Summary
Meeting on the Cell-Cycle Regulation of Meiosis
Keywords: cell cycle, cohesion, cyclin, meiosis, recombination
Introduction
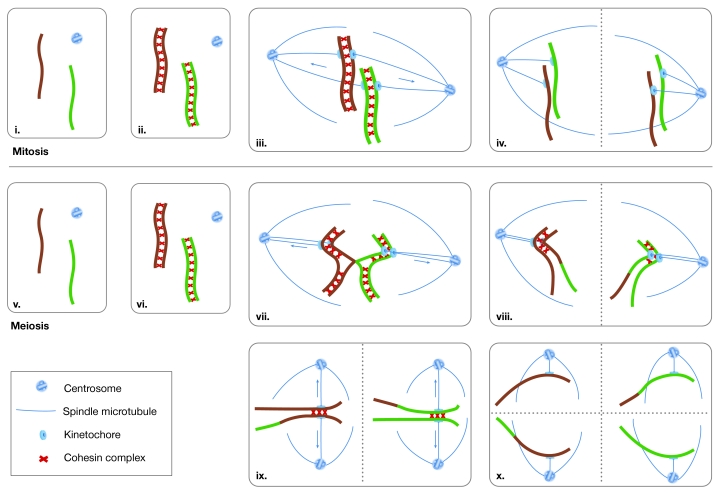
The British Society for Cell Biology autumn meeting on the Cell-Cycle Regulation of Meiosis encompassed a unique blend of disciplines from cytology to biochemistry, and covered topics ranging from recombination to human infertility. Meiosis shares much of the same cell-cycle machinery as mitosis, but also includes several unique features and regulatory pathways. As two consecutive rounds of chromosome segregation (meiosis I and II) follow a single round of chromosome replication, four haploid nuclei are made from a single diploid precursor cell. The unique event in meiosis is the segregation of homologous chromosomes (homologues) at meiosis I (Fig 1).
Figure 1.
Mitotic and meiotic chromosome cycles. In mitotic cells, connections called sister-chromatid cohesion are formed during replication and hold the sister chromatids together until they are ready to be segregated at anaphase (i–iii). When the two kinetochores of a sister-chromatid pair attach to microtubules that emanate from opposite poles of the cell, the pulling forces of the spindle are resisted by the cohesion between sister centromeres (iii). The resulting tension stabilizes microtubule attachments. When all chromatid pairs have achieved this biorientation on the spindle, sister-chromatid cohesion is destroyed and the chromatids are pulled to the cell poles (iv). During meiotic prophase I, maternal and paternal homologues pair and become connected by chiasmata (v–vii). The combination of chiasmata and cohesion allows the homologue pair to be orientated on the spindle, similar to the way in which a pair of sister chromatids would be aligned during mitosis (vii). When cohesin is destroyed, the homologues are segregated (viii). A second round of segregation then occurs without an intervening round of replication (ix–x). Note that centromere cohesion must be maintained until the onset of meiosis II. Arrows indicate the directions of the pulling forces that are generated by microtubules. Dashed lines indicate the planes of cell division.

The British Society for Cell Biology meeting on the Cell-Cycle Regulation of Meiosis took place in Newcastle, UK, between 13 and 15 September 2004, and was organized by M. Herbert, K. Jones, M. Whitaker and M. Levasseur
Recombination and meiotic chromosomes
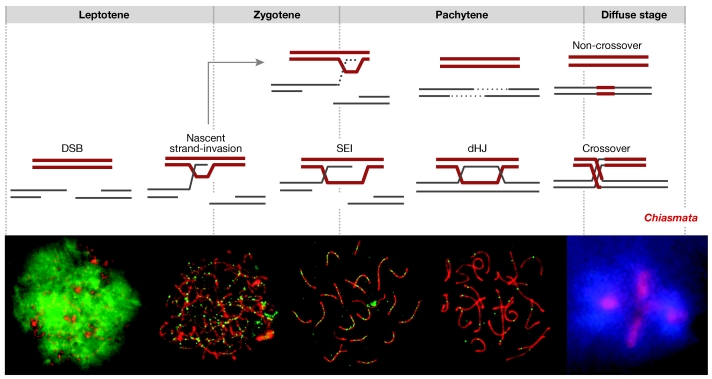
Meiotic recombination mediates two essential processes: the stable pairing of homologous chromosomes during the leptotene and zygotene stages, and the connection of homologues by chiasmata from the diplotene stage through to the anaphase I stage. N. Hunter (Davis, CA, USA) described the molecular events of recombination in Saccharomyces cerevisiae (Fig 2; Hunter & Kleckner, 2001; Borner et al, 2004). Recombination is initiated by programmed DNA double-strand breaks (DSBs). More than 250 DSBs are formed per meiotic cell, only a subset of which go on to produce crossovers that lead to chiasmata, whereas most form non-crossovers without the exchange of chromosome arms. Along the crossover pathway, the two ends of a DSB interact sequentially with a homologue through two joint molecule intermediates: single-end invasions (SEIs) and double-Holliday junctions (dHJs). dHJs are subsequently resolved to give mostly crossover products. The events that lead to non-crossovers are less clear, although evidence favours the synthesis-dependent strand-annealing mechanism. According to this model, one DSB-end invades a homologue and primes DNA synthesis. The nascent strand is then displaced and anneals to complementary sequences on the second DSB-end to seal the break.
Figure 2.
DNA events and cytological landmarks of meiotic recombination. The top row shows the classical stages of meiotic prophase I. The middle row shows the relative timeline of the molecular events of meiotic recombination based on physical assays in yeast, Saccharomyces cerevisiae (see main text for details). The bottom row shows the relative timeline of recombination markers in mouse spermatocytes. Each panel shows immunofluorescence images of spread meiotic chromosomes. The appearance and disappearance of immunostaining foci of recombination proteins indicate that the DNA pathway inferred in yeast also applies to mammals. Staining of the histone γ-H2AX and the recombinase Rad51 indicate that double-strand break (DSB) formation and the assembly of recombination complexes occurs during the leptotene stage. The disappearance of γ-H2AX and RAD51, and the formation of MutS homologue 4 (MSH4) foci indicate that strand invasion occurs during the zygotene or early pachytene stages. The appearance of crossover-specific MutL homologue 1 (MLH1) foci parallels the timing of the crossover-specific double-Holliday junction (dHJ) molecules. Crossover formation presumably follows during the late pachytene stage, before the appearance of chiasmata during the diplotene stage (shown in the final panel). Images provided courtesy of E. de Boer, Wageningen,the Netherlands.
Is the yeast pathway generally applicable to higher eukaryotes? The conservation of recombination proteins, their localization along meiotic chromosomes and the phenotypes of knockouts imply that this is the case. Most persuasively, the timing of the localization of recombination proteins in mice defines four molecular transitions, which parallel the DNA transitions that have been defined in yeast (Fig 2; Moens et al, 2002).
Chiasmata, cohesion and segregation. Several talks at the meeting highlighted the link between the number and location of crossovers, and the fidelity of chromosome segregation. Crossover failure carries a risk of aneuploidy (that is, the formation of gametes with abnormal numbers of chromosomes). Less obviously, homologues with a crossover that is located close to either the centromere or a telomere are also susceptible to missegregation (Koehler et al, 1996). Crossing over might cause a local disruption of sister-chromatid cohesion, which could lead to the premature separation of sister chromatids at the centromere or premature chiasma resolution at a telomere.
Support for the idea that recombination locally disrupts cohesion has been provided by images of diplotene chromosomes, which show that cohesion has been lost at the sites of chiasmata. Consistent with this, R. Jessberger (New York, NY, USA) showed that the cohesin REC8 is specifically absent from crossover sites (Eijpe et al, 2003). Local cohesion loss might relieve topological constraints on chromosomal exchange and/or chromosome condensation.
The maternal-age effect. Advancing maternal age is a second aneuploidy risk factor in human females; the aneuploidy level rises to ≥20% in older women and has an important effect on human fertility, miscarriage and birth defects. The nature of the maternal-age effect is unclear. One theory is that cohesion deteriorates over time. To test this, S. Bickel (Hanover, NH, USA) modelled the maternal-age effect in Drosophila (Jeffreys et al, 2003). In female flies that were deprived of protein-rich food and male company, oogenesis was arrested and the oocytes 'aged'. An age-dependent increase in missegregation was detected, but primarily when the chromosomes were achiasmate and cohesion was compromised. These data indicate that the normally efficient backup system for segregating achiasmate chromosomes in Drosophila deteriorates with age.
An achiasmate segregation system has also been found in yeast (Kemp et al, 2004), although the issue of whether there is a similar system in mammals remains contentious. The proteinaceous cores or axes of prophase chromosomes contain the synapto-nemal complex protein 3 (Scp3). In female mice that lack Scp3, approximately 30% of metaphase oocytes have achiasmate chromosomes and aneuploidy is increased. As in human females, aneuploidy increases with advancing maternal age in these mutant mice, but not in wild-type mice (Yuan et al, 2002). Perhaps, as in Drosophila and yeast, achiasmate chromosomes are segregated by a backup system some component of which deteriorates over time. If this is the case, the age-dependent phenomenon should not be peculiar to the Scp3−/− mouse; indeed, any mutation that gives rise to achiasmate chromosomes should cause an age-exacerbated increase in aneuploidy.
Recent studies have shown that prophase defects are common among women (M. Hulten, Coventry, UK). P. Hunt (Cleveland, OH, USA) showed that older oocytes often have disorganized meiosis I spindles and a failure of chromosome congression at the spindle equator. In mice, analogous defects and subsequent aneuploidy are caused by mutations that affect oocyte growth (Hodges et al, 2002). This supports the idea that advancing age alters the growth environment of maturing follicles and somehow causes congression failure.
Extrinsic risk factors for aneuploidy. Hunt also described the serendipitous discovery that the oestrogenic compound bisphenol A markedly induces aneuploidy in female mice (Hunt et al, 2003). A sudden rise in aneuploidy levels was traced to bisphenol A that had leached from damaged polycarbonate cages and drinking bottles. Interestingly, the main effect that is elicited by bisphenol A is congression failure, which is similar to that observed in older human oocytes. Therefore, extrinsic factors, such as exposure to bisphenol A, could exacerbate the intrinsic risk factors of altered recombination and advancing maternal age. The advice is to throw out your polycarbonate food and drink containers!
What is the link between the prenatal events of meiotic recombination and postnatal defects in chromosome congression? Failure to achieve a crossover and premature resolution of distal chiasmata both result in achiasmate chromosomes that might rely on a backup system for segregation. In Drosophila, this system is specifically disrupted by mutations that alter spindle morphology and chromosome movement along microtubules (Matthies et al, 1999). Therefore, human age-induced changes in spindle morphology and congression might somehow phenocopy Drosophila achiasmate segregation mutants. This proposal hinges on the assumption that mammals have an achiasmate segregation system. A rigorous test of this assumption is eagerly awaited.
Proteins that promote crossing over. In somatic cells, crossing over during DSB repair is generally suppressed. By contrast, meiotic cells must ensure that each chromosome pair has at least one crossover. The mechanism of this 'crossover assurance' is unclear; however, proteins that specifically promote the crossover outcome of recombination have been identified. For example, two homologues of the MutS family of DNA mismatch-recognition proteins, MSH4 and MSH5, have such a meiosis-specific role. C. Franklin (Birmingham, UK) presented an analysis of Arabidopsis msh4 mutants (Higgins et al, 2004). Similar to equivalent yeast and mouse mutants, synaptonemal complex (SC) formation and crossing over are defective in these plants. Interestingly, a residual ∼16% of crossovers forms independently of Msh4 and these events are randomly distributed among the chromosomes. These data are consistent with the proposal that there are two classes of meiotic crossovers: class I crossovers that have a regulated distribution to ensure that every pair of chromosomes obtains at least one chiasma, and class II crossovers that occur randomly, perhaps as the occasional outcome of the repair of DSBs that are not normally destined to become crossovers (Copenhaver et al, 2002; de los Santos et al, 2003; Borner et al, 2004).
The SC is the cytological hallmark of meiotic prophase. This proteinaceous structure forms between the lengths of paired homologues during the zygotene stage. SCs comprise two dense lateral elements that flank a central region, which contains a less dense central element. The lateral elements correspond to the rod-like homologue axes along which the chromatin of sister chromatids is organized. Transverse filaments (TFs) lie across the central region to create a zipper-like appearance. H. Cooke (Edinburgh, UK) described the identification of new SC components in the mouse. Synaptonemal complex protein 1 (SYCP1) is known to bridge the central region, and Cooke also introduced two new components, which are confined to the central element of the SC. These proteins might correspond to the pillars that form a ladder-like structure in the SC and could bolster its stability.
The Sycp1−/− mouse (C. Heyting, Wageningen, The Netherlands) is the first knockout of a mammalian TF protein. Sycp1−/− mice are sterile and males fail to produce sperm. The rare metaphase cells that do form have few chiasmata, which indicates that chiasmata formation is dependent on SYCP1. Beautiful immunostained images showed that chromosome pairing and the initiation of recombination occur normally in Sycp1−/− cells, but SCs are absent and progression to the later stages of recombination is defective. Together with studies in yeast, flies and worms, these data indicate a highly conserved function for SC in the maturation of crossover-designated DSBs (Hunter, 2003).
Three talks focused on the assembly and function of axial elements. T.-F. Wang (Taipei, Taiwan) showed that the yeast axial protein Red1 undergoes waves of phosphorylation and sumolation throughout meiotic prophase. He went on to present a general model for the role of these post-translational modifications in regulating the assembly/disassembly of macromolecular complexes along chromosomes. Jessberger suggested a role for the cohesin component, structural maintenance of chromosomes 1β (SMC1β), in limiting axial compaction. Chromosomes in Smc1β−/− cells make SCs that are 50% shorter than normal (Revenkova et al, 2004). This contrasts with data from Scp3−/− mice, the SCs of which are twice the length of those in the wild-type mice. Jessberger suggested that the structure imposed by SMC1b might limit the axial compaction function of SCP3. In addition, data presented by A. Kouznetsova (C. Hoog group, Stockholm, Sweden) suggest that the role of cohesins in prophase may be distinct from their function in sister-chromatid cohesion; in Scp3−/− mice, the cohesin axis is severely abnormal during prophase, but this does not lead to cohesion defects at later stages. The length and/or composition of axes is important for crossing over, as illustrated by the fact that both Smc1β−/− and Scp3−/− mice have crossover defects. Even in wild-type meiosis, axis length seems to determine the crossover frequency. Hulten showed that, despite having identical DNA content, chromosomes in oocytes have longer axes and SCs than those in spermatocytes (Tease & Hulten, 2004). Moreover, longer axes/SCs correlate with more crossing over. Two models have been proposed to explain this covariation (Kleckner et al, 2003): the first postulates that the number of DSBs is determined by axis length, whereas the second states that axis length determines the fraction of DSBs that will mature into crossovers. In the latter model, the number of DSBs per kilobase will not vary and so DSBs will be denser when the axes are shorter. Therefore, if crossover interference spreads over a fixed length of axis/SC, fewer crossovers will form when the axes are shorter (as is the case in males). Future studies should determine which of these two models is correct and identify the factors that modulate axis length.
Checkpoints. V. Boerner of the Kleckner group (Cambridge, MA, USA) emphasized the importance of distinguishing mutations that alter checkpoint monitoring from those that alter the process that is being monitored. He illustrated this point through analysis of the yeast pachytene checkpoint 2 (pch2) mutant, which suppresses the prophase arrest of cells that lack the SC zinc uptake transporter protein Zip1 (San-Segundo & Roeder, 1999). Pch2 is a member of the AAA-ATPase superfamily, the modes of action of which include the disruption or alteration of macromolecular assemblies. DNA physical assays were used to show that Pch2 alters the recombination process in a Zip1 background, which allows efficient DSB repair and restores some crossovers. Boerner suggested that Pch2 contributes to a regulatory barrier that prevents recombination in the absence of meiotic proteins, such as Zip1. By contrast, N. Bhalla of the Dernberg group (Berkeley, CA, USA) presented equally compelling evidence that Caenorhabditis elegans pch-2 is part of a checkpoint that monitors unsynapsed chromosomes. It is unclear how these two seemingly disparate conclusions will be reconciled.
Cell cycle and cohesion
Cohesin. F. Uhlmann (London, UK) opened the cell-cycle session by discussing cohesin, which is a complex of four proteins: the structural maintenance of chromosomes proteins SMC1 and SMC3, and the sister-chromatid cohesion proteins SCC1 and SCC3. According to the prevailing model, cohesin keeps sister chromatids together until the onset of the anaphase stage by forming a ring that captures two DNA duplexes. As pointed out by Jessberger, there could be at least four cohesin-like complexes in meiocytes: SMC1α-SMC3-RAD21-SA1/2; SMC1α-SMC3-REC8-STAG3; SMC1β-SMC3-REC8-STAG3; and an additional complex that is built on the SMC1β-SMC3 heterodimer. These complexes occur at different stages of meiosis and might have distinct functions, including chromosome-arm cohesion, centromeric cohesion and/or the support of meiotic recombination.
The separation of homologous chromosome pairs in meiosis I is known as homologue disjunction. In budding and fission yeasts, this process is dependent on the proteolytic cleavage of the cohesin subunit Rec8 by separase. Whether such a mechanism also operates during meiosis I of higher eukaryotes remains unclear. Observations that arm cohesin is released independently of separase during mitotic prophase, and that anaphase-promoting complex/cyclosome (APC/C) is dispensable in Xenopus meiosis I but not II, indicate that cohesin cleavage by separase might not be essential for vertebrate anaphase I onset. Conversely, recent data from mammals indicate that cyclin B and securin destruction are required, and that excess mitosis arrest-deficient 2 (MAD2) protein inhibits exit from meiosis I. This indicates that some aspects of the mechanism are conserved between yeast and mammals. To examine the role of REC8 cleavage in mammalian meiosis, N. Kudo of the Nasmyth group (Vienna, Austria) identified cleavage sites on mouse Rec8 and generated a transgenic mouse that expresses non-cleavable Rec8. He presented evidence that homologues non-disjoin during spermatogenesis of the transgenic mice. However, females expressing non-cleavable Rec8 were still fertile. Kudo also reported oocyte maturation in separase conditional-knockout mice. Again, he showed homologue non-disjunction during meiosis I, even though cyclin-dependent kinase (CDK) activity is reduced at anaphase I. Is it possible that males and females use different mechanisms to segregate their chromosomes? We look forward to the results of further analyses of these mice.
APC/C and cytostatic factor. F. Klein (Vienna, Austria) discussed a negative regulator of APC/Cama1 (a form of APC/C that functions in meiosis), which is known as mitotic nuclear division 2 (MND2). Deletion of MND2 leads to premature securin degradation and catastrophic prophase defects during budding yeast meiosis. In this situation, the Shugoshin protein, which protects centromeric cohesion, also seems to be destabilized. This indicates that Shugoshin degradation is promoted by APC/Cama1 during budding yeast meiosis.
Another negative regulator of the APC is early mitotic inhibitor 1 (Emi1). This was proposed to be part of the cytostatic factor (CSF) pathway in Xenopus, which ensures that egg development is arrested until fertilization takes place. P. Jackson (Stanford, CA, USA) reported that Emi1 is targeted for destruction by polo-like kinase 1 (Plk1), CDK1 and the β-transducin repeat-containing protein (β-TrCP) ubiquitin ligase, and that the Emi1 degron sequence EDSGVSSF is equivalent to the Plk1-interacting motif. Moreover, Plk1 is required for Emi1 destruction in egg extracts. K. Ohsumi of the Kishomoto group (Tokyo, Japan) showed that overexpressed Emi1 is an unstable protein; however, even when using a different Emi1 antibody, he could not detect endogenous Emi1 in metaphase II eggs. Jackson agreed that exogenous Emi1 is unstable, although he proposed the existence of a stabilizing factor that maintains endogenous Emi1 levels. He noted that there is a similar distinction between endogenous and exogenous protein stability for β-catenin, which is another β-TrCP substrate.
Preventing aneuploidy. Another theme of the meeting was the temporal pattern of destruction of cell cycle-associated proteins and its regulation by spindle-checkpoint proteins. J. Pines (Cambridge, UK) and C. Lehner (Bayreuth, Germany) discussed the temporal order of destruction. Pines reminded us that by inactivating the spindle checkpoint in HeLa cells, both cyclin B1 and securin are destroyed earlier and disappear at the time that cyclin A is normally destroyed. A similar situation occurs during female mammalian meiosis I. H. Homer from the groups of M. Herbert and A. McDougall (Newcastle, UK) showed that depletion of MAD2 and/or BubR1 advanced the timing of both cyclin B and securin destruction during meiosis I. This resulted in premature anaphase onset and increased aneuploidy in mouse oocytes. In addition, the hormonal environment of maturing oocytes could contribute to the maternal-age effect. Although it remains unclear why oocytes from human ovaries that are nearing the end of their reproductive life have markedly increased aneuploidy, the hormonal environment and falling levels of spindle-checkpoint proteins might contribute to this problem.
Meiotic gene expression
Gene-expression controls at the level of RNA are crucial for meiosis in animals. A principal regulator in mice is the RNA-binding protein Dazl. Targeted deletion of Dazl prevents mouse germ cells from progressing past the leptotene stage, which indicates that it regulates RNAs that encode proteins that are crucial for this process. The action of Dazl is probably of central importance to germ cells, as it is a member of an ancient gene family. The homologous Boule gene also regulates meiotic entry in Drosophila by controlling translation of an important cell-cycle regulator. In principle, translational control allows the coordinated regulation of many proteins, and the intriguing question remains as to which and how many RNAs might be regulated by Dazl. By analysing RNAs that were isolated by immunoprecipitating Dazl from pre-meiotic mouse testes using microarrays, and by similarly profiling changes in the RNA populations that were present in Dazl-knockout mice just before they showed a phenotype (day 7), N. Reynolds (Edinburgh, UK) identified the mouse homologue of the Drosophila gene Vasa, which is crucial for meiosis. The mouse Vasa transcript binds Dazl protein in vitro and contains sequences that are similar to previously identified optimal Dazl-binding sites. An important question raised by this analysis was one that is relevant to many researchers: how to interpret the mass of information obtained by microarray approaches.
Translational control is also crucial in females as meiosis is interrupted in prophase I, sometimes for many years. Many of the mechanisms that regulate re-entry into meiosis have been discovered in Xenopus laevis and involve the control of poly(A) tail length by cis-acting signals in the 3′ untranslated region of silenced RNAs, which are known as cytoplasmic polyadenylation elements (CPEs), and the CPE-binding protein (CPEB). CPEB acts as an on/off switch depending on its phosphorylation status. Before meiotic entry, unphosphorylated CPEB prevents translation by binding maskin, which masks the 5′ cap structure-initiation factor 4E (eIF4E) of the silenced messages from the ribosome. On meiotic re-entry, CPEB is phosphorylated and actively recruits cleavage and polyadenylation specificity factor (CPSF) and poly(A) polymerase, which elongate the poly(A) tails of the silent transcripts from less than 20 to more than 150 nucleotides. The binding of poly(A)-binding protein (PABP) in turn destabilizes the interaction of maskin with the cap structure and activates translation by allowing transcript association with polyribosomes. J. Richter (Worcester, MA, USA) discussed how his group have used co-immunoprecipitation and mass spectrometry to identify new CPEB-interacting proteins that are required for cytoplasmic polyadenylation. Crucially, one such protein is a Xenopus orthologue of a cytoplasmic poly(A) polymerase that is important in germ-cell development in C. elegans; it is presumably this protein that actively elongates the poly(A) tail of silenced messages, thereby activating them for translation.
A mysterious aspect of male meiotic gene expression is silencing of the X and Y chromosomes in a transcriptionally inactive structure that is called the XY body. Superficially, this is similar to dosage compensation in female cells, in which one of the X chromosomes is inactivated. X inactivation might not be established during early development, as was previously thought, but rather before birth in the XY body of the father. Meiotic sex-chromosome inactivation (MSCI) requires the phosphorylation of histone H2AX (J. Turner, London, UK) and the ubiquitylation of H2 (W. Baarends, Rotterdam, The Netherlands). These modifications affect the histone code that regulates the transcriptional availability of DNA by altering the chromosome structure. H2AX is phosphorylated to give γ-H2AX by the ATM and Rad3-related kinase (ATR), which is recruited to the XY body by the tumour suppressor breast cancer 1 (BRCA1). Surprisingly, all unsynapsed chromosomes or chromosome segments become inactivated during meiosis in male and female mice with sex chromosome aberrations. The silencing of unpaired DNA, as well as XY-body formation, resembles a phenomenon in fungi that is known as meiotic silencing by unpaired DNA.
In conclusion, this meeting brought together mitotic and meiotic cell-cycle and gene-expression researchers who have begun to dissect the cell-cycle regulation of meiosis. In the future, we can expect new information that relates to human health (for example, aneuploidy and its causes) to merge with important new data about the basic cell and molecular biology events that regulate meiotic division.



Acknowledgments
Many thanks go to the meeting organizers and particularly M. Herbert for her tireless efforts in organizing this captivating event.
References
- Borner GV, Kleckner N, Hunter N (2004) Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117: 29–45 [DOI] [PubMed] [Google Scholar]
- Copenhaver GP, Housworth EA, Stahl FW (2002) Crossover interference in Arabidopsis. Genetics 160: 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos T, Hunter N, Lee C, Larkin B, Loidl J, Hollingsworth NM (2003) The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C (2003) Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1β and SMC3. J Cell Biol 160: 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JD, Armstrong SJ, Franklin FC, Jones GH (2004) The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis. Genes Dev 18: 2557–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA (2002) Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod 17: 1171–1180 [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ (2003) Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13: 546–553 [DOI] [PubMed] [Google Scholar]
- Hunter N (2003) Synaptonemal complexities and commonalities. Mol Cell 12: 533–535 [DOI] [PubMed] [Google Scholar]
- Hunter N, Kleckner N (2001) The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106: 59–70 [DOI] [PubMed] [Google Scholar]
- Jeffreys CA, Burrage PS, Bickel SE (2003) A model system for increased meiotic nondisjunction in older oocytes. Curr Biol 13: 498–503 [DOI] [PubMed] [Google Scholar]
- Kemp B, Boumil RM, Stewart MN, Dawson DS (2004) A role for centromere pairing in meiotic chromosome segregation. Genes Dev 18: 1946–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N, Storlazzi A, Zickler D (2003) Coordinate variation in meiotic pachytene SC length and total crossover/chiasma frequency under conditions of constant DNA length. Trends Genet 19: 623–628 [DOI] [PubMed] [Google Scholar]
- Koehler KE, Hawley RS, Sherman S, Hassold T (1996) Recombination and nondisjunction in humans and flies. Hum Mol Genet 5: 1495–1504 [DOI] [PubMed] [Google Scholar]
- Matthies HJ, Messina LG, Namba R, Greer KJ, Walker MY, Hawley RS (1999) Mutations in the α-tubulin 67C gene specifically impair achiasmate segregation in Drosophila melanogaster. J Cell Biol 147: 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B (2002) The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA–DNA interactions without reciprocal recombination. J Cell Sci 115: 1611–1622 [DOI] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R (2004) Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 6: 555–562 [DOI] [PubMed] [Google Scholar]
- San-Segundo PA, Roeder GS (1999) Pch2 links chromatin silencing to meiotic checkpoint control. Cell 97: 313–324 [DOI] [PubMed] [Google Scholar]
- Tease C, Hulten MA (2004) Inter-sex variation in synaptonemal complex lengths largely determine the different recombination rates in male and female germ cells. Cytogenet Genome Res 107: 208–215 [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu JG, Hoja MR, Wilbertz J, Nordqvist K, Hoog C (2002) Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 296: 1115–1118 [DOI] [PubMed] [Google Scholar]