Abstract
TRANCE/TRAF6 signalling governs osteoclastogenesis in vivo. Only the TRANCE receptor (TRANCE-R) has been shown to induce osteoclastogenesis, even though other immune receptors, including CD40 and IL-1R/Toll-like receptor, use TRAF6 to activate overlapping signalling cascades. These observations led us to question whether qualitative or quantitative differences exist between the TRAF6-mediated signals induced by TRANCE and by other ligand–receptor pairs. Here we show that stimulation by overexpressed wild-type CD40 can induce osteoclastogenesis. Stimulation through modified CD40 containing increased numbers of TRAF6-binding sites in the cytoplasmic tails showed a dose-dependent increase in the activation of p38 kinase and more pronounced osteoclastogenesis. Moreover, precursors overexpressing TRAF6 differentiate into osteoclasts in the absence of additional signals from TRANCE. Our results suggest that differences in the osteoclastogenesis-inducing capacity of TRANCE-R versus other TRAF6-associated receptors may in part stem from a quantitative difference in the TRAF6-mediated signals.
Keywords: osteoclast, TRAF6, TRANCE, CD40, signal
Introduction
Osteoclasts are multinuclear cells (MNCs) derived from myeloid lineage cells and are responsible for bone resorption. The balance between resorption by osteoclasts and formation by osteoblasts is critical for bone homeostasis. Excessive osteoclastogenesis or activation of mature osteoclasts causes the bone destruction associated with diseases such as rheumatoid arthritis, osteoporosis, multiple myeloma and bone metastasis (Teitelbaum, 2000).
The differentiation of osteoclasts is regulated by two principal factors: macrophage-colony stimulating factor (M-CSF) and tumour necrosis factor (TNF)-related activation-induced cytokine (TRANCE, also known as RANKL; Suda et al, 1999). Bone marrow precursors differentiate into mature osteoclasts in vitro in the presence of M-CSF and TRANCE. Mice lacking either TRANCE or its cognate receptor TRANCE-R (also known as RANK) show severe osteopetrosis due to lack of mature osteoclasts (Dougall et al, 1999; Kong et al, 1999; Kim et al, 2000). These results indicate that the interaction between TRANCE and its receptor is essential for the differentiation of osteoclasts in vivo.
Like most TNF receptor (TNFR) family members, TRANCE-R binds to adaptor molecules called TNF receptor-associated factors (TRAFs). Six TRAFs (TRAF1–TRAF6) have been reported so far on the basis of their similarities in the carboxy-terminal TRAF domain (Walsh & Choi, 2003). Only TRAF6 seems to have a critical role in osteoclastogenesis. Like TRANCE-deficient mice, TRAF6-deficient mice show severe osteopetrosis due to impaired osteoclastogenesis (Lomaga et al, 1999; Naito et al, 1999; Kobayashi et al, 2003). In addition, TRAF6-deficient osteoclast precursors obtained from spleen fail to differentiate into mature osteoclasts in vitro even in the presence of both M-CSF and TRANCE. These results indicate that the TRANCE signalling essential for osteoclastogenesis is predominantly mediated by TRAF6.
TRAF6 can mediate signalling not only from TRANCE-R but also from other TNFR family members, such as CD40 and the IL-1R/Toll-like receptor (TLR) family members (Ye et al, 2002; Akira, 2003; Walsh & Choi, 2003). Despite the activation of apparently overlapping TRAF6-dependent signalling cascades by those receptors, only TRANCE-R has been shown to induce osteoclastogenesis. It has not been elucidated, however, whether qualitative or quantitative differences distinguish TRAF6-mediated signalling originating from TRANCE-R versus that from other receptors such as CD40. Here we show that increased usage of TRAF6 by CD40, characterized in part by pronounced activation of p38 kinase, leads to osteoclastogenesis, suggesting that quantitative differences in the induction of TRAF6-mediated signals by a given receptor may be important for functional differences leading to osteoclastogenesis between TRANCE-R and other TRAF6-associated receptors.
Results
Distinct TRAF6-binding sites of TRANCE-R
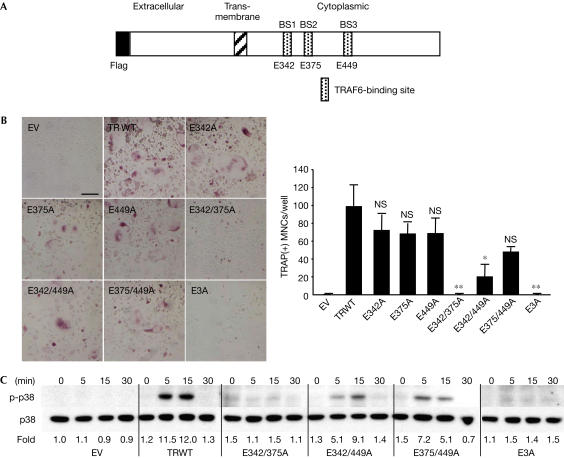
We have previously shown that three TRAF6-binding sites of TRANCE-R are functionally equivalent at least in terms of activating nuclear factor kappa B (NF-κB) in transient transfection assays (Ye et al, 2002). To determine whether there is any qualitative difference in TRAF6-binding sites for osteoclastogenesis, modified TRANCE-R with mutations in TRAF6-binding sites were transduced into osteoclast precursors (bone marrow macrophages, BMMs), selected for similar surface expression (supplementary Fig 1A online) and tested for their capacity to induce osteoclastogenesis (Fig 1A). When BMMs overexpressing wild-type (WT) TRANCE-R (TRWT) were stimulated by crosslinking the Flag motif added to the amino termini, tartrate-resistant acid phosphatase (TRAP)-positive MNCs (TRAP(+) MNCs) were formed (Fig 1B). Similar to TRWT, modified receptors with a single mutation, E342A, E375A or E449A (mutation in BS1, BS2 or BS3, respectively), still induced osteoclastogenesis. TRANCE-R mutants bearing two mutations, E342/449A or E375/449A, also induced osteoclastogenesis, albeit to a reduced extent. Furthermore, osteoclasts from these mutants formed actin rings and retained bone-resorptive capacity (supplementary Fig 1B online). However, neither E342/375A nor the triple mutant (E3A) induced osteoclastogenesis. Stimulation of E342/375A or E3A failed to induce the activation of p38 kinase (Fig 1C), which is critical for osteoclastogenesis (Lee et al, 2002; Li et al, 2002; Mansky et al, 2002). Interestingly, overexpression of the E342/375A or E3A mutants hampered osteoclastogenesis induced by the endogenous TRANCE-R, suggesting that they may act as dominant-negative mutants (supplementary Fig 1C online).
Figure 1.
TRAF6-binding sites of the TRANCE receptor. (A) Schematic representation of modified TRANCE-R and its mutants. (B) Osteoclastogenesis mediated by TRANCE-R mutants. BMMs were transduced with pMX-puro empty vector (EV) or pMX-puro with wild-type TRANCE-R (TRWT) or mutants, and stimulated by anti-Flag antibody (10 μg/ml). Transduced BMMs were stained for TRAP after 4 days. Statistical analysis was performed using the Student's t-test versus TRWT. NS, not significant, *P<0.01, **P<0.001. Scale bars, 100 μm. (C) Transduced BMMs were serum starved for 1 h, stimulated by anti-Flag antibody for the period indicated and analysed by western blotting.
These results demonstrate that either the BS1 or BS2 TRAF6-binding site has a greater ability to induce p38 kinase activation and osteoclastogenesis than the BS3 site and that at least one of the two membrane-proximal TRAF6-binding sites is required for optimal osteoclastogenesis. Although the contribution of the third binding site is minimal, enhanced osteoclastogenesis was seen in E342A compared with E342/449A, suggesting that the third motif has the ability to influence osteoclastogenesis. These results together suggest that all three TRAF6-binding sites in TRANCE-R have the potential, although with different capacities, to induce osteoclastogenesis.
Endogenous CD40 and osteoclastogenesis
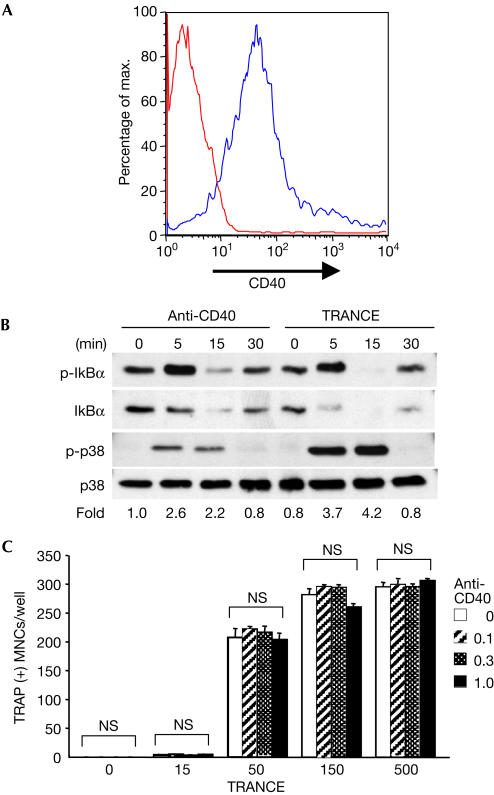
The cumulative effect of engaging the TRAF6-binding sites might be a contributing factor for osteoclast differentiation. To test this idea further, we examined the potency of CD40 for osteoclast differentiation. CD40 shares the highest homology among the TNFR family with TRANCE-R, but CD40 contains only one TRAF6-binding site in its cytoplasmic tail (Walsh & Choi, 2003). Moreover, osteoclast precursors express surface CD40 (Fig 2A), albeit at a lower level than mature macrophages or dendritic cells (data not shown). In addition, similar to TRANCE, anti-CD40 antibody induced the activation of NF-κB and p38 MAPK (Fig 2B). The activation of p38 kinase by CD40, however, was significantly lower than that by TRANCE. Despite the presence of functional CD40 on BMMs, CD40 stimulation did not induce osteoclast differentiation of BMMs (Fig 2C) and did not enhance the TRANCE-induced osteoclastogenesis (Fig 2C). Similar results are obtained using soluble CD40L (supplementary Fig 2 online). These results suggest that the endogenous level of CD40 is sufficient to trigger signalling cascades overlapping those induced by TRANCE in osteoclast precursor cells, but this signalling is insufficient for their differentiation.
Figure 2.
Stimulation of endogenous CD40 failed to induce osteoclastogenesis. (A) BMMs were stained for CD40 and analysed by FACS. Isotype (red line) and CD40-transduced (blue line) BMMs are shown. (B) BMMs were serum starved for 1 h, and stimulated with anti-CD40 (1 μg/ml) or TRANCE (500 ng/ml) for the period indicated. Total cell lysates were subjected to western blotting. (C) BMMs were cultured in the presence of anti-CD40 (0, 0.1, 0.3 or 1 μg/ml) and/or TRANCE (0, 15, 50, 150 or 500 ng/ml) for 4 days and stained for TRAP. Statistical analysis was performed using the Student's t-test versus anti-CD40 absent condition. NS, not significant.
Enhanced CD40 signals induce osteoclastogenesis
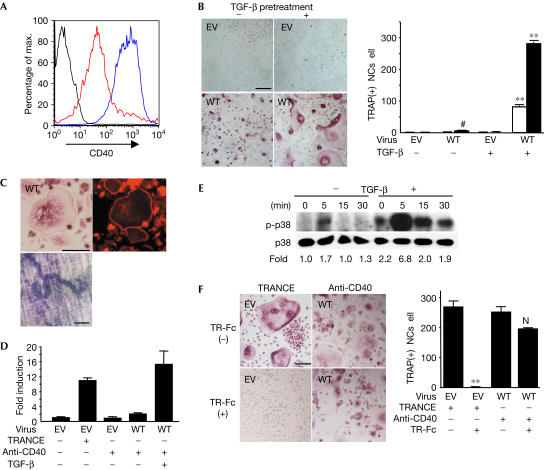
As the TRANCE-R mutant with only one TRAF6-binding site could successfully induce osteoclastogenesis when overexpressed (Fig 1), it was suggested that enhanced stimulation of endogenous, WT CD40 may induce osteoclast differentiation. To test this possibility, we overexpressed WT CD40 in BMMs (Fig 3A). When WT CD40-overexpressing BMMs were stimulated with anti-CD40, TRAP(+) cells were formed, although most were mononuclear (Fig 3B). These results suggest that when stronger stimuli are given by overexpression, CD40 can initiate osteoclast differentiation.
Figure 3.
Enhanced CD40 signalling has the potential to induce osteoclastogenesis. (A) BMMs were transduced with pMX-puro carrying WT CD40 and stained for CD40 after puromycin selection. Isotype (black line), empty vector-transduced (EV; red) and WT CD40-transduced (blue) BMMs are indicated. (B) Transduced BMMs were cultured in the absence or presence of TGF-β (1 ng/ml), stimulated with (closed bar) or without (open bar) anti-CD40 (1 μg/ml) for 4 days and stained for TRAP. (C) Osteoclasts from WT CD40-transduced BMMs with TGF-β pretreatment were stained for TRAP (top left) and F-actin (top right). Transduced BMMs were cultured on the bone slices with anti-CD40 for 6 days, and bone slices were stained with 0.5% toluidine blue (bottom). (D) NFATc1 mRNA induction. Transduced BMMs were stimulated with anti-CD40 or TRANCE (500 ng/ml) for 48 h. NFATc1 mRNA induction, normalized with HPRT expression, was determined by real-time PCR. (E) WT CD40-transduced BMMs, prepared in the absence or presence of TGF-β, were serum starved for 1 h, and stimulated with anti-CD40 for the period indicated. Total cell lysates were subjected to western blotting. (F) Transduced BMMs, prepared in the presence of TGF-β, were cultured with anti-CD40 or TRANCE in the absence or presence of TR-Fc (5 μg/ml) for 4 days, and stained for TRAP. Statistical analysis was performed using the Student's t-test versus the EV (B) or TR-Fc absent condition (F). NS, not significant; #P<0.05; **P<0.001. Scale bars, 100 μm.
To test whether osteoclast differentiation by CD40 can be further enhanced, WT CD40-overexpressing BMMs were treated with transforming growth factor beta (TGF-β), a known costimulatory molecule for osteoclastogenesis (Chambers, 2000). In the presence of TGF-β, TRAP(+) MNCs can be detected in culture, even without anti-CD40. This result is most probably due to the constitutive activation of CD40 in the cells following overexpression. When cells were treated with anti-CD40 or CD40L, there was a profound increase in the number of TRAP(+) MNCs (Fig 3B; supplementary Fig 3A online). Like mature osteoclasts, these cells expressed high levels of NFATc1 messenger RNA, formed actin rings and resorbed bone slices (Fig 3C,D). In addition, CD40-induced activation of p38 kinase was enhanced and sustained in the presence of TGF-β. How TGF-β enhances p38 kinase activation or osteoclastogenesis is not yet clear, but TGF-β did not alter the level of CD40 expression or the interaction between CD40 and TRAF6 (Fig 3E; supplementary Fig 3B,C online).
To confirm that the osteoclast differentiation observed was due to the intrinsic effect of CD40-mediated signals rather than an indirect effect of altered participation of TRANCE and its receptor, we cultured CD40-overexpressing BMMs in the presence of a soluble form of TRANCE-R (TR-Fc, 5 μg/ml). As previously shown, TR-Fc completely inhibited TRANCE-induced osteoclastogenesis, but it failed to inhibit CD40-induced osteoclastogenesis (Fig 3F). These results suggest that amplified CD40 signalling can induce osteoclastogenesis.
TRAF6 signals can mediate osteoclastogenesis
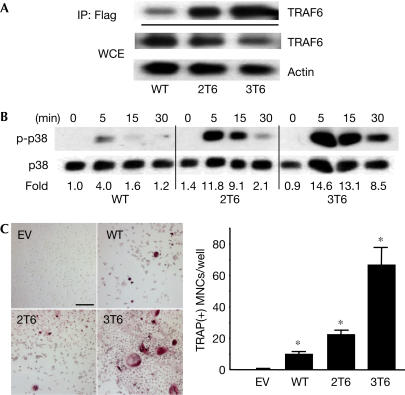
To explore further the possibility that enhanced signalling by CD40, in particular by its TRAF6-binding motif, may be critical for osteoclastogenesis, we constructed CD40 mutants with an increasing number of TRAF6-binding motifs. We inserted one or two additional TRAF6-binding motifs, identical to the endogenous CD40 TRAF6-binding motif, into CD40. These CD40 mutants were transduced into BMMs, selected for similar surface expression (supplementary Fig 4A online) and stimulated with anti-CD40. Even though all the mutants were similarly expressed, CD40 mutants with additional TRAF6-binding sites (CD40-2T6 or CD40-3T6) recruited more TRAF6 than WT CD40 (Fig 4A). In addition, there was a concurrent increase in the activation of p38 MAPK with additional TRAF6-binding sites (Fig 4B). Although CD40-2T6 was still not more efficient than WT CD40, CD40-3T6 stimulation showed a marked increase in the formation of mature osteoclasts (Fig 4C). These results suggest that when CD40 induces more pronounced and persistent activation of p38 kinase by recruiting more TRAF6 to the receptor, it can induce osteoclast differentiation in the absence of costimulatory factors (e.g. TGF-β), and that the TRAF6-binding site of CD40 has an intrinsic capacity to induce osteoclast differentiation. As overexpression of TRAF6 alone without any stimuli can activate downstream signalling cascades (Ishida et al, 1996; Franzoso et al, 1997), we tested the effect of TRAF6 overexpression on osteoclastogenesis. Surprisingly, overexpressed TRAF6 induced the formation of bone-resorbing TRAP(+) MNCs, even in the absence of exogenous TRANCE (Fig 5).
Figure 4.
CD40 TRAF6-binding site and osteoclastogenesis. (A) BMMs were transduced with pMX-puro empty vector (EV), WT CD40 or CD40 mutants with two (2T6) or three (3T6) TRAF6-binding sites. Total cell lysates were collected after puromycin selection and subjected to immunoprecipitation using anti-Flag antibody for transduced CD40 and western blotting for TRAF6. (B) Transduced BMMs were serum starved for 1 h and stimulated with anti-CD40 antibody (1 μg/ml) for the indicated period. Total cell lysates were subjected to western blotting. (C) Transduced BMMs were cultured with anti-CD40 for 4 days and stained for TRAP. Statistical analysis was performed using the Student's t-test versus EV. *P<0.01. Scale bars, 100 μm.
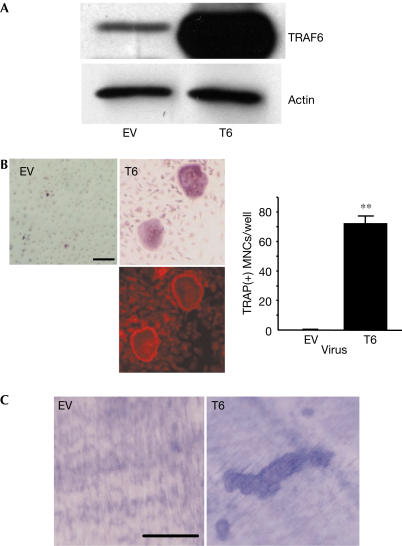
Figure 5.
TRAF6-mediated signal is sufficient for osteoclastogenesis. (A) BMMs were transduced with pMX-puro empty vector (EV) or WT TRAF6 (T6). Total cell lysates were subjected to western blotting. (B) Transduced BMMs were cultured in the presence of only M-CSF for a further five days and stained for TRAP (top) or F-actin (bottom). (C) Transduced BMMs were cultured on the bone slices in the presence of only M-CSF for 7 days, and bone slices were stained with 0.5% toluidine blue. Statistical analysis was performed using the Student's t-test versus EV. **P<0.001. Scale bars, 100 μm.
Discussion
The TRANCE–TRAF6 axis is known to have a central role in osteoclastogenesis (Dougall et al, 1999; Kong et al, 1999; Lomaga et al, 1999; Naito et al, 1999; Kim et al, 2000; Kobayashi et al, 2003). Even though many different cytokines use TRAF6 as a signalling adaptor molecule, TRANCE-R is unique in that it induces osteoclastogenesis from bone marrow precursors. Therefore, we have addressed, in this study, whether there is a qualitative or quantitative difference in TRAF6-mediated signals from TRANCE-R or other receptors. Our data show that TRANCE-R with only one TRAF6-binding site did not efficiently induce osteoclastogenesis. Rather, at least two TRAF6-binding sites (any combination) are required for optimal osteoclastogenesis. These results suggest, in terms of osteoclastogenic potential, that TRANCE-R has quantitatively but not qualitatively different TRAF6-binding sites in its cytoplasmic tail.
On the supposition that the quantitative difference in TRAF6-mediated signals can be a determining factor for distinct osteoclastogenic potential of various receptors that use TRAF6, we tested the potential of CD40 for its ability to induce osteoclastogenesis. When BMMs were stimulated with anti-CD40, they failed to induce osteoclastogenesis, as expected. However, when CD40 stimulation was enhanced by overexpression of WT CD40, by the addition of TGF-β or by multiplication of TRAF6-binding sites in the cytoplasmic tail of CD40, there was prolonged TRAF6-mediated signalling, measured by p38 kinase activation, and a subsequent increase in osteoclast differentiation. Additionally, overexpression of TRAF6 alone in bone marrow precursors induced osteoclastogenesis in the absence of any additional differentiation factor such as TRANCE. Taken together, these results are consistent with the idea that there may not be any qualitative difference between TRANCE-R- and CD40-mediated signals in osteoclast precursors. Hence, we suggest that a quantitative difference in TRAF6 activation, manifested by the degree of its recruitment to the surface receptor and p38 kinase activation, can be one of the key mechanisms that distinguish TRANCE-R from other receptors that use TRAF6 in terms of osteoclastogenic potential.
Similar mechanisms of controlling distinct biological outcomes by growth/differentiation factors, despite their use in overlapping signalling cascades, have been reported (Traverse et al, 1994; Vaudry et al, 2002). For example, although both epidermal growth factor (EGF) and nerve growth factor (NGF) use the same kinase cascade, only NGF can induce neuronal differentiation. When the EGF receptor was overexpressed, EGF could induce neuronal differentiation similar to NGF (Traverse et al, 1994). In the case of osteoclast differentiation, any TRAF6-binding receptor might have the potential, but only TRANCE-R can meet the threshold to induce osteoclastogenesis.
However, an alternative interpretation of our data is possible, such that multiplication of TRAF6-binding sites in the CD40 mutant receptors may result in an increased avidity, commensurate with TRWT, for osteoclastogenic TRAF6-interacting proteins. Therefore, when the number of TRAF6-binding sites are increased in the receptor or when TRAF6 expression is highly increased, other distinct signalling cascades could be activated that are necessary for osteoclast differentiation.
Methods
Reagents. Soluble TRANCE and TR-Fc have been described previously (Kim et al, 2000). CD40L was from R&D systems (Minneapolis, MN, USA). Antibodies specific for phospho-IκBα, IκBα, phospho-p38 and p38 were from Cell Signaling Technology (Beverly, MA, USA), and TRAF6 was from MBL (Woburn, MA, USA), F-actin from Sigma (St Louis, MO, USA), anti-CD40 (HM40-3) from BD Bioscience Pharmingen (San Diego, CA, USA) and anti-Flag (M2) from Sigma (St Louis, MO, USA).
Retrovirus preparation. All complementary DNAs were inserted into pMX-puro. To make retrovirus, we transfected PLAT-E cells and collected the supernatant 24–48 h after transfection (Morita et al, 2000). To generate CD40-2T6 and CD40-3T6, the TRAF6-binding site (residues 212–250) of CD40 was inserted behind the original TRAF6-binding site (supplementary information online). Flag-tagged TRANCE-R mutants have been described previously (Ye et al, 2002), and cDNAs were transferred into pMX-puro.
FACS analysis. BMMs were collected with cell dissociation solution (Sigma). Cells were stained for specific antibody and then run on a BD FACSCalibur (BD Bioscience). Data were analysed using FlowJo (Tree Star, Ashland, OR, USA).
Real-time PCR. Total RNA was isolated from BMMs using Trizol (Invitrogen, Carlsbad, CA, USA). cDNA was generated using SuperScript II (Invitrogen) and used for TaqMan PCR. Primers for mouse NFATc1 and HPRT were from Applied Biosystems (Foster City, CA, USA). TaqMan PCR was performed on an ABI Prism 7000 Sequence Detection System (Applied Biosystems).
Osteoclast differentiation, immunoprecipitation and western blot analysis. Details are shown in supplementary information online.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v6/n2/extref/7400345s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank D. Fremont for recombinant human M-CSF, T. Kitamura for pMX vectors and PLAT-E cells, S. Tanaka for helpful discussion, and O. Smirnova and Y. Kadono for assistance. This work was supported in part by National Institutes of Health grants AR49078 and AR48521 (to Y.C.) and the Molecular and Cellular BioDiscovery Research Program grant M1-0401-00-0045 from the Ministry of Science and Technology, Korea (to S.Y.L.).
References
- Akira S (2003) Toll-like receptor signaling. J Biol Chem 278: 38105–38108 [DOI] [PubMed] [Google Scholar]
- Chambers TJ (2000) Regulation of the differentiation and function of osteoclasts. J Pathol 192: 4–13 [DOI] [PubMed] [Google Scholar]
- Dougall WC et al. (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13: 2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11: 3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T et al. (1996) Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem 271: 28745–28748 [DOI] [PubMed] [Google Scholar]
- Kim N, Odgren PR, Kim DK, Marks SC Jr, Choi Y (2000) Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA 97: 10905–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T et al. (2003) TRAF6 is a critical factor for dendritic cell maturation and development. Immunity 19: 353–363 [DOI] [PubMed] [Google Scholar]
- Kong YY et al. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323 [DOI] [PubMed] [Google Scholar]
- Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH (2002) The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone 30: 71–77 [DOI] [PubMed] [Google Scholar]
- Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N (2002) p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 143: 3105–3113 [DOI] [PubMed] [Google Scholar]
- Lomaga MA et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13: 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansky KC, Sankar U, Han J, Ostrowski MC (2002) Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-κB ligand signaling. J Biol Chem 277: 11077–11083 [DOI] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7: 1063–1066 [DOI] [PubMed] [Google Scholar]
- Naito A et al. (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4: 353–362 [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20: 345–357 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289: 1504–1508 [DOI] [PubMed] [Google Scholar]
- Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A (1994) EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol 4: 694–701 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296: 1648–1649 [DOI] [PubMed] [Google Scholar]
- Walsh MC, Choi Y (2003) Biology of the TRANCE axis. Cytokine Growth Factor Rev 14: 251–263 [DOI] [PubMed] [Google Scholar]
- Ye H et al. (2002) Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418: 443–447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information