; 1UN6 |
| First and second zinc fingers (CCCH- type) of TIS11d | Single strand | 2/2 | – | RNA processing and degradation | Hudson et al, 2004; 1RGO |
aTwo coaxially stacked dsRNA (each 10-bp long).
b12-bp duplex capped by a non-physiologically relevant UUCG tetraloop.
cConsists of loop A, loop E and helices I, IV and V. CBP20, cap-binding protein 20; dsRBM, double-stranded RNA-binding motif; hnRNP, heterogeneous nuclear ribonucleoprotein; HuD, Hu protein D; PABP, poly(A)-binding protein; RBD, RNA-binding domain; Rnt1p, RNase III homologue; RRM, RNA-recognition motif; TFIIIA, transcription factor IIIA; Xlrbpa2, Xenopus laevis RNA-binding protein A.
RNA sequence- and shape-dependent recognition by an RRM
The RRM is the most common RNA-binding motif. It is a small protein domain of 75–85 amino acids with a typical βαββαβ topology that forms a four-stranded β-sheet packed against two α-helices (Mattaj, 1993). RRMs are found in about 0.5%–1% of human genes (Venter et al, 2001) and are often present in multiple copies (up to six per protein). RRM-domain-containing proteins are involved in many cellular functions, particularly messenger RNA and ribosomal RNA processing, splicing and translation regulation, RNA export and RNA stability (Dreyfuss et al, 2002).
So far, ten structures of an RRM in complex with RNA have been determined using either NMR spectroscopy or X-ray crystallography (Table 1). These structures reveal the complexity of protein–RNA recognition mediated by the RRM, which often involves not only protein–RNA interactions but also RNA–RNA and protein–protein interactions. All ten structures reveal some common features. The main protein surface of the RRM involved in the interaction with the RNA is the four-stranded β-sheet, which usually contacts two or three nucleotides (exemplified here by the RRM1 of sex-lethal; Fig 2A; Handa et al, 1999). The nucleotides are located on the surface of the β-sheet, with the bases oriented parallel to the β-sheet plane and often packed against conserved hydrophobic side-chains (usually aromatics). These two or three nucleotides are recognized sequence-specifically by interactions with the protein side-chains of the β-sheet and with the main-chain and side-chains of the residues carboxy-terminal to the β-sheet. Interestingly, it seems that almost all possible sequences (doublets or triplets) can be accommodated on such a surface as the RNA sequences are different in each structure (Table 1).
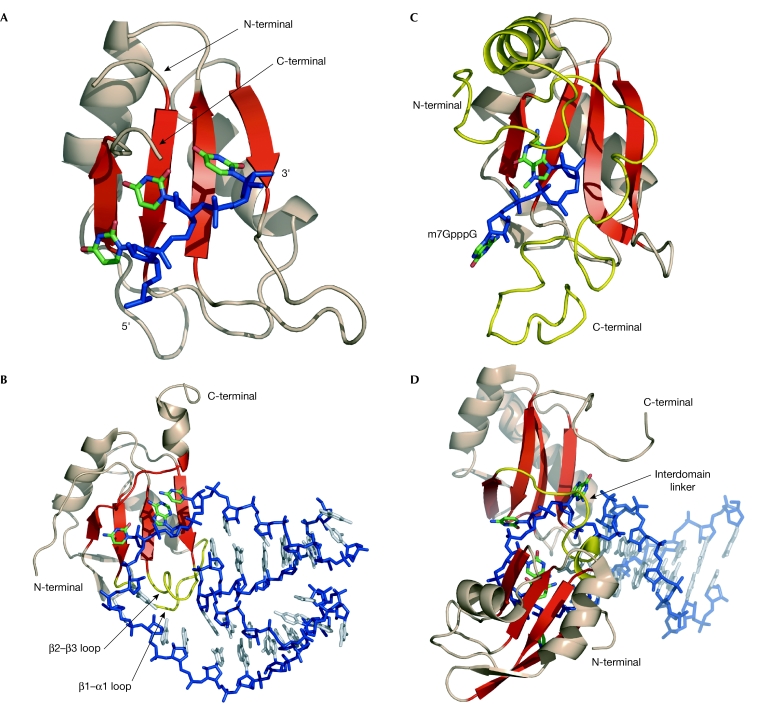
Figure 2.
RNA recognition by RNA-recognition motifs. The similarities and differences are highlighted in red and yellow, respectively. (A) RNA-recognition motif 1 (RRM1) of sex-lethal (shown as a ribbon model) interacts with the triplet UUU (shown as a stick model; Handa et al, 1999). The four-stranded β-sheet (in red) recognizes three nucleotides—this is the canonical mode of RRM–RNA interaction. (B) RRM1 of U1A bound to an RNA internal loop (Allain et al, 1997). The four-stranded β-sheet (in red) recognizes three nucleotides. The β2–β3 loop and the β1–α1 loop (in yellow) contact additional RNA residues. (C) RRM of CBP20 complexed with m7GpppG (7-methyl-G(5′)ppp(5′)G; Mazza et al, 2002). C- and N-terminal extensions (in yellow) provide additional protein–RNA contacts that creates a specific binding pocket for 5′ capped RNAs. (D) RRMs 1 and 2 of nucleolin bound to an RNA stem–loop (Allain et al, 2000). The four-stranded β-sheet of RRM2 (in red) recognizes only two nucleotides. The interdomain linker (in yellow) participates in the recognition of the hairpin architecture.
Often, RRM-containing proteins bind more than three nucleotides and recognize longer single-stranded RNA (for example, poly(A)-binding protein (PAPB; Deo et al, 1999), sex-lethal (Handa et al, 1999), Hu protein D (HuD; Wang & Hall, 2001), heterogeneous nuclear RNP A1 (hnRNP A1; Ding et al, 1999), nucleolin (Allain et al, 2000; Johansson et al, 2004), RNA stem–loops U1A (Oubridge et al, 1994), U2B″ (Price et al, 1998), nucleolin (Allain et al, 2000)) or even internal loops (U1A; Allain et al, 1997; Varani et al, 2000), all with high affinity (Kd ≈ 10−9M−1). In U1A, U2B″, nucleolin and sex-lethal, two loops between the secondary-structure elements of the RRM (the β2–β3 loop and the β1–α1 loop) are essential for additional contacts with the RNA (Fig 2B). These loops vary significantly in size and amino-acid sequence between the different RRMs. In the RRM of CBP20, the C- and N-terminal extensions (which are stabilized by the cognate protein CBP80) provide a tight binding pocket for the 5′ capped RNAs (7-methyl-G(5′)ppp(5′)N, where N is any nucleotide; Fig 2C; Mazza et al, 2002). In proteins that contain several RRMs, high-affinity binding can only be achieved by the cooperative binding of at least two RRMs to the RNA (for example, in nucleolin (Fig 2D), PABP and sex-lethal). In addition to the β-sheet–RNA contacts, interactions between the inter-domain linker and the RNA and between the RRMs themselves contribute to the marked increase in affinity compared with the binding of the individual domain alone. These structures show that the RRM is a platform with a large capacity for variation in order to achieve high RNA-binding affinity and specificity. For example, it is remarkable that a single domain like nucleolin RRM2 contacts only two nucleotides, whereas U1A RRM1 contacts 12 nucleotides and the RRM of Y14 (Fribourg et al, 2003) does not contact RNA but rather another protein. This fascinating plasticity of the RRM explains why it is so abundant and why it is involved in so many different biological functions; however, this plasticity makes it difficult to predict how the RRM achieves RNA recognition.
RNA recognition by zinc fingers
CCHH-type zinc-finger domains are the most common DNA-binding domain found in eukaryotic genomes. Typically, several fingers are used in a modular fashion to achieve high sequence-specific recognition of DNA (Miller et al, 1985). Each finger displays a ββα protein fold in which a β-hairpin and an α-helix are pinned together by a Zn2+ ion. DNA-sequence-specific recognition is achieved by the interactions between protein side-chains of the α-helix (at position −1, 2, 3 and 6, for the canonical arrangement) and the DNA bases in the major groove (Fig 3A; Wolfe et al, 2000). However, there is increasing evidence that zinc fingers are also used to recognize RNA (Finerty & Bass, 1997; Mendez-Vidal et al, 2002; Picard & Wegnez, 1979; Theunissen et al, 1992). The crystal structure of three zinc fingers (fingers 4–6) of transcription factor IIIA (TFIIIA) in complex with a 61-nucleotide fragment of the 5S RNA (Lu et al, 2003) provided the first insight into RNA recognition by CCHH-type zinc fingers. In this structure, finger 4 binds to loop E, finger 5 to helix V, and finger 6 to loop A (Fig 3B). Finger 4 recognizes loop E by specifically interacting with a bulged guanosine (Fig 3C) and, similarly, finger 6 recognizes loop A by specifically interacting with two bases (an adenine and a cytosine) that also bulge out from the rest of the RNA (Fig 3B). The specific recognition of the RNA by both fingers 4 and 6 is achieved by side-chain contacts from the N-terminal parts of the α-helix (at position −1, 1 and 2; Fig 3C). The interaction of finger 5 with helix V differs from the ones made by fingers 4 and 6. In this case, finger 5 recognizes a short RNA double helix by multiple contacts between basic amino acids of the α-helix and the RNA sugar-phosphate backbone (Fig 3D).
Figure 3.
DNA vs RNA recognition by CCHH-type zinc fingers. (A) Zinc finger 2 of Zif 268 bound to double-stranded DNA (Pavletich & Pabo, 1991). The α-helix of the zinc finger (in red) inserts into the DNA major groove; base contacts are made from positions −1, 2, 3 and 6 of the α-helix (the protein side-chains are shown as an element-type coloured stick model). The DNA bases that are recognized by the finger are coloured yellow. (B) Overall view of the complex of transcription factor IIIA (TFIIIA) fingers 4–6 (F4–F6) and 61-nucleotide 5S RNA (Lu et al, 2003). The protein and RNA are represented as ribbon models. The bulged bases involved in the recognitions are highlighted in yellow. Cyan balls represent zinc ions. (C) TFIIIA finger 4 (F4) bound to loop E (Lu et al, 2003). The α-helix (in red) of the finger 4 specifically interacts with a guanosine base that bulges out (in yellow); the base contacts are made from the side-chain at position −1, 1 and 2 of the α-helix. (D) TFIIIA finger 5 (F5) bound to helix V (Lu et al, 2003). The α-helix (in red) of finger 5 recognizes the dsRNA shape by non-sequence-specific contacts to the RNA sugar-phosphate backbone.
In contrast to the above-mentioned CCHH zinc fingers, another class of zinc fingers (CCCH-type) was recently found to adopt a different fold and to recognize sequence-specifically single-stranded RNA (Hudson et al, 2004). In this NMR structure, sequence-specific RNA recognition is achieved by a network of intermolecular hydrogen bonds between the protein main-chain functional groups and the Watson–Crick edges of the bases (Hudson et al, 2004). These structures reveal that zinc fingers bind to RNA differently to the way they do to DNA. The CCHH-type zinc fingers have two modes of RNA binding. First, the zinc fingers interact non-specifically with the backbone of a double helix, and second, the zinc fingers specifically recognize individual bases that bulge out of a structurally rigid element. The CCCH-type zinc fingers show a third mode of RNA binding, in which the single-stranded RNA is recognized in a sequence-specific manner. Taken together, zinc fingers represent a unique class of nucleic-acid-binding proteins that are capable of a direct readout of the DNA sequence within a DNA double helix, a direct readout of the RNA sequence within single-stranded RNA, and an indirect readout of the RNA as they recognize the shape of the RNA rather than its sequence. Of course, more structures of CCHH-type and CCCH-type zinc fingers in complex with RNA will need to be determined to generalize their mode of RNA recognition.
Conclusions
Proteins that contain RNA-binding domains and their interactions with RNA have important roles in all aspects of gene expression and regulation. The enormous diversity of interactions observed in protein–RNA complexes indicates that a simple recognition code is unlikely to exist in the world of protein–RNA interactions. However, two unifying themes may be inferred from the known complexes: the recognition of the primary RNA sequence and/or the recognition of the RNA shape by individual RBPs. In a simplistic view, the RRMs, dsRBMs and CCHH-type zinc fingers seem to be shaped to recognize single-stranded RNA, double-stranded RNA and RNA bulges, respectively. However, we have shown here by reviewing several recent protein–RNA complex structures that, the RRM and, to a lesser extent, the dsRBMs and the CCHH-type zinc fingers have evolved to recognize specifically a rich repertoire of RNAs in terms of length, sequence and structure. This is achieved in three ways: first, by the subtle amino-acid change in variable regions of the domains, namely the β2–β3 and the β1–α1 loops in the RRM, α-helix 1 in the dsRBM and the α-helix in the zinc fingers; second, by multiplication of the domains to achieve higher affinity through cooperative binding; and third, by extension of the protein domain. Although more structures still need to be determined, it might soon be possible to predict which RBP binds to which RNA, and how it recognizes its target. As a consequence, post-transcriptional gene expression and its regulation could be understood and controlled at the atomic level.
Richard Stefl, Lenka Skrisovska & Frédéric H.-T. Allain, who is an EMBO Young Investigator
Acknowledgments
We apologize to authors whose work could not be cited due to space constraints. The authors are supported by the Swiss National Science Foundation (No. 31-67098.01), the Roche Research Fund for Biology at the ETH Zurich (F.H.-T.A.), and the European Molecular Biology Organization and the Human Frontier Science Program postdoctoral fellowships (R.S.).
References
- Allain FH, Howe PW, Neuhaus D, Varani G (1997) Structural basis of the RNA-binding specificity of human U1A protein. EMBO J 16: 5764–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain FH, Bouvet P, Dieckmann T, Feigon J (2000) Molecular basis of sequence-specific recognition of pre-ribosomal RNA by nucleolin. EMBO J 19: 6870–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo RC, Bonanno JB, Sonenberg N, Burley SK (1999) Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell 98: 835–845 [DOI] [PubMed] [Google Scholar]
- Ding J, Hayashi MK, Zhang Y, Manche L, Krainer AR, Xu RM (1999) Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes Dev 13: 1102–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M, Jantsch MF (2002) New and old roles of the double-stranded RNA-binding domain. J Struct Biol 140: 147–153 [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Kim VN, Kataoka N (2002) Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol 3: 195–205 [DOI] [PubMed] [Google Scholar]
- Fierro-Monti I, Mathews MB (2000) Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem Sci 25: 241–246 [DOI] [PubMed] [Google Scholar]
- Finerty PJ, Bass BL (1997) A Xenopus zinc finger protein that specifically binds dsRNA and RNA–DNA hybrids. J Mol Biol 271: 195–208 [DOI] [PubMed] [Google Scholar]
- Fribourg S, Gatfield D, Izaurralde E, Conti E (2003) A novel mode of RBD-protein recognition in the Y14–Mago complex. Nat Struct Biol 10: 433–439 [DOI] [PubMed] [Google Scholar]
- Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S (1999) Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature 398: 579–585 [DOI] [PubMed] [Google Scholar]
- Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE (2004) Recognition of the mRNA AU-rich element by the zinc finger domain of TIS11d. Nat Struct Mol Biol 11: 257–264 [DOI] [PubMed] [Google Scholar]
- Johansson C, Finger LD, Trantirek L, Mueller TD, Kim S, Laird-Offringa IA, Feigon J (2004) Solution structure of the complex formed by the two N-terminal RNA-binding domains of nucleolin and a pre-rRNA target. J Mol Biol 337: 799–816 [DOI] [PubMed] [Google Scholar]
- Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, Schultz J, Ponting CP, Bork P (2004) SMART 4.0: towards genomic data integration. Nucleic Acids Res 32: D142–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulliot N, Quevillon-Cheruel S, Graille M, Van Tilbeurgh H, Leeper TC, Godin KS, Edwards TE, Sigurdsson ST, Rozenkrats N, Nagel RJ, Ares M, Varani G (2004) A new α-helical extension promotes RNA binding by the dsRBD of Rnt1p RNAse III. EMBO J 23: 2468–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch JR (2002) RNA chaperones exist and DEAD box proteins get a life. Cell 109: 797–800 [DOI] [PubMed] [Google Scholar]
- Lu D, Searles MA, Klug A (2003) Crystal structure of a zinc-finger—RNA complex reveals two modes of molecular recognition. Nature 426: 96–100 [DOI] [PubMed] [Google Scholar]
- Mattaj IW (1993) RNA recognition: a family matter? Cell 73: 837–840 [DOI] [PubMed] [Google Scholar]
- Mazza C, Segref A, Mattaj IW, Cusack S (2002) Large-scale induced fit recognition of an m(7)GpppG cap analogue by the human nuclear cap-binding complex. EMBO J 21: 5548–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Vidal C, Wilhelm MT, Hellborg F, Qian W, Wiman KG (2002) The p53-induced mouse zinc finger protein wig-1 binds double-stranded RNA with high affinity. Nucleic Acids Res 30: 1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messias AC, Sattler M (2004) Structural basis of single-stranded RNA recognition. Acc Chem Res 37: 279–287 [DOI] [PubMed] [Google Scholar]
- Miller J, Mclachlan AD, Klug A (1985) Repetitive zinc-binding domains in the protein transcription factor Iiia from Xenopus oocytes. EMBO J 4: 1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman M, Kallman AM, Bass BL (2000) In vitro analysis of the binding of ADAR2 to the pre-mRNA encoding the GluR-B R/G site. RNA 6: 687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubridge C, Ito N, Evans PR, Teo CH, Nagai K (1994) Crystal structure at 1.92 Å resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature 372: 432–438 [DOI] [PubMed] [Google Scholar]
- Pavletich NP, Pabo CO (1991) Zinc finger–DNA recognition: crystal structure of a Zif68-DNA complex at 2.1 Å. Science 252: 809–817 [DOI] [PubMed] [Google Scholar]
- Picard B, Wegnez M (1979) Isolation of a 7s particle from Xenopus laevis oocytes—5s RNA–protein complex. Proc Natl Acad Sci USA 76: 241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SR, Evans PR, Nagai K (1998) Crystal structure of the spliceosomal U2B″–U2A′ protein complex bound to a fragment of U2 small nuclear RNA. Nature 394: 645–650 [DOI] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St Johnston D, Varani G (2000) RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J 19: 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC (1998) Molecular basis of double-stranded RNA–protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J 17: 7505–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Barber GN (2003) The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J 17: 961–983 [DOI] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M (1992) A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci USA 89: 10979–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens OM, Haudenschild BL, Beal PA (2004) The binding selectivity of ADAR2's dsRBMs contributes to RNA-editing selectivity. Chem Biol 11: 1239–1250 [DOI] [PubMed] [Google Scholar]
- Theunissen O, Rudt F, Guddat U, Mentzel H, Pieler T (1992) RNA and DNA-binding zinc fingers in Xenopus Tfiiia. Cell 71: 679–690 [DOI] [PubMed] [Google Scholar]
- Varani L, Gunderson SI, Mattaj IW, Kay LE, Neuhaus D, Varani G (2000) The NMR structure of the 38 kDa U1A protein–PIE RNA complex reveals the basis of cooperativity in regulation of polyadenylation by human U1A protein. Nat Struct Biol 7: 329–335 [DOI] [PubMed] [Google Scholar]
- Venter JC et al. (2001) The sequence of the human genome. Science 291: 1304–1351 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Hall TMT (2001) Structural basis for recognition of AU-rich element RNA by the HuD protein. Nat Struct Biol 8: 141–145 [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Nekludova L, Pabo CO (2000) DNA recognition by Cys(2)His(2) zinc finger proteins. Annu Rev Biophys Biomol Struct 29: 183–212 [DOI] [PubMed] [Google Scholar]
- Wu H, Henras A, Chanfreau G, Feigon J (2004) Structural basis for recognition of the AGNN tetraloop RNA fold by the double-stranded RNA-binding domain of Rnt1p RNase III. Proc Natl Acad Sci USA 101: 8307–8312 [DOI] [PMC free article] [PubMed] [Google Scholar]