Abstract
We have previously demonstrated that low-shear modeled microgravity (low-shear MMG) serves to enhance the virulence of a bacterial pathogen, Salmonella enterica serovar Typhimurium. The Salmonella response to low-shear MMG involves a signaling pathway that we have termed the low-shear MMG stimulon, though the identities of the low-shear MMG stimulon genes and regulatory factors are not known. RpoS is the primary sigma factor required for the expression of genes that are induced upon exposure to different environmental-stress signals and is essential for virulence in mice. Since low-shear MMG induces a Salmonella acid stress response and enhances Salmonella virulence, we reasoned that RpoS would be a likely regulator of the Salmonella low-shear MMG response. Our results demonstrate that low-shear MMG provides cross-resistance to several environmental stresses in both wild-type and isogenic rpoS mutant strains. Growth under low-shear MMG decreased the generation time of both strains in minimal medium and increased the ability of both strains to survive in J774 macrophages. Using DNA microarray analysis, we found no evidence of induction of the RpoS regulon by low-shear MMG but did find that other genes were altered in expression under these conditions in both the wild-type and rpoS mutant strains. Our results indicate that, under the conditions of these studies, RpoS is not required for transmission of the signal that induces the low-shear MMG stimulon. Moreover, our studies also indicate that low-shear MMG can be added to a short list of growth conditions that can serve to preadapt an rpoS mutant for resistance to multiple environmental stresses.
Modulation of virulence in response to environmental cues is a common trait of pathogenic bacteria. Osmolarity, starvation, temperature, pH, and growth phase have all been shown to affect the expression of numerous virulence parameters in a wide range of pathogens (10, 29). Recently, we demonstrated that a low-shear culture environment which models microgravity and in vivo low-shear conditions in tissues serves as a novel signal to increase the virulence potential of the invasive enteric bacterial pathogen Salmonella enterica serovar Typhimurium (13, 14, 36, 40). Specifically, Salmonella grown under conditions of low-shear modeled microgravity (low-shear MMG), compared to Salmonella grown under identical culture conditions but with normal gravity (1 × g), had a decreased 50% lethal dose, a shortened host time to death, and increased colonization of the liver and spleen in the murine model of infectivity. In addition, low-shear MMG-grown Salmonella were more resistant to acid stress and macrophage killing than 1 × g-grown cells and exhibited significant differences in protein synthesis. This study was the first demonstration that a mechanical force such as low-shear MMG can serve as an environmental signal to increase the virulence of a microbial pathogen.
Cultivation of cells under low-shear MMG is facilitated by the use of a novel cell culture apparatus called a high-aspect-ratio vessel (HARV; Synthecon, Inc., Houston, Tex.) (39). The HARV is a rotating bioreactor in which cells are maintained in a gentle fluid orbit that creates a sustained low-shear (<1 dyne/cm2), low-turbulence environment for cell growth (14). Under these conditions, a form of optimized suspension culture is achieved that is currently being utilized for a number of applications that allow both eukaryotic and prokaryotic cells to assume phenotypes that cannot be observed under conventional culture conditions. These phenotypes include the formation of three-dimensional cell aggregates that morphologically and physiologically resemble in vivo tissue and the expression and secretion of molecules that are normally expressed in vivo but are not efficiently expressed by using standard in vitro culture techniques (11, 22, 26, 35, 41). The low-shear growth environment achieved through optimized suspension culture is thought to provide growth cues similar to those encountered during normal in vivo tissue development in utero and in certain low-shear areas of the body such as between the brush border microvilli of epithelial cells (3, 4, 8, 13, 40). The in vivo environment of brush border microvilli is particularly relevant to studies involving Salmonella since the organism is likely to occupy this niche between the microvilli of epithelial cells in the intestine and other tissues during the natural course of infection. In addition, since several cellular responses observed under low-shear optimized suspension culture mimic those observed during cell culture in space (aboard the space shuttle and the International Space Station), this culture environment is also used for ground-based studies of the effects of MMG on both eukaryotic and prokaryotic cellular physiologies. This aspect of the low-shear MMG studies using Salmonella will provide information about how infectious bacterial pathogens respond in the environment of space, a critical issue to address since the duration of and numbers of crew members on space missions are increasing and since crew members are becoming increasingly dependent on regenerative biosystems that have the significant potential to become contaminated (32).
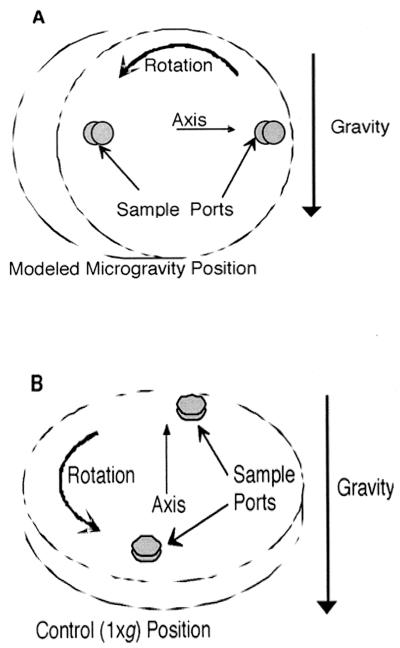
Though designed to provide an environment of low-shear MMG, the HARV can also be used to grow cells at 1 × g by simply changing the physical position of the bioreactor so that the normal amount of gravitational shear is exerted on the contents of the vessel (Fig. 1). Thus, the MMG and 1 × g conditions in the HARV are identical except for the physical orientation of the apparatus. By growing cells in the HARV under both low-shear MMG and 1 × g conditions, it is possible to observe the effects of the mechanical force of low-shear MMG on cellular physiology. Experiments performed under low-shear MMG during optimized suspension culture will provide important advances that will have an impact on both earth- and space-based cellular and physiological research.
FIG. 1.
A HARV bioreactor in the low-shear MMG orientation (A) and in the 1 × g orientation (B) is shown. When completely filled with liquid so that air bubbles cannot cause turbulence, the HARV in the MMG position suspends cells in a gentle fluid orbit in which the gravitational-shear forces are greatly reduced. When the HARV is in the 1 × g control position, gravitational-shear forces are exerted normally. (Reprinted from reference 36.)
A major cellular response that occurs when a cell senses an environmental signal, such as low-shear MMG, is alteration of gene expression (6, 10, 15, 17-19, 29, 36). We have previously shown that the expression of several Salmonella proteins is altered when the cells are grown under conditions of low-shear MMG (36). Thus, Salmonella organisms are able to sense a change in mechanical force (low-shear MMG) and respond with changes in gene expression and a corresponding change in phenotype. This global change in gene expression in response to low-shear MMG indicates the existence of a regulatory network that we term the low-shear MMG stimulon. However, there is currently no information available regarding how the cells of any species, including bacteria, are able to accomplish the transmission of this novel signal. Studies to elucidate how Salmonella responds to low-shear MMG will allow us to begin to determine the mechanisms by which cells process this novel and largely uncharacterized signal and identify the genes that are altered in expression under these conditions. However, whether Salmonella uses a known environmental signaling pathway or a previously uncharacterized pathway to process the low-shear MMG signal remains to be seen.
RpoS, or σ38, encoded by the rpoS gene, is the primary sigma factor responsible for the expression of genes that are required for resistance to environmental stresses and has accordingly been described as the master regulator of the general stress response in Escherichia coli and Salmonella (17, 28). This fact is clearly demonstrated by the fact that rpoS-deficient strains are highly sensitive to a wide range of environmental stresses and cannot induce the stress resistance that is observed under certain culture conditions, such as stationary phase and carbon starvation (24, 30). Accordingly, the genes of the RpoS regulon are induced in response to several types of environmental signals, including acid shock, carbon starvation, osmotic shock, and entry into macrophages and epithelial cells (5, 16, 25, 30). In addition, Salmonella rpoS mutants are avirulent in the murine model of infection, most likely due to deficient expression of several genes required for full pathogenicity (6, 7, 9, 34, 42). Since growth under low-shear MMG enhances the resistance of Salmonella to acid stress and macrophage killing and increases Salmonella virulence in the murine model of infection, we decided to extend our characterization of the effects of low-shear MMG on Salmonella physiology in both the wild type and an isogenic rpoS-deficient strain. We reasoned that if RpoS plays a role in the transmission of the low-shear MMG environmental signal in Salmonella, we would see altered responses to low-shear MMG in the rpoS mutant compared to those observed in the wild-type strain.
In this study, we examined the ability of low-shear MMG to confer cross-resistance to other stresses (osmotic, thermal, and oxidative) in addition to lowered pH in both the wild-type and rpoS mutant strains and we examined the effects of low-shear MMG on the growth kinetics of both strains in minimal medium. We also investigated the potential of low-shear MMG to alter the virulence profile of the rpoS mutant using both macrophage survival and mouse infection assays. In addition, we used DNA microarray analysis to determine if similar changes in gene expression in response to low-shear MMG occur in both the wild-type and rpoS mutant strains. Our results show that transmission of the low-shear MMG signal does indeed occur in the rpoS mutant strain as it does in the wild-type strain and that MMG induces similar changes in gene expression in the wild-type and rpoS backgrounds. Our findings indicate that RpoS is not required for the low-shear MMG response to occur in Salmonella; thus, the newly discovered low-shear MMG stimulon is an RpoS-independent signaling pathway. Moreover, the results provide striking evidence that growth at low-shear MMG allows Salmonella to overcome multiple stress resistance defects caused by an rpoS mutation. To our knowledge, this is the first study to demonstrate that low-shear MMG represents a novel example of a growth condition that can serve to preadapt an rpoS mutant for resistance to multiple environmental stresses.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All studies were performed with wild-type S. enterica serovar Typhimurium χ3339 (12) (which is an animal-passaged isolate of SL1344) and χ4973, an isogenic rpoS mutant derivative (34). Bacterial cells were grown in Lennox broth (LB) (27) for all experiments, except to obtain the growth curves, for which cells were grown in M9 minimal medium (37). To facilitate the low-shear MMG growth conditions, cells were grown in the HARV (Synthecon, Inc.) as described previously (36). Briefly, static overnight cultures grown at 37°C were diluted 1:200 in fresh medium and introduced into the HARV such that the bioreactor was completely filled with culture medium and no air bubbles were present. The bioreactor was oriented to grow cells under conditions of either low-shear MMG (Fig. 1A) or 1 × g (Fig. 1B). All incubations in the HARV were done at 37°C with a rotation rate of 25 rpm. The HARV cultures were harvested after 10 h of growth for all experiments unless otherwise indicated. Both the MMG and 1 × g cultures were at similar culture densities at this time point, which corresponds to late log phase.
Environmental-stress assays.
Strains grown in HARV bioreactors at MMG and 1 × g were harvested after 10 h of growth and immediately subjected to the particular stress being tested. For acid stress, the pH of the harvested cultures was lowered to 3.5 by the addition of an amount of concentrated citrate buffer that had been previously determined to give this pH value. The pH level during the assay was monitored with pH strips and then confirmed with a pH electrode at the end of the assay. The acid stress assay was performed statically at room temperature for 30 min. For thermal stress, the harvested cultures were immediately transferred to heating blocks set at the desired temperature and assayed for 30 min. For osmotic stress, sodium chloride was added to the cultures to a concentration of 2.5 M and the samples were incubated statically at 37°C for 10 h. For oxidative stress, hydrogen peroxide was added to the cultures from a fresh 30% stock solution (Sigma Chemical Co., St. Louis, Mo.) to the desired final concentration, and the assay was performed statically at room temperature for 30 min. For all the stress assays, samples were removed at time zero (before the addition of stress) and at various time points thereafter and then plated on LB agar to determine the numbers of viable CFU. Percent survival was calculated as the number of CFU at each time point divided by the number of CFU at time zero. At least three independent trials were performed for each stress experiment.
Growth measurements in minimal medium.
Cultures of Salmonella were grown in the HARV bioreactors in M9 minimal medium under low-shear MMG and 1 × g conditions as described above. The growth of the cultures was monitored over the course of 12 h by using measurements of absorbance at 600 nm, and numbers of viable CFU were counted after cultures were plated on LB agar. The data presented in Fig. 4A are from a single experiment but are representative of results obtained from three independent trials, all of which reflected the same growth differences between the MMG and 1 × g cultures. The generation times of the bacterial cultures were calculated with the CFU data by using standard equations as described previously (33).
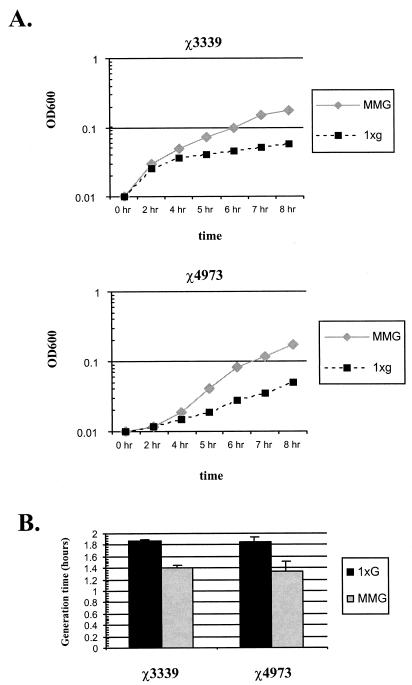
FIG. 4.
Low-shear MMG decreases the generation times of both wild-type Salmonella χ3339 and the rpoS mutant χ4973 in minimal medium. Static overnight cultures of strains χ3339 and χ4973 were diluted into fresh M9 minimal medium and grown under 1 × g or MMG conditions in the HARV. The growth of the cultures was monitored by determining the optical densities at 600 nm (A), and the generation times (based on data from monitoring numbers of viable CFU during the assay) were calculated (B). The data presented in panel A are from a single experiment but are representative of results obtained from three independent trials, all of which reflected the same growth differences between the MMG and 1 × g cultures.
Invasion and survival within J774 macrophages.
To examine the effect of low-shear MMG on the ability of the Salmonella strains to invade and survive within J774 macrophages, an in vitro assay was performed as described previously (34).
Mouse virulence assays.
Eight-week-old female BALB/c mice were perorally and intraperitoneally infected with serial dilutions of Salmonella strains that had been grown at MMG and 1 × g. Prior to peroral infection, the mice were fasted for 4 to 6 h, and food and water were returned to the animals 30 min postinfection. The health and viability of the mice were monitored over the course of 30 days. Quantitation of viable Salmonella organisms in tissues and organs was performed as described previously (34).
Microarray analysis.
Salmonella serovar Typhimurium microarrays (generously provided from the laboratory of Michael McClelland, Sidney Kimmel Cancer Center, San Diego, Calif. [31]) were prepared by printing PCR amplicons, suspended in a high-concentration salt solution, onto aminosilane-coated glass microscope slides by using a high-speed robotic system (GeneMachine OmniGrid Array Maker; Genomic Instrumentation Services, San Carlos, Calif.). Total cellular Salmonella RNA was obtained with an RNeasy kit (QIAGEN Inc., Valencia, Calif.) from MMG and 1 × g cultures grown for 10 h. Twenty micrograms of total RNA template was added to 9 μg of random hexamer primers (Operon Technologies, Inc., Almeda, Calif.). Annealing was accomplished by incubation for 10 min at 70°C and then for 10 min at room temperature. cDNA probes were synthesized with SuperScript II reverse transcriptase (10 U/ml; Life Technologies, Inc., Gaithersburg, Md.) in the presence of deoxyribonucleoside triphosphates (dATP, dGTP, and dCTP, each at 0.5 mM; dTTP at 0.17 mM) and Cy3- or Cy5-dUTP at 0.1 mM. Dye-swap reactions were performed to control for any possible differences in Cy3- or Cy5-dUTP incorporation. cDNA synthesis proceeded at 42°C for 2.5 h, and the reaction was terminated by the addition of EDTA to 10 mM. The RNA templates were hydrolyzed with 0.25 M NaOH, and the reaction was neutralized by the addition of an equimolar amount of HCl. The labeled cDNA was purified with a PCR purification kit (QIAGEN Inc.), dried, and stored at −20°C. Microarrays were probed by cohybridizing the fluorescently labeled cDNAs from the MMG and 1 × g cultures of the same Salmonella strain to the same microarray by using a Genomic Solutions (Ann Arbor, Mich.) automated hybridization chamber. Following hybridization, the microarrays were scanned for the Cy3 and Cy5 fluorescent signals with a ScanArray 3000 from GSI Lumonics (Watertown, Mass.). Stored images were analyzed with Imagene analysis software (Biodiscovery, Los Angeles, Calif.) and Gene Spring software (Silicon Genetics, Palo Alto, Calif.). For both the Cy3 and Cy5 images, the intensity of each spot was defined as the summed intensities of each pixel within a circle that was precisely positioned over the spot. Background was defined as the summed intensity of an identical number of pixels directly surrounding the spot. The Cy3 and Cy5 values for each spot were normalized to account for any difference in total intensity between the two scanned images. To calculate the expression ratio, the normalized MMG and 1 × g signal intensity values at each spot were adjusted to subtract background and the resulting MMG value was divided by the resulting 1 × g value. The RpoS regulon genes analyzed for this report were bolA, csgC, ftsQ, ftsZ, glgS, katE, katG, narV, narW, narY, narZ, osmB, osmY, otsB, poxB, proP, spvA, spvB, spvC, spvD, spvR, treA, yciE, yciG, and yohF. The expression ratios of these genes in both the wild-type χ3339 and rpoS mutant χ4973 strains did not deviate from 1 by more than twofold.
RESULTS
Low-shear MMG provides cross-resistance to acid, thermal, and osmotic stresses for both the wild-type and rpoS mutant strains of Salmonella.
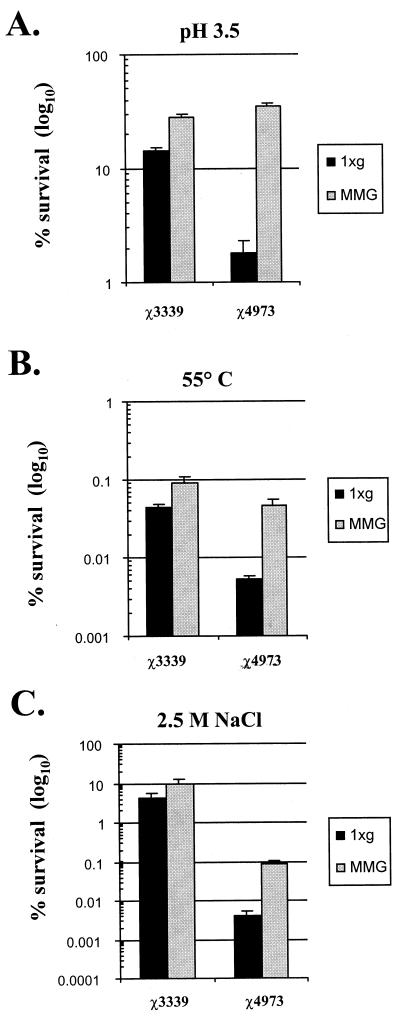
We have previously shown that, in addition to enhancing virulence potential, the growth of Salmonella under conditions of low-shear MMG increases the resistance of the wild-type bacteria to acid stress (pH 3.5). This prompted us to ask two questions: (i) can this phenotype be observed after low-shear MMG cultivation of a strain that is deficient in rpoS, which is required for the expression of a global regulon of genes needed for stress resistance, and (ii) does low-shear MMG alter the resistance of Salmonella to other stresses besides acid in both the wild type and the rpoS mutant? To address the first question, we grew the wild-type strain (χ3339) and an isogenic rpoS-deficient strain (χ4973) under conditions of both low-shear MMG and 1 × g, harvested the cultures, and immediately challenged the bacteria at pH 3.5 for 30 min (Fig. 2A). Comparison of the survival profile of each strain cultured at 1 × g showed the expected enhanced sensitivity of the rpoS mutant χ4973 to this stress. As expected, the wild-type strain χ3339 exhibited an increased resistance to acid stress when it was cultured at low-shear MMG. Strikingly, however, χ4973 also exhibited increased acid resistance when it was cultured at low-shear MMG (a 19-fold increase in percent survival compared to that at 1 × g), and this resistance was at the same level as or greater than that of the MMG-grown wild-type strain. Similar results were also observed for both thermal and osmotic stress (Fig. 2B and C). Growth at low-shear MMG increased the resistance of the wild-type strain to these stresses compared to that at 1 × g, indicating that low-shear MMG does confer cross-resistance to other stresses besides lowered pH. The rpoS mutant χ4973 exhibited the expected and previously characterized enhanced sensitivity to these stresses at 1 × g. But as with acid challenge, growth at low-shear MMG clearly endowed χ4973 with increased resistance to both thermal and osmotic stress (8.6-fold and 20.1-fold increases in percentages of survival compared to those at 1 × g, respectively). Therefore, the environmental stresses to which low-shear MMG confers cross-resistance in Salmonella include acid shock, thermal shock, and high osmolarity. Moreover, we observed increased resistance to all three stresses in the MMG-grown rpoS mutant χ4973.
FIG. 2.
Cross-resistance to multiple stresses induced by low-shear MMG in both wild-type Salmonella χ3339 and the rpoS mutant χ4973. Strains χ3339 and χ4973 were grown under 1 × g or low-shear MMG and subjected to the following environmental stresses as described in Materials and Methods: acid shock (pH 3.5, 30 min) (A), thermal shock (55°C, 26 min) (B), or osmotic shock (2.5 M NaCl, 10 h) (C). The percentages of the initial number of viable bacteria present before the addition of the stress that survived the assay are plotted. At least three independent trials of each stress experiment were performed, and the error bars correspond to the standard errors of the means.
Low-shear MMG increases the sensitivity of Salmonella to oxidative stress for both the wild-type and rpoS mutant strains.
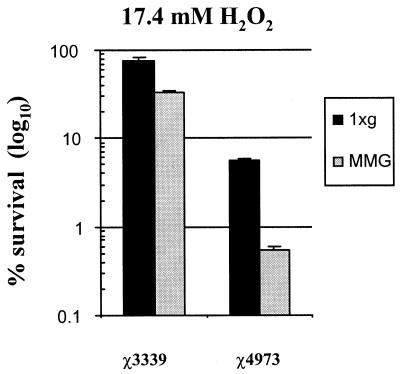
The next environmental challenge used in the course of our experiments was that of oxidative stress. In these experiments, both the wild-type and rpoS mutant strains were grown at low-shear MMG and 1 × g and subsequently challenged with the addition of hydrogen peroxide (17.4 mM) to facilitate hyperoxidative conditions (Fig. 3). Surprisingly, the wild-type strain χ3339 grown at low-shear MMG was more sensitive to oxidative challenge than the same strain grown at 1 × g. As expected, the rpoS mutant χ4973 grown at 1 × g displayed characteristic hypersensitivity to peroxide compared to the sensitivity of the wild-type strain cultured under the same conditions. But, as with the wild-type strain, χ4973 cultured under low-shear MMG was even more sensitive to the oxidative stress than the same strain grown at 1 × g. Thus, growth at low-shear MMG increased the sensitivity of Salmonella to oxidative stress, and this phenotype was observed both in the wild-type and rpoS mutant strains. The reason for the increased sensitivity of the low-shear MMG-grown strains to oxidative stress is not known at this time, but the phenotype is RpoS independent, as it was observed in both the wild-type and rpoS mutant strains.
FIG. 3.
Increased sensitivity to hydrogen peroxide induced by low-shear MMG in both wild-type Salmonella χ3339 and the rpoS mutant χ4973. Strains χ3339 and χ4973 were grown under 1 × g or low-shear MMG and subjected to hyperoxidative stress (17.4 mM H2O2, 30 min) as described in Materials and Methods. The percentages of the initial number of viable bacteria present before addition of the stress that survived the assay are plotted. At least three independent trials of each stress experiment were performed, and the error bars correspond to the standard errors of the means.
Low-shear MMG shortens the generation time of both the wild-type and rpoS mutant strains of Salmonella in minimal medium.
Both MMG- and 1 × g-grown Salmonella exhibit very similar growth profiles in rich medium (i.e., LB broth) (36). However, growth in minimal medium alters the metabolic profile of the bacterial cell compared to that after growth in rich medium. Accordingly, bacterial growth kinetics in minimal media are commonly different than those observed in rich media. In light of this, we monitored the growth of both wild-type χ3339 and the rpoS mutant χ4973 in minimal medium at both low-shear MMG and 1 × g. Upon diluting overnight cultures into fresh minimal medium and commencing growth at both low-shear MMG and 1 × g, we observed that both strains started growing at a higher rate in the low-shear MMG cultures than in the 1 × g cultures (Fig. 4A). In addition to observing the higher growth rate from the optical densities at 600 nm plotted in Fig. 4, we also observed this phenomenon by using CFU plate count methods of monitoring growth (data not shown). From the CFU data at selected time points, we determined the generation times of both strains under low-shear MMG and 1 × g culture conditions (Fig. 4B). For wild-type χ3339 and the rpoS mutant χ4973, the generation times of the low-shear MMG cultures were 0.474 and 0.505 h less than those of the 1 × g cultures, respectively. Thus, low-shear MMG shortens the generation time of Salmonella in minimal medium by approximately 30 min in both wild-type and rpoS mutant strains, indicating that this phenotype is RpoS independent.
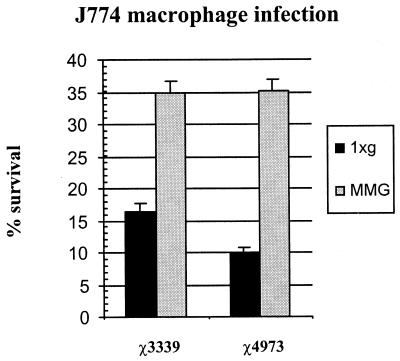
Low-shear MMG increases the ability of both wild-type and rpoS mutant Salmonella to survive within J774 macrophages.
We have previously demonstrated that MMG-grown wild-type Salmonella is better able to survive within J774 macrophages than 1 × g-grown Salmonella (36). To determine whether the rpoS allele is required for this phenotype, we examined the effect of low-shear MMG on the survival of the rpoS mutant χ4973 in J774 cells. When cultured under conventional conditions in an aerated flask, χ4973 does not have an in vitro macrophage survival defect and infects J774 macrophages at the same frequency as does the wild-type strain (34). We performed a J774 macrophage survival assay with both wild-type χ3339 and the rpoS mutant χ4973 grown at low-shear MMG and 1 × g (Fig. 5). As expected, the wild-type strain χ3339 grown under low-shear MMG was better able to survive within the macrophage cells than the same strain grown under 1 × g. Consistent with the environmental-stress assay results and the minimal medium growth phenotype, the rpoS mutant χ4973 displayed the same phenotype as the wild-type strain in the macrophage survival assay. Thus, RpoS is not required for low-shear MMG to increase the ability of Salmonella to survive within macrophages.
FIG. 5.
Survival of MMG- and 1 × g-grown wild-type Salmonella χ3339 and the rpoS mutant χ4973 within J774 macrophage cells. Cultures of χ3339 and χ4973 grown under 1 × g and low-shear MMG were used to infect J774 macrophages for a total of 40 min as described previously (34). The percentages of the initial inoculum recovered at the end of the assay are plotted. At least three independent trials of each stress experiment were performed, and the error bars correspond to the standard errors of the means.
Growth under low-shear MMG does not restore virulence to an rpoS mutant Salmonella strain in the murine model of infection.
Strains of Salmonella defective for rpoS are avirulent in the murine model of infection compared to the wild-type strain (7, 9, 34, 42). Our results in this study have shown that growth at low-shear MMG can reverse the sensitivity of an rpoS mutant Salmonella strain to several environmental stresses relevant to those encountered in vivo and allows increased survival of this strain in J774 macrophages. Therefore, we examined the ability of low-shear MMG to confer virulence to the rpoS mutant χ4973 in a murine model of infection. We infected 8-week-old female BALB/c mice with a range of doses of both MMG- and 1 × g-grown χ4973 using both the peroral and intraperitoneal routes of infection. It has been previously shown that χ4973 is not lethal to mice following peroral infection when it is grown under conventional conditions, even at the highest possible dosage (34). We observed the same phenotype with both the MMG- and 1 × g-grown cultures of χ4973, indicating that growth at low-shear MMG does not confer on an rpoS mutant Salmonella strain the ability to cause lethal infection in mice (data not shown). While there was no difference in host lethality between the χ4973 low-shear MMG and 1 × g cultures, we monitored the tissue distribution of this strain in infected mice. At 1, 3, 5, and 7 days postinfection, we harvested the livers and spleens of mice infected with MMG- and 1 × g-grown χ4973. We also harvested the Peyer's patches, intestinal walls, and intestinal luminal contents at 1, 2, and 3 days postinfection. Comparisons of the numbers of viable bacteria recovered from these tissues showed no difference between the MMG- and 1 × g-grown χ4973 cultures (data not shown). However, there was a detectable difference in the physical appearances of the two sets of infected mice. Specifically, the mice infected with MMG-grown χ4973 had a “scruffier” coat appearance (which lasted throughout the 30-day infection period) than that of mice infected with the 1 × g-grown bacteria. Since this type of coat appearance generally reflects a deleterious health condition, this result suggests that the mice infected with the MMG-grown bacteria may have experienced more stress due to infection. It is also worth noting that the severity and duration of the scruffy-coat phenotype in mice infected with MMG-grown χ4973 had not been observed in our previous studies using mice perorally infected with the same strain grown under conventional conditions.
Low-shear MMG does not induce the transcription of RpoS regulon genes but does alter the expression of a separate group of genes in both the wild-type and rpoS mutant strains.
We have shown that growth at low-shear MMG results in similar alterations in stress resistance in both the wild-type and rpoS mutant Salmonella strains. One possible model to explain this finding may be that low-shear MMG induces the RpoS regulon, even in the absence of RpoS, through an unknown mechanism. An alternative explanation is that low-shear MMG does not induce the RpoS regulon and instead changes the levels of expression of a different set of genes. Therefore, we used microarray-mediated gene expression profiling to determine whether low-shear MMG turns on genes that are part of the Salmonella RpoS regulon.
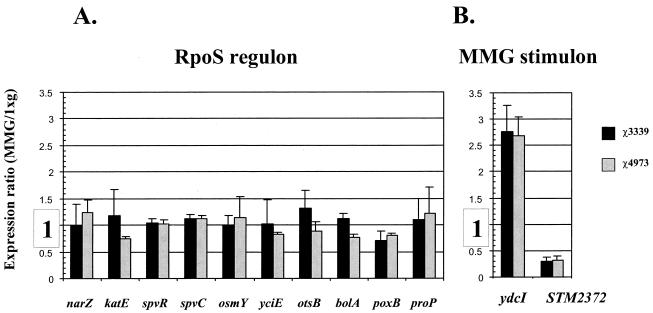
We are currently using whole-genome DNA microarray analysis to monitor the global gene expression of Salmonella in response to a number of growth conditions, including low-shear MMG (43). We grew the wild-type χ3339 and rpoS mutant χ4973 strains under conditions of both low-shear MMG and 1 × g and harvested the total cellular RNA from the cultures. Fluorescently labeled cDNAs synthesized from the harvested RNA were hybridized to glass DNA microarrays spotted with 99% of the open reading frames of the Salmonella genome. The experiments were set up as cohybridizations in the following manner. Samples from χ3339 MMG- and 1 × g-grown cultures were cohybridized to a single array, and the samples from the same culture conditions for χ4973 were cohybridized on a separate array. After hybridization, scanning, and quantitation, we calculated an expression ratio of the signal at each spot from the MMG fluorescence channel to the signal from the 1 × g channel. For the purposes of this study, we focused on 25 genes that are part of the RpoS regulon (see Materials and Methods for a complete list of the analyzed genes). For both the wild-type χ3339 and rpoS mutant χ4973 strains, we found that the low-shear MMG/1 × g expression ratios for all 25 genes did not deviate from 1 by more than twofold; the results for 10 of those genes (narZ, katE, spvR, spvC, osmY, yciE, otsB, bolA, poxB, and proP) are presented in Fig. 6. This finding indicates that the RpoS regulon of Salmonella is not induced in response to the low-shear MMG growth conditions. However, we have detected changes in the levels of expression of numerous other Salmonella genes in response to low-shear MMG. We have presented the expression ratios of two of these genes, one of which is induced by low-shear MMG (ydcI) and the other of which is down-regulated (STM2372) (Fig. 6). The ydcI gene encodes a putative transcriptional regulator of the LysR family, while the STM2372 gene is predicted to encode a putative membrane transporter. Both open reading frames are functionally uncharacterized at present. The changes in ydcI and STM2372 expression are seen in both the wild-type and rpoS mutant backgrounds, indicating that low-shear MMG-induced, RpoS-independent changes in gene expression occur.
FIG. 6.
DNA microarray analysis of gene expression in response to low-shear MMG in Salmonella strains χ3339 and χ4973. Strains χ3339 and χ4973 were grown under 1 × g and MMG conditions and processed for microarray analysis as described in Materials and Methods. The expression ratio of the MMG fluorescent signal divided by the 1 × g fluorescent signal for each specified gene was calculated as described in Materials and Methods and plotted graphically. (A) Those genes that are part of the RpoS regulon; (B) two genes that are part of the low-shear MMG stimulon.
DISCUSSION
Studies to characterize the transmission of the low-shear MMG signal will provide knowledge about how cells sense the low-shear growth cues that are present during optimized suspension culture. This will have important ramifications in understanding how tissues develop, how cells respond to in vivo low-shear environments, and how conditions of reduced gravity, such as in space, will impact both eukaryotic and prokaryotic physiologies. Additionally, studies to learn how Salmonella responds to low-shear MMG will expand our knowledge of how Salmonella modulates its virulence both on earth and in space and may lead to new insights about how previously characterized Salmonella virulence systems function or to the identification of novel, previously uncharacterized bacterial virulence strategies.
In this study, we have extended our characterization of the effects of low-shear MMG on Salmonella physiology in both wild-type and rpoS mutant strains. Given the central role of RpoS as the major regulator of the general stress response upon exposure to different environmental signals, we reasoned that RpoS would be a likely candidate for regulating the Salmonella low-shear MMG response. The results presented here indicate that transmission of the environmental signal provided by low-shear MMG occurs in both the wild-type and rpoS mutant strains of Salmonella. This finding demonstrates that, under the conditions used here, RpoS is not required for Salmonella to process the low-shear MMG signal and produce the corresponding responses that result in the various low-shear MMG-induced phenotypes. We have shown that both the wild-type and rpoS mutant strains grown under low-shear MMG respond in the same manner to four different environmental stress conditions as the 1 × g-grown cultures. Both the wild-type and rpoS mutant strains grown under low-shear MMG also exhibited the same decrease in generation time in minimal media and the same increased ability to survive in macrophages as 1 × g cultures. We have also presented evidence that similar changes in levels of gene expression in response to low-shear MMG occur in both the wild-type and rpoS mutant strains and that the RpoS regulon is not induced by the low-shear MMG conditions used in these studies. Given that we observed an array of common phenotypes in both strains, we conclude that the newly described low-shear MMG stimulon is an RpoS-independent signaling pathway and that multiple RpoS-independent responses occur under low-shear MMG. However, the possibility that certain RpoS-dependent responses to low-shear MMG exist and have yet to be observed cannot be ruled out. If an RpoS-dependent low-shear MMG response does exist, it is not required for observing the changes in resistance to environmental stress, increased growth rate in minimal medium, increased survival in macrophages, and alterations of gene expression that have been reported in this study.
Based on our initial observation of low-shear MMG-induced acid resistance, we subjected both the wild-type and rpoS mutant strains to other environmental stresses to determine whether low-shear MMG confers cross-resistance to multiple stress conditions and whether the rpoS allele is necessary to observe this phenotype. We found that low-shear MMG does indeed confer cross-resistance to acid shock, thermal shock, and hyperosmolarity. These findings further strengthen the notion that low-shear MMG enhances those properties that allow Salmonella to be more virulent since these stresses might be encountered during the bacterial life cycle. Moreover, we observed this cross-resistance in both the wild-type and rpoS mutant strains. Thus, low-shear MMG represents a novel example of a growth condition that can reverse certain stress resistance defects of an rpoS mutant and preadapt the rpoS mutant for a stress challenge to which it is normally hypersensitive. This finding indicates that low-shear MMG is able to induce a stress response that is RpoS independent. The existence of RpoS-independent stress responses has been demonstrated for E. coli and Salmonella (1, 2, 18), but whether low-shear MMG induces these previously characterized responses or another novel stress response remains to be determined.
As part of our examination of low-shear MMG-induced cross-resistance to environmental stresses, we measured the resistance of both MMG- and 1 × g-grown wild-type and rpoS mutant Salmonella strains to hydrogen peroxide. To our surprise, we found that both strains were more sensitive to oxidative stress when they were cultured under low-shear MMG than under 1 × g. The reasons why growth under low-shear MMG increases virulence and decreases resistance to oxidative stress are not known, but we have evidence that at least two genes involved with protection against oxidative stress are down-regulated under low-shear MMG growth conditions (43). Future studies will undoubtedly help us to characterize this aspect of the low-shear MMG response.
We have also demonstrated that the generation times of both the wild-type and rpoS mutant strains in minimal medium are decreased by 25 to 30 min under low-shear MMG growth conditions compared to those under 1 × g conditions. We believe that this decreased generation time may reflect the global changes in levels of gene expression that occur under low-shear MMG and that allow Salmonella to more efficiently propagate in the stringent growth environment of minimal medium. This result helps to better characterize low-shear MMG as a signal that has direct effects on Salmonella metabolism and that acts to reprogram the physiological state of the bacterium. Alternatively, the reduced generation time might also be due to differences in levels of mass diffusion in the low-shear MMG environment that might affect nutrient uptake and metabolism independently of the other changes associated with the MMG stimulon. Regardless of which possibility is true, it is interesting that the reduced generation time is consistent with the results of experiments performed on various space missions that found that bacteria grew to higher densities in liquid culture in space flight than did equivalent ground-based controls (20, 21, 23, 32). Together, these findings indicate that bacteria can more readily proliferate in an environment of low-shear MMG (similar to space or certain in vivo niches [4, 13, 14]) and suggest that bacteria in this environment are more readily able to initiate growth that could lead to contamination, colonization, and infection.
Since we previously demonstrated that MMG-grown wild-type Salmonella strains are better able to survive within J774 macrophages (36), we determined whether the rpoS allele is required for this phenotype. Our results indicate that growth under low-shear MMG does confer on the rpoS mutant strain an increased ability to survive in J774 macrophages. We also determined whether low-shear MMG could restore virulence to an rpoS mutant Salmonella strain in the murine infection model. Growth at low-shear MMG (or 1 × g) in the HARV did not confer to the rpoS mutant Salmonella strain the ability to cause lethal infection in mice. In addition, there was no difference between the numbers of viable bacteria recovered from the livers, spleens, and intestines of mice infected with the rpoS mutant cultured at low-shear MMG and at 1 × g. However, we did see a difference between the physical appearance (i.e., the scruffier coat) of the mice infected with the MMG-grown rpoS mutant cultures and that of mice infected with the 1 × g-grown cultures throughout the 30-day infection period, and this phenotype was observed in several separate experiments. This difference indicates that the reactions of mice to the bacteria grown under low-shear MMG were different from the reactions of mice to bacteria grown under 1 × g. We believe the results of the murine infection studies underscore the importance of RpoS in Salmonella virulence. The results of this study show that the low-shear MMG signal is received by the rpoS strain and that corresponding responses are made. However, in the murine model, we do not see a marked increase in the quantifiable virulence of either the MMG- or the 1 × g-grown rpoS mutant because this strain lacks functions that are required for full virulence under any conditions. Thus, it appears that the rpoS mutation causes an “avirulence hurdle” in the murine infection model that is too large for the effects of low-shear MMG to overcome. The difference in coat appearance between mice infected with the MMG- and 1 × g-grown rpoS mutant bacteria may indicate, however, that low-shear MMG has the potential to alter the way the host responds to this strain. Further study will help us to better understand this potentially informative effect.
As part of our characterization of the low-shear MMG response in Salmonella, we are currently using whole-genome microarray analysis to detect global changes in gene expression in cells grown under low-shear MMG compared to that of cells grown under 1 × g (43). For this study, we used this technique to focus on those genes that are part of the RpoS regulon since these genes are expressed in response to environmental signals in an RpoS-dependent manner and encode functions directly related to stress resistance. We found no significant changes in 25 RpoS-dependent genes in either the wild type or the rpoS mutant in response to low-shear MMG. Thus, it appears that the RpoS regulon of Salmonella is not induced by low-shear MMG. However, we have detected changes in levels of expression of many other genes in response to low-shear MMG in both the wild-type and rpoS mutant strains (43). This important molecular information will lead to significant advances in understanding how Salmonella responds to low-shear MMG and in the identification of which cellular functions are targeted by this response. To our knowledge, the microarray experiments in this study and in another recent study are the first to identify genes that are altered in expression by low-shear MMG in a prokaryotic species (43). We have presented data here for two such genes that are altered in expression in both the wild-type and rpoS backgrounds: ydcI (which is up-regulated) and STM2372 (which is down-regulated). This finding shows that the same low-shear MMG-induced changes in gene expression are able to occur in both strains, which further supports the conclusion that rpoS is not required for the low-shear MMG signal to be processed by Salmonella. The ydcI gene encodes a putative transcriptional regulator that is related to members of the LysR family that act to regulate the expression of a diverse group of genes in response to different coinducers (38). It is possible that ydcI is induced by low-shear MMG and subsequently changes the expression of genes that play a role in the reprogramming of Salmonella physiology in response to this stimulus. The STM2372 gene is predicted to encode a putative membrane transport protein. The down-regulation of this protein in response to low-shear MMG may result in the decreased transport of a particular substrate across the Salmonella cell envelope. This decreased transport may help the cell to better adapt to the low-shear MMG growth conditions. The identification of other genes that change expression in response to low-shear MMG will undoubtedly provide clues about the transmission of this environmental signal.
Acknowledgments
We are grateful to Tim Hammond, Pat Allen, Luis Cubano, and Laurel Stewart for providing invaluable assistance in the DNA microarray analysis. We thank Bill Halford for critical review of the manuscript.
This work was supported, in part, by NASA-Ames grants NAG2-1378 and NAG9-1350.
REFERENCES
- 1.Audia, J. P., C. C. Webb, and J. W. Foster. 2001. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int. J. Med. Microbiol. 291:97-106. [DOI] [PubMed] [Google Scholar]
- 2.Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. [DOI] [PubMed] [Google Scholar]
- 3.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder, W. Chai, A. M. Lawson, M. E. Molyneux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, Z., J. Xin, D. M. Pollock, and J. S. Pollock. 2000. Shear stress-mediated NO production in inner medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 279:F270-F274. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. Y., L. Eckmann, S. J. Libby, F. C. Fang, S. Okamoto, M. F. Kagnoff, J. Fierer, and D. G. Guiney. 1996. Expression of Salmonella typhimurium rpoS and rpoS-dependent genes in the intracellular environment of eukaryotic cells. Infect. Immun. 64:4739-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements, M., S. Eriksson, D. Tezcan-Merdol, J. C. Hinton, and M. Rhen. 2001. Virulence gene regulation in Salmonella enterica. Ann. Med. 33:178-185. [DOI] [PubMed] [Google Scholar]
- 7.Coynault, C., V. Robbe-Saule, and F. Norel. 1996. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol. Microbiol. 22:149-160. [DOI] [PubMed] [Google Scholar]
- 8.Creasy, R., and R. Resnik. 1984. Maternal-fetal medicine: principles and practice. W. B. Saunders Co, Philadelphia, Pa.
- 9.Fang, F. C., S. J. Libby, N. A. Buchmeier, J. Switala, J. Harwood, and D. Guiney. 1992. The alternative sigma factor KatF (RpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 11.Freed, L. E., R. Langer, I. Martin, N. R. Pellis, and G. Vunjak-Novakovic. 1997. Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 94:13885-13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo, P., A. M. Weinstein, and S. Weinbaum. 2000. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am. J. Physiol. Renal Physiol. 279:F698-F712. [DOI] [PubMed] [Google Scholar]
- 14.Hammond, T. G., and J. M. Hammond. 2001. Optimized suspension culture: the rotating-wall vessel. Am. J. Physiol. Renal Physiol. 281:F12-F25. [DOI] [PubMed] [Google Scholar]
- 15.Hammond, T. G., F. C. Lewis, T. G. Goodwin, R. M. Lennehan, D. A. Wolf, K. P. Hire, W. C. Campbell, E. Benes, K. C. O'Reilly, R. K. Globus, and J. H. Kaysen. 1999. Gene expression in space. Nat. Med. 4:359. [DOI] [PubMed] [Google Scholar]
- 16.Hengge-Aronis, R. 1996. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 21:887-893. [DOI] [PubMed] [Google Scholar]
- 17.Hengge-Aronis, R. 2000. The general stress response in Escherichia coli. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 18.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1512. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 19.Johanson, K., P. Allen, F. Lewis, L. Cubano, L. Hyman, and T. Hammond. Saccharomyces cerevisiae gene expression changes during rotating wall vessel suspension culture. J. Appl. Physiol., in press. [DOI] [PubMed]
- 20.Kacena, M. A., P. E. Leonard, P. Todd, and M. W. Luttges. 1997. Low gravity and inertial effects on the growth of E. coli and B. subtilis in semi-solid media. Aviat. Space Environ. Med. 68:1104-1108. [PubMed] [Google Scholar]
- 21.Kacena, M. A., B. Manfredi, and P. Todd. 1999. Effects of space flight and mixing on bacterial growth in low volume cultures. Microgravity Sci. Technol. 12:74-77. [PubMed] [Google Scholar]
- 22.Kaysen, J. H., W. C. Campbell, R. R. Majewski, F. O. Goda, G. L. Navar, F. C. Lewis, T. J. Goodwin, and T. G. Hammond. 1999. Select de novo gene and protein expression during renal epithelial cell culture in rotating wall vessels is shear stress dependent. J. Membr. Biol. 168:77-89. [DOI] [PubMed] [Google Scholar]
- 23.Klaus, D., S. Simske, P. Todd, and L. Stodieck. 1997. Investigation of space flight effects on Escherichia coli and a proposed model of underlying physical mechanisms. Microbiology 143:449-455. [DOI] [PubMed] [Google Scholar]
- 24.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 25.Lee, I. S., J. Lin, H. K. Hall, B. Bearson, and J. W. Foster. 1995. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol. Microbiol. 17:155-167. [DOI] [PubMed] [Google Scholar]
- 26.Lelkes, P. I., D. L. Galvan, G. T. Hayman, T. J. Goodwin, D. Y. Chatman, S. Cherian, R. M. Garcia, and B. R. Unsworth. 1998. Simulated microgravity conditions enhance differentiation of cultured PC12 cells towards the neuroendocrine phenotype. In Vitro Cell. Dev. Biol. Anim. 34:316-325. [DOI] [PubMed] [Google Scholar]
- 27.Lennox, E. S. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 28.Loewen, P., and R. Hengge-Aronis. 1994. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 29.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1996. Environmental regulation of virulence gene expression in Escherichia, Salmonella, and Shigella spp., p. 2803-2815. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C.
- 30.McCann, M. P., J. P. Kidwell, and A. Matin. 1991. The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J. Bacteriol. 173:4188-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 32.Mishra, S., and D. Pierson. 1992. Space flight, effects on microorganisms. In J. Lederberg (ed.), Encyclopedia of microbiology, vol. 4, p. 53-60. Academic Press, Inc., San Diego, Calif.
- 33.Neidhardt, F., J. Ingraham, and M. Schaechter. 1990. Physiology of the bacterial cell: a molecular approach. Sinauer Associates, Inc., Sunderland, Mass.
- 34.Nickerson, C. A., and R. Curtiss III. 1997. Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect. Immun. 65:1814-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickerson, C. A., T. J. Goodwin, J. Terlonge, C. M. Ott, K. L. Buchanan, W. B. Uicker, K. Emami, C. L. Cedor, R. Ramamurthy, M. S. Clarke, T. Hammond, and D. L. Pierson. 2001. Three-dimensional tissue assemblies: novel models for the study of Salmonella pathogenesis. Infect. Immun. 69:7106-7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickerson, C. A., C. M. Ott, S. J. Mister, B. J. Morrow, L. Burns-Keliher, and D. L. Pierson. 2000. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 68:3147-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz, R. P., and D. A. Wolf. January 1991. Rotating bioreactor cell culture apparatus. U.S. patent 4,988,623.
- 40.Stock, U. A., and J. P. Vacanti. 2001. Cardiovascular physiology during fetal development and implications for tissue engineering. Tissue Eng. 7:1-7. [DOI] [PubMed] [Google Scholar]
- 41.Unsworth, B. R., and P. I. Lelkes. 1998. Growing tissues in microgravity. Nat. Med. 4:901-907. [DOI] [PubMed] [Google Scholar]
- 42.Wilmes-Riesenberg, M. R., J. W. Foster, and R. Curtiss III. 1997. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 65:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, J. W., R. Ramamurthy, S. Porwollik, M. McClelland, T. Hammond, P. Allen, C. M. Ott, D. L. Pierson, and C. A. Nickerson. Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]