Summary
Meeting on Steroid Hormone Receptors
Keywords: kinase cascades, membrane, non-genomic, nuclear receptor, steroid receptor
Introduction
This unifying conference provided new insights into the connections between the membrane and nuclear cellular actions of the main classes of steroid hormone receptors (progesterone, oestrogen, androgen, mineralocorticoid and glucocorticoid), and other important members of the nuclear receptor superfamily (receptors for thyroid hormone (TH) and vitamin D). Speakers at the conference addressed important questions about the characteristics and actions of steroid membrane receptors, many of which remain unresolved. The topics ranged from the cell biology of sex steroids in hormonally responsive cancers to new aspects of steroid receptor (SR) signalling at non-classical sites of action. Here, we present highlights from the meeting that address 'burning questions' about the integration of membrane- and nuclear-receptor-initiated signalling. We concentrate primarily on new information about the characteristics of membrane SRs, their relationship to their nuclear counterparts and new experimental approaches for addressing these issues.
The 2004 Federation of American Societies for Experimental Biology (FASEB) Summer Research Conference on 'Steroid Hormone Receptors: Integration of Plasma Membrane- and Nuclear-Initiated Signalling in Hormone Action' took place from 31 July to 5 August, 2004, in Tucson, Arizona, USA, and was organized by R. Pietras, E. Levin and M. Farach-Carson
What are the general features of membrane SR actions?
Almost 30 years ago, Richard Pietras and Clara Szego reported that cell-surface forms of oestrogen receptors (ERs) were coupled to the rapid activation of intracellular signalling pathways (Pietras & Szego, 1975, 1977); this finding 'rocked the boat' in a field that was dominated by the study of transcription. Since that time, some general concepts that characterize the so-called membrane-initiated or rapid actions of SRs have emerged. These actions occur on a timescale of milliseconds to minutes and alter the levels or activities of signalling molecules, such as lipids, ions, enzymes or protein complexes. They are therefore separable from the transcriptional activities of their classically defined (nuclear) receptors, although they can eventually culminate in genomic events. Many groups have reported a minority (approximately 5–10%) plasma membrane SR population, the members of which are often associated with lipid rafts or caveolae and are known to be sites that are enriched for signalling molecules.
E. Levin (Irvine, CA, USA) noted that caveolin-1 association can facilitate classical ER transport to the membrane, but cannot explain all membrane ER localization (such as ER localized to the mitochondrial membrane). Similarly, A. Norman (Riverside, CA, USA) characterized the membrane vitamin D receptor (VDR) as the classical VDR that is found in caveolar membrane fractions and in close association with caveolin 1 in several tissues from numerous species. However, I. Nemere (Logan, UT, USA) and M. Farach-Carson (Newark, DE, USA) described a distinct membrane vitamin D3-binding protein in chicken and rat intestinal epithelial cells, which is known as membrane-associated rapid response to steroids (1,25D3-MARRS) protein. It contains phosphorylation sites for numerous protein kinases and interaction (Rel homology) domains. MARRS is myristoylated and might function as a membrane-associated scaffold molecule that couples hormone binding to numerous rapid signalling events, including Ca2+ channel activity, phosphate uptake, and the activation of protein kinase C (PKC) and protein kinase A (PKA). By contrast, M. Freeman (Cambridge, MA, USA) described the ligand-dependent movement of androgen receptors (ARs) into caveolin-1-negative cholesterol-rich lipid rafts (flat rafts), where they associate with protein kinase B (PKB/AKT) in prostate cancer cells.
Protein associations and signalling events that are elicited by ligand-bound SRs at the cell surface include the coupling of SRs to G proteins and/or other signalling molecules, such as adaptor molecules or scaffold proteins, the liberation of second messengers, the direct and indirect activation of diverse protein and lipid kinases (including the cross-activation of tyrosine kinase growth-factor receptors), and the regulation of ion channels and co-transporters or pumps. Although the details of how SRs mediate specific signalling events are largely unknown, new mechanistic data from several speakers showed that SRs often seem to act by direct association with kinases or adaptor molecules to mediate downstream phosphatidylinositol 3-kinase (PI3K) or mitogen-activated protein kinase (MAPK) activation. For example, Levin described the ramifications of coupling between membrane ER dimers and G proteins. In breast cancer cells, G-protein activation leads to the cross-activation of epidermal-growth-factor receptor (EGFR) by the c-SRC-dependent activation of matrix metalloproteinases (MMPs) and the liberation of heparin-bound EGF. The activation of several kinases, including PI3K and extracellular-signal-regulated kinases (ERKs), by EGFR mediates changes in gene expression and cell behaviours, such as migration, survival and proliferation. In vascular and cardiac models, oestradiol mediates vasodilation and prevents cardiac hypertrophy by PI3K-dependent mechanisms. J. Bender (New Haven, CT, USA) showed membrane localization of the 46K splice variant of ER into caveolae in human endothelial cells, which required the open structure of c-SRC and occurred in an oestrogen-induced palmitoylation-dependent manner. In this system, oestradiol-mediated nitric oxide (NO) release is also c-SRC-dependent, and occurs through a PI3K/AKT/endothelial nitric-oxide synthase (eNOS) pathway. Similarly, P. Shaul (Dallas, TX, USA) showed that c-SRC and PI3K associate with ER-α upon ligand activation to stimulate eNOS, whereas the nuclear-localization signals and the DNA-binding domain of the receptor are required for c-SRC activation and PI3K interaction, respectively. D. Edwards (Denver, CO, USA) defined an independent PXXPXR domain in the amino-terminus of the human progesterone receptor (PR) that interacts directly with the Src homology 3 (SH3) domain of c-SRC to mediate the rapid activation of ERKs in breast epithelial cells. Similarly, S. Hammes (Dallas, TX, USA) showed that testosterone induces the release of frog and mouse oocytes from meiotic arrest through the AR-dependent activation of MAPKs. However, in this case, c-SRC-independent activation overcomes meiotic arrest by constitutive Gβγ-mediated signals and is assisted by the adaptor protein MNAR (modulator of non-genomic activity of ER) through as-yet-unknown mechanisms.
A great deal of experimentation and discussion still surrounds the identity of membrane (or at least non-nuclear) SRs. In addition to the diverse receptor proteins described above, P. Thomas (Port Aransas, TX, USA) described a large family of non-classical SRs. These G-protein-coupled serpentine receptors, which include those for many steroid classes, have mammalian homologues; this implies a wider functional applicability beyond their original description for the reproductive readiness of fish gametes. Along with the MARRS protein, these fascinating examples of alternative steroid-binding proteins are a reminder that steroids bind with high affinity to numerous classes of protein. This raises the issue of the detailed subtasks that are involved in the continuum of steroid actions, which might begin at the membrane and end in the nucleus. Even transmitting the initial signal (hormone) from blood-borne binding proteins to, and across, the membrane might involve different steroid-binding subtypes that act in a sequence that remains unknown. Alternatively, these diverse steroid-binding proteins might provide the mechanisms through which steroid actions branch off into different signalling cascades that end in different functions.
The role of MAPK activation in nongenomic steroid signals?
The hormonal activation of MAPKs is a common theme in understanding the cell proliferation- and survival-promoting effects of SRs. E. Faivre (Minneapolis, MN, USA) showed that liganded PR stimulates oscillations in MAPK activity in breast cancer cells through the c-SRC-dependent activation of MMPs, which leads to the cross-activation of EGFR. The activation of MAPK is independent of PR transcriptional activity, but requires both transcription by other factors and translation; this provides an example of how rapid PR signalling can interdigitate with genomic responses. The cell-growth-promoting effects of progestins are also MAPK-dependent, but are independent of PR transcriptional activity (C. Lange, Minneapolis, MN, USA). On a similar theme, R. Santen (Charlottesville, VA, USA) showed that long-term oestrogen-deprived (LTED) breast cancer cells markedly upregulate ER-α and show increased oestrogen-induced cell growth without apparent changes in gene expression. In these oestrogen-hypersensitive cells, oestradiol co-opts growth-factor-signalling pathways by promoting the direct binding of ER to the SHC adaptor protein and insulin-like growth factor receptor (IGFR) or EGFR, which leads to activation of the ERK cascade and interaction with the p85 subunit of PI3K to initiate AKT signalling. Functionally, this leads to growth and anti-apoptotic effects and is a model of adaptive hypersensitivity that has been observed clinically in breast cancer patients.
The distinctions between the membrane and nuclear actions of steroids became more blurred as further examples of the downstream targets of hormone-activated MAPKs were presented during the meeting. For example, P. Davis (Albany, NY, USA) showed that thyroxine activation of MAPKs leads to an association with, the serine phosphorylation of and the nuclear accumulation of, several components of the multiprotein transcription complexes that are known as enhanceosomes, which include signal transducer and activator of transcription 1 (STAT1) and STAT3, thyroid hormone receptor (TR)-β1, ER-α, p53, retinoid X receptor (RXR) and co-activators. Enhanceosomes are thought to help uncoil nucleosomal DNA and facilitate transcription. Davis noted that as TH levels are constant in vivo, membrane TRs might act to 'set' basal MAPK activity levels and so facilitate the nucleation of pre-enhanceosomes. It therefore seems that the endpoint of membrane SR actions is often regulation of their nuclear actions. On a similar theme, E. O'Neill (Chicago, IL, USA) showed that Ca2+/calmodulin-dependent protein kinase II directly associates with liganded ER-α and its kinase activity is required for ER transcriptional activity. Similarly, R. Peitras (Los Angeles, CA, USA) and N. Weigel (Houston, TX, USA) showed that the same rapidly activated kinases (c-ERBB2, cyclin-dependent protein kinase 2 (CDK2) and MAPKs) can mediate the phosphorylation of SR co-activators for ER (SRC3/AIB1) and PR (SRC1), and thereby regulate their nuclear accumulation and activities. Weigel noted that PR transcriptional activity is highest during the S phase of the cell cycle, at a time when SRC1 and PR are nuclear and heavily phosphorylated by CDK2. Lange also showed a positive regulatory role for specific MAPK and CDK2 phosphorylation sites on human PR. Therefore, SRs initiate rapid signalling responses, which sometimes lead to phosphorylation events that culminate in altered transcriptional regulation.
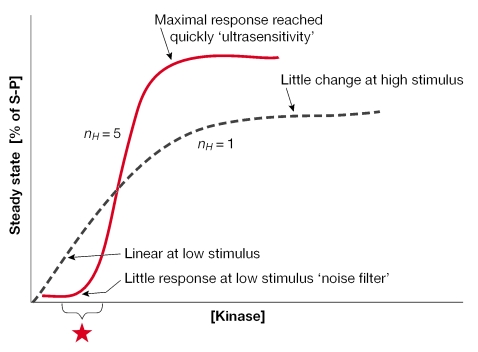
What is the purpose of the modest, but notable, MAPK activation by membrane-localized SRs? Perhaps some insight might be gained by understanding the nature of signalling by kinase cascades (for a review, see Ferrell, 2002). A 'three-kinase' cascade or MAPK module introduces ultrasensitivity to signalling pathways, which provides a way to filter out noise but respond decisively to stimuli. This might increase the sensitivity of responses to subtle changes in local hormone concentrations by setting the basal MAPK activity closer to the inflection point (Fig 1). Additionally, switch-like responses (steep activation curves) ensure that successful feed-forward control can occur. For example, the rapid SR-induced activation of ERKs could, in turn, cause the phosphorylation of the same SRs (membrane, nuclear or both) and other accessory proteins, thereby allowing the potentiation of subsequent receptor, or associated factor, activity. Such short-term potentiation or rapid response building might provide enough sustained stimulation to engage a more permanent genomic SR action.
Figure 1.
Steroid receptor activation of mitogen-activated protein kinase cascades might contribute to ultrasensitivity. Activation curves and Hill coefficients (nH) for classical Michaelis–Menten (dashed line) or cooperative (solid line) enzymes are shown. The steep activation curve (sigmoidal shape; cooperative system) also defines a rapid and decisive response that is mediated by a three-kinase cascade, where '% of S-P' is the percentage of phosphorylated substrate molecules at steady state or mitogen-activated protein kinase (MAPK) activity. The shape of the curve at low stimulus levels indicates a weak response and functions as a 'noise filter'. Membrane steroid receptors (SRs) might set the basal level of MAPK activity or the starting point (asterisk) closer to the steep part of the activation curve, so that a maximal response is reached rapidly after slight changes in the input stimulus. The 'switch-like' nature of ultrasensitive systems might ensure that signals will be successfully propagated and translated into the long-term regulation of cellular processes or genomic events, so the final response seems to have an 'all-or-nothing' quality. Figure modified from Ferrell, 1996.
What is the spectrum of membrane-initiated steroid action?
This meeting added to the long list of tissues that are now known to be regulated by steroid-induced actions at the membrane, the nucleus and numerous points in between. Actions in classic target tissues were described, such as sex-steroid actions in reproductive tissues, and a wide range of non-traditional target tissues for SR action were also discussed. In addition to those mentioned above, R. Miesfeld (Tucson, AZ, USA) began to define mechanistically the steroid-induced cytoskeletal reorganizations that underlie cell-shape changes. Brain-signalling pathways were brought more into focus by the work of several investigators. M. Kelly (Portland, OR, USA) described how oestradiol reduces the inhibitory potency of γ-aminobutyric acid (GABA) ligands by closing K+ channels, which involves Gq-mediated actions on PKA and PKC. K. Catt (Rockville, MD, USA) described ER-α and -β on neuronal processes, and outlined their roles in rapidly decreasing cyclic AMP levels and in modulating the activity of Ca2+ and K+ channels. L. Brewer (Lexington, KY, USA) described how vitamin D signalling decreases Ca2+ channel densities in the hippocampus.
Further definition was brought to the rapidly elicited functions of steroids in cardiovascular tissues. Oestrogenic and TH effects on the vascular system, which often involve eNOS activation, were the focus of talks by Bender, Shaul, Levin and J. Liao (Cambridge, MA, USA). J. Funder (Melbourne, Australia) described some newly discovered actions of classical intracellular mineralocorticoid receptors (which are probably located in the endoplasmic reticulum) in vascular smooth muscle cells, and in cardiomyocytes in which the effects of aldosterone seem to be mediated by PKCε. Liao described TR and other SR interactions with PI3K that cause a decrease in vascular resistance through eNOS and AKT phosphorylation.
These examples indicate that membrane-involved steroid actions share another feature with nuclear SRs: the context-specificity of their interactions and functions. It seems that the membrane-involved steroid-signalling machinery is relatively flexible, and 'mixes and matches' with whatever partnering proteins are at hand in a given cell type. Steroids elicit diverse functional endpoints in different tissues; however, in doing so, they often function to coordinate cohorts of tissues to respond to gross developmental or stage-specific changes, such as pregnancy. Some of the responses must occur immediately, whereas others are delayed; however, all are necessary for the development of the response of the whole organism. Overall, it seems that most of the tissue types that were originally thought to be the targets of steroids have now also been shown to have rapid non-genomic SR effects. Non-traditional target tissues are providing many examples of non-genomic pathway use. Steroids have been clearly implicated in both rapid and prolonged responses in most of the tissues investigated so far, and more examples are sure to come.
Are membrane and nuclear-receptor actions connected?
Several speakers discussed creative ways in which to distinguish signalling events that are initiated by membrane and nuclear SRs, including the use of membrane-selective SR ligands. For example, Farach-Carson compared short- (3-h) versus long-term (24-h) transcriptional regulation in osteoblasts with an analogue of vitamin D3 (1,25(OH)2D3) that mediates Ca2+ influx through voltage-sensitive Ca2+ channels, but does not bind to the nuclear VDR. Similarly, S. Kousteni (Fayetteville, AR, USA) presented studies in rodent bone using the synthetic ER/AR ligand oestren, which elicits membrane-involved signalling effects of these sex steroids without affecting their transcriptional activities. Oestren reversed bone loss in ovariectomized females and orchidectomized males without affecting their reproductive organs, through a mechanism that involved the activation of MAPKs through the c-SRC/SHC pathway and the phosphorylation of numerous downstream transcription factors. Norman described the identification of an alternative ligand-binding pocket (the A-pocket), which was identified through computer-docking studies using non-genomic ligands that are specific for the VDR. This A-pocket partially overlaps the classically defined genomic (or G-) pocket, and predicts how SRs might accommodate differentially shaped or flexible ligands that sample various equilibrium conformations of unliganded receptors. Ligands that choose the A-pocket rather than the G-pocket are predicted to cause non-genomic SR signalling. On a similar theme, C. Watson (Galveston, TX, USA) observed the ability of picomolar to nanomolar concentrations of xenoestrogens to activate MAPKs and elicit Ca2+ influx through membrane ERs, which leads to the differential release of prolactin, according to her pituitary cell model. This is in stark contrast to the inability of these compounds to initiate nuclear actions at low concentrations. Indeed, several investigators recognized the need to develop and test additional highly selective ligands. J. Katzenellenbogen (Champaign, IL, USA) presented a unique approach to probe the interactions of diverse ligands with ER-α and -β using ligand-binding domains (LBDs) that were chemically tethered to glass slides and were allowed to recruit co-activators. Such high-throughput screening could identify ligands that recruit signalling molecules, such as c-SRC-to-ER hetero- or homodimers, and thereby specify rapid versus nuclear responses.
Studies in knockout mice also provided useful new insights into the question of the membrane-versus-nuclear effects of SRs. According to Levin, knockout mice for either ER gene failed to undergo oestrogen-induced vasodilation, which implies that both ER-α and ER-β (perhaps heterodimerized) are required to mediate vascular responses to oestrogens. The comparison made by Norman of osteoblasts from VDR-knockout and wild-type mice implicated the classically described receptor protein in the effects on ion-channel activities that are related to exocytosis, in contrast to the MARRS protein described by Nemere and Farach-Carson. However, it is possible that the classical VDR might also regulate the expression of signalling proteins that are required for the rapid actions of vitamin D3 at the membrane in osteoblasts (Farach-Carson), similar to the way in which functional ER-α is required for intact IGF1 signalling in reproductive tissues (K. Korach, Research Triangle Park, NC, USA). The theme of the SR-dependent upregulation of proteins that are required for rapid signalling events is an important point of integration of membrane and nuclear hormone action. Finally, talks by B. Katzenellenbogen (Champaign, IL, USA), Korach and J. Cidlowski (Research Triangle Park, NC USA ) highlighted the usefulness of gene-array studies in understanding the isoform-specific nuclear action of these receptors, and the great potential of this technology for the dissociation of membrane and nuclear effects.
Future challenges
The meeting concluded with a discussion session that was led by Watson, which addressed many of the pivotal issues discussed above and also focused on some unresolved problems. Similar to discussions during the 1999 and 2002 iterations of this meeting, there were spirited debates on whether the effects of supraphysiological steroid concentrations are of interest, if not in normal functioning, then perhaps in toxic or therapeutic situations. However, it was interesting to note some evolution in this discussion. Watson reported that steroid mimetics (such as xenoestrogens), which were previously deemed non-toxic or ineffective because transcription-endpoint assays required high effective concentrations, have now been shown to be potent when assessed by appropriate non-genomic-signalling assays. It therefore seems wise to consider the many known mechanisms and modes of non-genomic SR signalling before concluding that high ligand concentrations are required, or that compounds are either harmful (for example, environmental oestrogens) or of particular interest for their beneficial selective actions (for example, vitamin D analogues or phytoestrogens).
In conclusion, although we were treated to many new revelations about SRs in different cellular locations, which mediate unique and versatile signals with numerous cross-connections, much remains unclear about the integrated participation of membrane and nuclear SR forms in mediating hormone action. When the same protein is found in several cellular locations, we must determine whether these populations emigrate (that is, exchange with one another in different locations) or are born and raised (that is, originate) in different regions of the cell. We are just beginning to ask the questions that will eventually lead to answers about when and if these SR 'cousins' ever get together for a reunion. In the meantime, stay tuned for a further blend of discussions on SR membrane and nuclear actions at the August 2006 FASEB Summer Conference.


Acknowledgments
We thank all participants for their contributions to a highly stimulating meeting, each subtopic of which could merit a separate review. We apologize to those colleagues whose work could not be cited owing to space constraints.
References
- Ferrell JE Jr (1996) Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci 21: 460–466 [DOI] [PubMed] [Google Scholar]
- Ferrell JE Jr (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14: 140–148 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM (1975) Endometrial cell calcium and oestrogen action. Nature 253: 357–359 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM (1977) Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature 265: 69–72 [DOI] [PubMed] [Google Scholar]