Abstract
Recently, a model of the flux of amino acids through transfer RNAs (tRNAs) and into protein has been developed. The model predicts that the charging level of different isoacceptors carrying the same amino acid respond very differently to variation in supply of the amino acid or of the rate of charging. It has also been shown that ribosome bypassing is specifically stimulated at ‘hungry' codons calling for an aminoacyl-tRNA in short supply. We have constructed two reporters of bypassing, which differ only in the identity of the serine codon subjected to starvation. The stimulation of bypassing as a function of starvation differed greatly between the two serine codons, in good agreement with the quantitative predictions of the model.
Keywords: amino acid, bypassing, charging, protein synthesis, tRNA
Introduction
Elf et al (2003) have developed a theoretical model of the response to amino-acid limitation of the different members (isoacceptors) of each family of transfer RNAs (tRNAs) that carry the same amino acid. The theory rests on considerations of supply and demand. The parameters of supply are the rate of synthesis of the limiting amino acid and the abundance of each isoacceptor tRNA in question; the parameters of demand are the frequencies with which the codons read by a given isoacceptor are found in translating ribosomes, as well as the overall capacity of ribosomes to consume the amino acid. The model leads to the counterintuitive prediction that amino-acid limitation can elicit very different degrees of residual aminoacyl charging among the different isoacceptors. Those that have a high concentration relative to the frequency of their cognate codons in messenger RNAs will retain a much higher level of charging than those in which the ratio is lower. We report here a test of this prediction in the case of two serine-tRNA isoacceptors, using ribosome bypassing as a reflection of the starvation experienced by codons calling for the two different isoacceptors.
Results And Discussion
It has been demonstrated elsewhere that ‘hungry' codons calling for a tRNA, the acylated form of which is in short supply, are the sites of reading frame errors (Gallant & Lindsley, 1992; Peter et al, 1992; Lindsley & Gallant, 1993; Barak et al, 1996; Gallant et al, 2000) and ribosome bypassing (Gallant & Lindsley, 1998; Gallant et al, 2003; Lindsley et al, 2003). Furthermore, minigene experiments have indicated that it is the absence of the aminoacylated tRNA, rather than any positive effect of the deacylated species, that gives rise to these ribosome errors (Lindsley et al, 2003). Therefore, the stimulation of either one of these ribosome gymnastic feats at a hungry codon should reflect the reduced charging level of that codon's cognate tRNA, an analytic approach that has been introduced already in the case of isoleucine tRNAs (Kaplun et al, 2002; Elf et al, 2003).
To test the model of selective tRNA charging, we have constructed reporters of bypassing, which differ only at the serine codon subjected to starvation. The reporter is a plasmid-borne, modified lacZ gene in which a bypass from a ‘take-off site' to a synonymous ‘landing site' 16 nucleotides downstream is the only way in which the ribosome can leave its initial, incorrect reading frame, avoid blocking terminators and access the β-galactosidase reading frame in a single event, and thus at a detectable frequency (Gallant & Lindsley, 1998; Lindsley et al, 2003). The serine codon is placed immediately after the take-off site codon; when the cognate seryl-tRNA is deacylated, the consequent ribosome pause increases the probability that the peptidyl-tRNA in the P-site will bypass from its take-off site, and its capture at the synonymous landing site results in synthesis of active β-galactosidase (Gallant & Lindsley, 1998; Gallant et al, 2003, 2004). In one reporter, the serine codon was UCC, which is read only by tRNA-Ser 5, an isoacceptor that the model predicts will be severely discharged (supplementary information online and see Elf et al, 2003). The other reporter had AGC, read by tRNA-Ser 3, which should retain a much higher charging level (supplementary information online and see Elf et al, 2003). The model predicts, therefore, that the same degree of inhibition of serine-tRNA synthetase should stimulate bypassing much more in the UCC construct than in the AGC construct. The charging of all serine-tRNA isoacceptors was inhibited with the analogue serine-hydroxamate (SHX; Tosa & Pizer, 1971; Pizer & Merlie, 1973). The prediction was confirmed by a qualitative, visual test of the analogue's effect on β-galactosidase synthesis encoded by the two reporters (Fig 1).
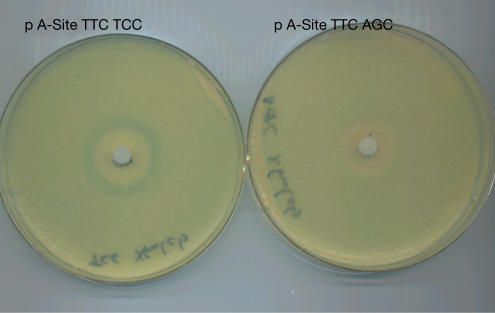
Figure 1.
Visualization of bypassing in response to SHX inhibition. Overnight cultures of host cells carrying the two plasmids were pour-plated in soft agar (about 2 × 108 cells) on M63-glucose minimal plates containing 40 μg/ml of X-GAL. After the agar hardened, filter discs containing 0.6 mg SHX were placed in the centres of the plates, which were then incubated at 37°C for about 72 h. Note the identical clear zones of growth inhibition due to SHX; note the blue ring, indicating β-galactosidase synthesis, in the region of partial growth inhibition in the plate of the TCC construct, and the absence of a blue ring in the plate of the AGC construct.
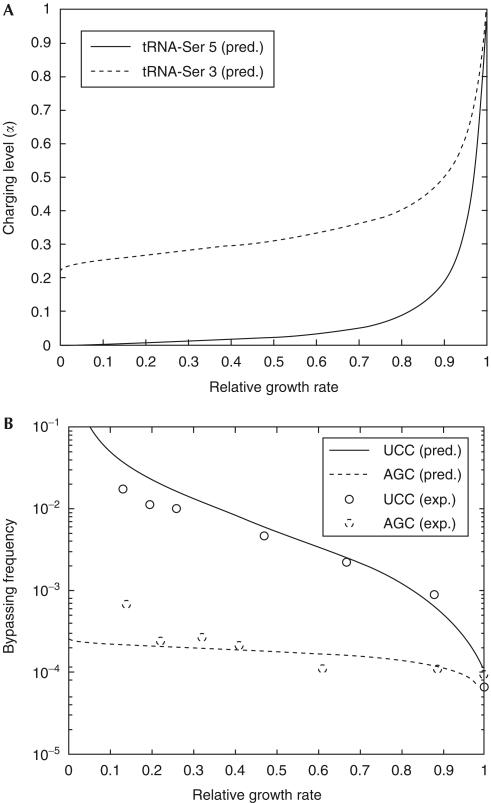
Quantitative results are shown in Table 1. First, we note that constructs with UCC and AGC in the position just after the take-off site support bypassing at about the same frequency in growing, uninhibited cells. This result argues that the bypass event per se is fairly unaffected by the nature of the serine codon. However, the effects of SHX on bypassing in the two reporters differed markedly. At every point of the curve, SHX stimulated bypassing much more at the UCC codon than at the AGC codon. Fig 2B compares the observed bypassing results with the quantitative predictions of the model. It can be seen that the agreement is very good for both codons, which is to say for both tRNAs. Conversely, the results are grossly inconsistent with any model that requires a uniform decline in the charging levels of synonymous isoacceptors during limitation.
Table 1.
Growth and β-galactosidase synthesis as a function of SHX concentration
| SHX (μg/ml) | lacZ+ DR of β-galactosidase synthesis (× 10−3) |
UCC |
AGC |
||||
|---|---|---|---|---|---|---|---|
| GR | DR | BP frequency (× 104) | GR | DR | BP frequency (× 104) | ||
| 0 |
925 |
1 |
60 |
0.65 |
1 |
85 |
0.92 |
| 27 |
906 |
0.88 |
796 |
8.8 |
0.89 |
103 |
1.1 |
| 40 |
570 |
0.67 |
1,276 |
22 |
0.61 |
64 |
1.1 |
| 50 |
304 |
0.47 |
1,428 |
47 |
0.41 |
64 |
2.1 |
| 60 |
140 |
0.26 |
1,408 |
101 |
0.31 |
38 |
2.7 |
| 72 |
91 |
0.195 |
1,060 |
116 |
0.22 |
22 |
2.4 |
| 100 | 39 | 0.13 | 691 | 177 | 0.14 | 26 | 6.7 |
DR is the differential rate of β-galactosidase synthesis in mEU/mg protein, over the one mass doubling with the lac promoter induced. GR is the growth rate relative to the uninhibited culture with no SHX, which typically had a doubling time of about 70 min. Bypassing (BP) frequency is the differential rate of the bypass reporter at each SHX concentration divided by that in control cells of the same host strain carrying the corresponding lacZ+ gene on the plasmid.
Figure 2.
Predicted charging levels of tRNAs, and predicted and observed bypassing in response to SHX inhibition. (A) Selective charging of Ser 3 and Ser 5 isoacceptors under different degrees of serine limitation (supplementary information online). (B) Experimentally determined (circles) and predicted (lines) bypassing frequencies at different growth rates. The predictions are on the basis of predicted selective charging levels in (A) (lines) and the model that fbp=rbp/(rbp+(kcat/Km)αt0i), where fbp is the bypassing frequency, t0i is the total concentration of the isoacceptor (i=3 or 5) and rbp=1.33 × 10−3 s−1.
Of course, our assay is less direct than would be a chemical measurement of the aminoacyl-tRNAs concerned. However, it has the virtue that it reflects directly the response of the translation system at hungry codons, which is why the model has general biological implications. The extremely different responses of our two bypassing reporters to inhibition of serine-tRNA synthetase illustrates the general implication of selective tRNA charging: namely, that reduced aminoacyl-tRNA supply has very different consequences at synonymous codons cognate to different isoacceptors. Bypassing is only one of the alternatives to normal translation that can occur at a hungry codon. The others include frameshifting, missense errors and erroneous peptide chain termination (Kurland, 1992; Parker, 1992; Kurland & Gallant, 1996). Clearly, the selective pressures that give rise to codon biases will be affected by the sensitivity with which individual synonymous codons experience aminoacyl-tRNA limitation, and are hence exposed to the costs of these various types of error.
Methods
Bypass reporter construction, plasmid manipulation, and methods of bacterial cultivation and β-galactosidase assay were all as described previously (Gallant & Lindsley, 1998; Gallant et al, 2003). The relevant sequence of the coding strand of the bypass reporter is ATG…TTC [TCC or AGC] ATC TAG C TAA TTT → → lacZ coding sequence, where both the take-off and landing sites are in bold type, and the landing site is underlined; the terminators blocking the outgoing and incoming reading frames are in italic type; and the hungry codon after the take-off site is in brackets. The corresponding sequence in the 0-frame lacZ+ control was ATG…TTC TCC GTC TAC CAG TTC → → lacZ coding sequence, and is identical to the bypass reporters everywhere else. The 0-frame control thus has a serine codon after the TTC take-off triplet. It differs slightly from the bypass reporters only in the absence of blocking terminators and retention of the starting reading frame into the lacZ coding sequence. Reporter plasmids were transformed into our standard Escherichia coli K12 host C92 (relA1 spoT) carrying the O6 deletion in lacZ. The lac promoter was induced by addition of 2 mM isopropyl-β-D-thiogalactopyranoside and 2.5 mM cyclic adenosine 3′-5′-monophosphate. Host cells carrying the indicated plasmid were cultivated in M63-glucose minimal medium. Exponential cultures were induced and divided into subcultures: one was allowed to grow exponentially and the others were inhibited by various concentrations of SHX. Samples were harvested for assay after one mass doubling. (Control samples that were harvested before induction had essentially zero activity.)
The predicted frequency of bypassing. The hydroxamate treatment reduces the growth rate to different levels by serine limitation. Fig 2A shows how the theoretically predicted aminoacylated fractions α3 and α5 of the serine isoacceptors 3 and 5, respectively, depend on the relative growth rate (supplementary information online). The rate rcog of accommodating seryl-tRNA3 when reading the cognate AGC and seryl-tRNA5 when reading the cognate UCC is modelled as rcog=(kcat/Km)(t3α3) and rcog=(kcat/Km)(t5α5), respectively. The total concentrations of tRNA-Ser 3 and tRNA-Ser 5 are taken to be t3=2.3 μM and t5=1.3 μM, respectively (Elf et al, 2003). kcat/Km is the association rate constant for the binding of a ternary complex (EF-Tu·GTP·aminoacyl-tRNA) containing either Ser 3 or Ser 5 to the A site of the ribosome multiplied with the probability that binding leads to peptidyl transfer.
The predicted frequency fbp of bypassing at a hungry codon is the ratio between the intrinsic rate rbp of bypassing and the sum of rbp and rcog, that is, fbp=rbp/(rbp+rcog). Experimental data were used to estimate the parameter rbp to be 1.33 × 10−3 s−1, which was used together with theoretical predictions of charged fractions of tRNA-Ser 3 and tRNA-Ser 5 (supplementary information online; Elf et al, 2003) to obtain the predicted bypassing frequencies, which are compared with the experimentally measured frequencies in Fig 2B.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v6/n2/extref/7400332s1.doc).
Note added in proof. A parallel test of the selective charging model, based on direct tRNA measurements for different amino-acid families to those tested here, is reported in Dittmar et al, this issue (doi:10.1038/sj.embor.7400341).
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by grant R01 13626 from the National Institutes of Health and the Swedish Research Council.
References
- Barak Z, Lindsley D, Gallant J (1996) On the mechanism of leftward frameshifting at several hungry codons. J Mol Biol 256: 676–684 [DOI] [PubMed] [Google Scholar]
- Elf J, Nilsson D, Tenson T, Ehrenberg M (2003) Selective charging of tRNA isoacceptors explains patterns of codon usage. Science 300: 1718–1722 [DOI] [PubMed] [Google Scholar]
- Gallant JA, Lindsley D (1992) Leftward ribosome frameshifting at a hungry codon. J Mol Biol 223: 31–40 [DOI] [PubMed] [Google Scholar]
- Gallant JA, Lindsley D (1998) Ribosomes can slide over and beyond ‘hungry' codons, resuming protein chain elongation many nucleotides downstream. Proc Natl Acad Sci USA 95: 13771–13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J, Lindsley D, Masucci JP (2000) The unbearable lightness of peptidyl-tRNA. In The Ribosome: Structure, Function, Antibiotics, and Cellular Interactions, Garrett RA, Douthewaite SR, Liljas A, Matheson AT, Moore PB, Noller HF (eds) pp 385–396. Washington, DC: ASM Press [Google Scholar]
- Gallant J, Bonthuis P, Lindsley D (2003) Evidence that the bypassing ribosome travels through the coding gap. Proc Natl Acad Sci USA 100: 13430–13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J et al. (2004) On the role of the starved codon and the takeoff site in ribosome bypassing in Escherichia coli. J Mol Biol 342: 713–724 [DOI] [PubMed] [Google Scholar]
- Kaplun A, Chipman DM, Barak Z (2002) Isoleucine starvation caused by sulfometuron methyl in Salmonella typhimurium measured by translational frameshifting. Microbiology 148: 713–717 [DOI] [PubMed] [Google Scholar]
- Kurland CG (1992) Translational accuracy and the fitness of bacteria. Annu Rev Genet 26: 29–50 [DOI] [PubMed] [Google Scholar]
- Kurland C, Gallant J (1996) Errors of heterologous protein expression. Curr Opin Biotechnol 7: 489–493 [DOI] [PubMed] [Google Scholar]
- Lindsley D, Gallant J (1993) On the directional specificity of ribosome frameshifting at a ‘hungry' codon. Proc Natl Acad Sci USA 90: 5469–5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D, Gallant J, Guarneros G (2003) Ribosome bypassing elicited by tRNA depletion. Mol Microbiol 48: 1267–1274 [DOI] [PubMed] [Google Scholar]
- Parker J (1992) Variations in reading the genetic code. In Transfer RNA in Protein Synthesis, Hatfield DL, Lee BJ, Pirtle RM (eds) pp 191–267. Boca Raton, FL: CRC Press [Google Scholar]
- Peter K, Lindsley D, Peng L, Gallant JA (1992) Context rules of rightward overlapping reading. New Biol 4: 520–526 [PubMed] [Google Scholar]
- Pizer LI, Merlie JP (1973) Effect of serine hydroxamate on phospholipid synthesis in Escherichia coli. J Bacteriol 114: 980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T, Pizer LI (1971) Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol 106: 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information