Summary
Reactive oxygen species connect nuclear factor-κB and c-Jun amino-terminal kinase
Keywords: apoptosis, c-Jun amino-terminal kinase, JNK, necrosis, NF-κB, reactive oxygen species (ROS)
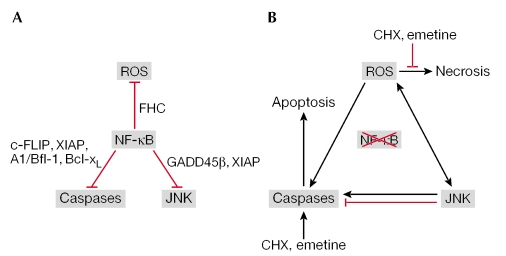
Numerous studies have shown that nuclear factor-κB (NF-κB) inhibits various types of cell death by upregulating anti-apoptotic genes, such as cellular FADD-like interleukin-1-converting enzyme (FLICE)-inhibitory protein (c-FLIP), X-chromosome-linked inhibitor of apoptosis (XIAP), A1 (also known as Bfl-1) and B-cell leukaemia/lymphoma-XL (Bcl-XL). However, the biological role of c-Jun amino-terminal kinase (JNK) activation in cell death remains unclear (Karin & Lin, 2002). Generally, transient JNK activation promotes cell survival, whereas sustained JNK activation promotes cell death. Although, until recently, these signalling cascades were usually discussed independently, two reports have described intimate crosstalk between the JNK and NF-κB signalling pathways (De Smaele et al, 2001; Tang et al, 2001). Tumour necrosis factor-α (TNF-α)-induced activation of JNK is prolonged in cells that are deficient in NF-κB activation, such as RelA- and IκB kinase-β (IKKβ)-knockout murine embryonic fibroblasts (MEFs). Consistent with previous findings (Chen et al, 1996), prolonged JNK activation in these cells promotes apoptosis. Together, these studies have shown that one of the anti-apoptotic functions of NF-κB is to downregulate JNK activation by upregulating growth arrest and DNA-damage-inducing protein-β (GADD45-β) or XIAP. A subsequent study reported that reactive oxygen species (ROS) have an essential role in mediating long-lasting JNK activation and necrotic cell death in NF-κB activation-deficient cells (Sakon et al, 2003; Nakano, 2004). Given that enhanced ROS accumulation by TNF-α is not observed in wild-type cells (Sakon et al, 2003; Ventura et al, 2004), it seems that NF-κB suppresses TNF-α-induced ROS accumulation under normal conditions (Fig 1). Although this function is thought to be mediated by upregulating antioxidant enzymes, the precise mechanism has not yet been clarified (Sakon et al, 2003).
Figure 1.
A model for nuclear factor-κB (NF-κB)-dependent survival signals. (A) Under normal conditions, NF-κB inhibits potentially apoptotic and necrotic signalling cascades, caspases that are induced by tumour necrosis factor-α (TNF-α), c-Jun amino-terminal kinase (JNK) and reactive oxygen species (ROS). (B) Under conditions in which NF-κB activation is blocked, three pro-apoptotic and necrotic signalling cascades are activated and amplify each other, which results in apoptosis or necrosis in a context-dependent manner. In the presence of a protein-synthesis inhibitor, such as cycloheximide (CHX) or emetine, TNF-α preferentially induces apoptosis. The black and red lines indicate positive and negative regulation, respectively.
Recently, two groups reported an essential role of ROS in long-lasting JNK stimulation in NF-κB activation-deficient cells (Pham et al, 2004; Ventura et al, 2004). After several rounds of transfection of cDNA libraries into RelA-knockout MEFs, followed by the isolation of transfected cDNAs from surviving cells in the presence of TNF-α, Pham and colleagues found that several antioxidant enzymes were enriched in the recovered cDNAs compared with the original cDNA libraries (Pham et al, 2004). Among these, expression of the ferritin heavy chain (fhc) gene was induced in a RelA-dependent fashion. FHC is one of two subunits of ferritin, which is a heteropolymer that also has light chains (FLCs). Importantly, the ectopic expression of FHC suppressed TNF-α-induced apoptosis, as well as ROS accumulation, in RelA-knockout MEFs. These results showed that the iron-binding activity of FHC was required for the elimination of ROS, and that the knockdown of endogenous FHC using small-interfering RNA (siRNA) rendered NIH3T3 cells sensitive to TNF-α-induced apoptosis.
By contrast, Ventura and colleagues (2004) reported on the anti- and pro-apoptotic roles of JNK in TNF-α-induced cell death under different experimental conditions. When cells were treated with TNF-α in the presence of the protein-synthesis inhibitor emetine, the sensitivity to TNF-α-induced apoptosis was greatly enhanced in JNK1/JNK2-double-knockout (the term JNK-knockout is used hereafter) cells compared with wild-type controls. This result, along with the findings of a previous study (Lamb et al, 2003), indicates that JNK protects cells from apoptosis under these experimental conditions. Interestingly, TNF-α stimulation alone induced ROS-dependent necrotic cell death in wild-type MEFs that stably expressed degradation-resistant IκBα (ΔN-IκBα). Surprisingly, TNF-α stimulation did not induce ROS accumulation or necrosis in JNK-knockout cells that expressed ΔN-IκBα, which points to essential roles for JNK in TNF-α-induced ROS accumulation and necrotic cell death.
Although these two studies, along with previous work (Tobiume et al, 2001; Sakon et al, 2003), highlighted the crucial contribution of ROS to TNF-α-induced, sustained JNK activation, several apparent inconsistencies should be considered. For example, it is unclear whether the activation of JNK occurs upstream or downstream of ROS accumulation. Pham et al (2004), along with a previous study (Sakon et al, 2003), reported that pretreatment with antioxidants inhibited TNF-α-induced delayed and long-lasting, but not early and transient, JNK activation, whereas pretreatment with the JNK inhibitor SP600125 did not inhibit TNF-α-induced ROS accumulation (H. Nakano et al, unpublished data); this indicates that ROS function upstream of JNK. However, these results seem to conflict with the finding that TNF-α-induced ROS accumulation was abolished in JNK-knockout cells that expressed ΔN-IκBα (Ventura et al, 2004). One possible explanation for this discrepancy is that the residual JNK activity, even in the presence of JNK inhibitor, might induce small amounts of ROS that can activate JNK, thereby resulting in the further enhancement of ROS accumulation.
Another controversial issue is whether the accumulation of ROS induces cell death through apoptosis or necrosis (Pham et al, 2004; Ventura et al, 2004). Protein-synthesis inhibitors, such as cycloheximide (CHX) and emetine, which are usually required for TNF-α to induce cell death in wild-type cells, might affect the fate of the TNF-α-treated cells and determine whether they die from apoptosis or necrosis. Indeed, TNF-α-induced necrotic cell death was only observed in cells that were stimulated with TNF-α alone (Ventura et al, 2004), and not in those stimulated with TNF-α plus CHX or emetine (Pham et al, 2004; Ventura et al, 2004). This implies that TNF-α-induced apoptosis might prevail over necrosis in the presence of protein-synthesis inhibitors (Fig 1).
These recent advances in the NF-κB and JNK fields clearly illustrate a newly discovered and important function of NF-κB: the inhibition of ROS accumulation through the upregulation of antioxidant enzymes, such as FHC. However, these studies have also raised important questions that will need to be addressed through further experiments. For example, the mechanism by which ROS induce long-lasting JNK activation remains to be identified. ROS might inactivate mitogen-activated protein (MAP) kinase phosphatases that normally suppress JNK activation, which would result in long-lasting JNK activation. However, taking into account the fact that TNF-α-induced sustained JNK activation was abolished in MAP kinase kinase 4 (MKK4)/MKK7-double-knockout MEFs (Ventura et al, 2004), MAP kinase kinase kinases (MAPKKKs), such as apoptosis-signal-regulating kinase 1 (ASK1), might respond to ROS and activate MKK4 or MKK7, which would result in the activation of JNK. However, the molecular mechanism by which TNF-α induces ROS accumulation is largely unknown. Further studies will therefore be necessary to establish whether ROS are upstream or downstream targets of JNK. Moreover, it is unclear how ROS induce necrosis under specific conditions. Therefore, future studies should focus on identifying the mechanism of ROS-dependent necrosis, as well as JNK activation, to allow the manipulation of various pathological conditions in which ROS are involved.

References
- Chen YR, Wang X, Templeton D, Davis RJ, Tan TH (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet X and γ radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem 271: 31929–31936 [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G (2001) Induction of gadd45b by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414: 308–313 [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A (2002) NF-κB at the crossroads of life and death. Nat Immunol 3: 221–227 [DOI] [PubMed] [Google Scholar]
- Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ (2003) JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell 11: 1479–1489 [DOI] [PubMed] [Google Scholar]
- Nakano H (2004) Signaling crosstalk between NF-κB and JNK. Trends Immunol 25: 402–405 [DOI] [PubMed] [Google Scholar]
- Pham CG et al. (2004) Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 119: 529–542 [DOI] [PubMed] [Google Scholar]
- Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H (2003) NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22: 3898–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A (2001) Inhibition of JNK activation through NF-κB target genes. Nature 414: 313–317 [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2: 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS Jr, Davis RJ (2004) JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev 18: 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]