Abstract
The Twisted gastrulation (Tsg) proteins are modulators of bone morphogenetic protein (BMP) activity in both vertebrates and insects. We find that the crossveinless (cv) gene of Drosophila encodes a new tsg-like gene. Genetic experiments show that cv, similarly to tsg, interacts with short gastrulation (sog) to modulate BMP signalling. Despite this common property, Cv shows a different BMP ligand specificity as compared with Tsg, and its expression is limited to the developing wing. These findings and the presence of two types of Tsg-like protein in several insects suggest that Cv represents a subgroup of the Tsg-like BMP-modulating proteins.
Keywords: BMP, crossvein, crossveinless, Drosophila melanogaster, twisted gastrulation
Introduction
Extracellular gradients of bone morphogenetic protein (BMP) signalling direct many tissue patterning events including dorsoventral (D/V) patterning in both vertebrates and insects. In Drosophila, Twisted gastrulation (Tsg; Zusman & Wieschaus, 1985; Mason et al, 1994, 1997), Short gastrulation (Sog; Ferguson & Anderson, 1992; Piccolo et al, 1996), Crossveinless-2 (Cv-2; Conley et al, 2000), Tolloid (Tld; Marques et al, 1997), Tolloid-related-2 (Tlr-2; Nguyen et al, 1994) and Shrew (Srw; Ferguson & Anderson, 1992; Vilmos et al, 2001) modulate gradients of inductive signals from the BMP ligands Decapentaplegic (Dpp; Ferguson & Anderson, 1992), Glass-bottom-boat (Gbb/60A; Khalsa et al, 1998; Wharton et al, 1999) and Screw (Scw; Arora et al, 1994). Full-length Tsg can bind BMPs in a ternary complex with Sog or Chordin (Chd) proteins and prevent receptor activation (Chang et al, 2001; Ross et al, 2001; Scott et al, 2001; Shimmi & O'Connor, 2003). The Tld and Tlr-2 metalloproteases can act as switches by cleaving the Sog/Chd inhibitors in a reaction that is influenced by Tsg (Yu et al, 2000; Larrain et al, 2001), and in some settings the carboxy-terminal domain of Tsg can promote BMP activity by displacing Tld-generated Sog fragments from BMP (Larrain et al, 2001; Garcia Abreu et al, 2002; Oelgeschlager et al, 2003). Differences in substrate specificities of these BMP modulators may contribute to the wide range of biological specificities seen with BMP signalling (Scott et al, 1999; Garcia Abreu et al, 2002). As Tsg is only expressed during D/V patterning of the fly embryo (Mason et al, 1994), although BMP ligands signal many times in fly development, we searched for additional tsg-like genes in the Drosophila genome.
Results
The tsg2/cv genomic region
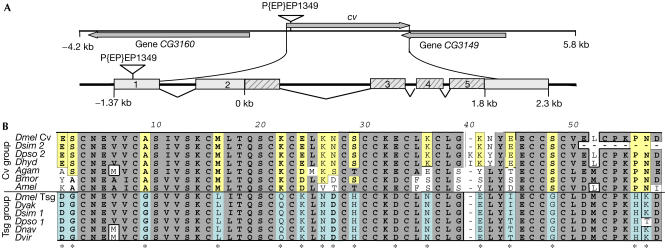
A tsg-like gene (CG12410) was found on the first chromosome between CG3160 and CG3149 (Fig 1A). It encodes a protein with about 50% homology to the Tsg protein and the same molecular topology: two cysteine-rich (CR) domains connected by a variable hinge domain. Ambiguities and gaps in the public sequence were resolved by resequencing (supplementary Fig S1 online; accession number AE003435, bases 63001–65992). A comparison of tsg-like genes in insects suggests two subgroups in the tsg-like family typified by cv and tsg (Fig 1B; supplementary Fig S2 online). Of the five insects for which complete genomes are available, Drosophila melanogaster, Drosophila pseudoobscura and Drosophila simulans have both a tsg-like and a cv-like gene, whereas the mosquito and bee seem to have only a cv-like gene (Fig 1B; supplementary Figs S2,S3 online).
Figure 1.
Organization of the cv gene region and alignment of the first cysteine-rich domain (CR1) of the tsg-related loci. (A) Arrows show the direction of transcription. Bottom: predicted exon–intron structure of cv. Striped boxes indicate protein-coding exons. (B) Sequences that distinguish Cv from Tsg-like proteins are indicated with an asterisk and highlighted in yellow and light blue, respectively (for alignment of the full proteins, see supplementary Fig S3 online). Agam, Anopheles gambiense; Amel, Apis mellifera; Bmor, Bombyx mori; Dhyd, Drosophila hydei; Dmel, Drosophila melanogaster; Dnav, Drosophila navajoa; Dpso, Drosophila pseudoobscura; Dsim, Drosophila simulans; Dvir; Drosophila virilis; Dyak, Drosophila yakuba.
CG12410 is allelic to crossveinless
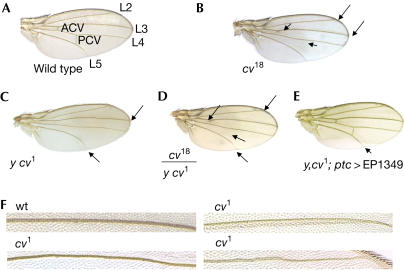
To determine the function of CG12410, we excised the element EP1349 that shows no mutant phenotype and is located about 700 base pairs (bp) 5′ of the exon containing the predicted start codon (Fig 1A; supplementary Fig S1 online). Four strains were recovered that delete portions of CG12410 and all showed a recessive visible crossveinless phenotype with loss of the anterior crossveins (ACV) and the posterior crossveins (PCV; Fig 2A,B). Two of the four mutants were strict recessive visibles (cv18, cv43), whereas the other two (cv34, cv51) showed semilethality (22% and 53%) not linked to the cv locus.
Figure 2.
Characterization of cv. (A–E) Arrows indicate the delta tips of the longitudinal veins and loss of crossveins. (F) These high magnifications show examples of the meanderings and other vein abnormalities not previously described for cv.
As flies heterozygous for cv18 and cv1 (Fig 2C,D) have the same phenotype as cv1 homozygotes, CG12410 is allelic to cv. Further evidence for allelism was obtained by rescuing both cv1 and cv18 hemizygotes with CG12410 using UAS>EP1349 under the control of the ptc>Gal4 driver (Fig 2E).
Although the most obvious phenotype is the absence of crossveins and a delta at the tips of the L3 and L4 veins as originally described (Bridges, 1920; Waddington, 1940), we also found that the longitudinal veins in cv mutants show poorly defined edges and trajectories often broadening and meandering along their length in a manner similar to that seen in sog− wing tissue (Yu et al, 1996; Fig 2F), suggesting that cv has a role in refining the domains where veins and crossveins form.
Deletions define the cv functional region
The nature of the cv mutations was determined by PCR (supplementary Fig S4 online) and sequencing the P-induced deletions (supplementary Fig S1 online). As cv18, cv34, cv43 and cv51 alleles delete a region that extends from the P-element insertion site (supplementary Fig S1 online) past the ATG start codon to the second intron of cv, we consider these alleles as physically verified nulls. The cv1 mutation is due to a 412 retrotransposon (Strobel et al, 1979; Will et al, 1981) inserted in the second intron of cv that introduces two poly(A) addition signals that should terminate the cv transcript prematurely.
The cv52 and cv12 mutations show no phenotype; however, they delete all the DNA from the insertion to either the adjacent gene CG3160 (cv52) or to within 475 bp of the translation start site (cv12; supplementary Fig S1 online). Thus, regulatory sequences necessary for cv function do not extend past 475 bp upstream of the cv12 breakpoint.
Cv is expressed in the developing wing
Endogenous cv messenger RNA was detected only in the developing pupal wing, with no evidence of earlier expression. Expression of cv first appears as diffuse staining in the regions of the vein primordia 24–28 h after pupariation (APF) and later refines to stripes of 2–3 cells localized at the vein–intervein boundaries (Fig 3A–F) and disappears by 40 h APF. By comparison, dpp and the sog-like cv-2 are expressed in the vein domain at these times, whereas sog and gbb are expressed in the intervein regions with concentrations at the boundaries that are coincident with cv (Yu et al, 1996; Conley et al, 2000; Fig 3H).
Figure 3.
Expression of crossveinless. (A–F) In situ hybridization to pupal wings. The timepoints indicate hours after pupariation (APF), and arrows indicate crossveins. Note that expression refines to two parallel stripes along the vein primordia (black arrowheads (F)). Elevated cv is also present (white arrowheads (F)) where deltas form at vein tips. (G) A leg disc with cv misexpressed under the control of the ptc promoter was used as a control for both the probe and background. (H) Diagram depicts the relative expression domains of genes involved in vein formation.
Cv is diffusible and not interchangeable with Tsg
We found that expression of UAS>cv along the anterior–posterior (A/P) border rescues both the ACV and the PCV in cv mutants, whereas tsg does not rescue either crossvein (Fig 4A). Thus, anterior expression of cv can restore function in posterior cells. Similarly, cv expressed in the embryo does not rescue tsg mutants (Fig 4B). Thus, Tsg and Cv are not functionally interchangeable proteins.
Figure 4.
Misexpression experiments using the widely expressed A9 wing Gal4 driver and two restricted Gal4 drivers, ptc and en. (A) UAS>tsg driven by ptc>Gal4 does not restore the crossveins, the longitudinal veins or the L4 and L5 vein tips, typical of cv1 mutants. (B) Conversely, cv driven by the tsg promoter cannot rescue tsg mutants. Results of crossing males homozygous for the tsg>cv transgene on the second chromosome to tsgYN97/FM7 females are tabulated. Despite carrying the tsg>cv transgene, no hemizygous tsg males are viable. (C–J) Comparison of the activity of different combinations of cv and tsg with sog and cv-2 is shown. (K–R) BMP ligand specificity of Cv with or without Sog is shown. (S–V) Tsg shows different ligand specificity.
Cv modulates BMP activity
It has been well documented that the loss of BMP signalling in wings produces two phenotypes, one being reduction of wing size and the other loss of veins (Posakony et al, 1990; Wharton et al, 1999; Ray & Wharton, 2001). The first phenotype involves an early abrogation of long-range BMP signalling, whereas the second results from a late local loss of signalling in veins.
It has also been shown that Tsg can inhibit BMP-like ligands by synergizing with Sog, or in other environments can promote BMP activity by displacing an inhibitory fragment of Sog generated by proteolytic cleavage (Larrain et al, 2001). To compare the activities of Cv and Tsg, we expressed transgene combinations under the control of the wing driver A9>Gal4. Excess cv alone can induce small fragments of extra veins and a delta phenotype (Fig 4C), consistent with a mild pro-BMP activity. In contrast, coexpression of cv and sog produces a phenotype resembling early loss of BMP signalling in the organizer that runs along the A/P boundary (i.e. reduction in size and loss of intervein regions; Posakony et al, 1990; Fig 4E,F). Interestingly, coexpression of cv and sog along the A/P border affects structures throughout the wing (Fig 4G), whereas expression of these proteins in the posterior compartment affects only posterior structures (Fig 4E). The asymmetric activity of cv+sog suggests a restricted mobility due either to local inhibition of a long-range signal such as Dpp or Gbb or to the existence of an asymmetric inhibitor of diffusion of Cv/Sog-containing complexes (Park et al, 2000; Fujise et al, 2003).
It has been postulated that Cv might synergize with Cv-2, a second Sog-like protein (Larrain et al, 2001); however, when Cv-2 is coexpressed with either Cv or Tsg, we see no evidence of the strong inhibition of BMP signalling observed when Cv or Tsg is coexpressed with Sog (compare Fig 4I,J with 4E,F). On further underscoring a difference between Sog and Cv-2, both Cv and Sog mutants show similar effects on wing veins (loss of crossveins and expanded vein tips), whereas Cv-2 mutants show loss of vein tips as well as crossveins. Thus, Cv-2 is not interchangeable with Sog.
BMP inhibition by Cv+Sog is ligand specific
To evaluate ligand specificity, we tested the ability of Cv and Sog to suppress the wing disruptions caused by overexpressing Dpp and Gbb (Fig 4K,L). Expression of cv, tsg or sog alone has no detectable effect on the overexpression of dpp (Fig 4M,O,S). However, when expressed together with sog, cv and tsg behave fairly differently when challenged with excess Dpp. Although tsg with sog can rescue the effect of excess dpp, cv with sog does not suppress the dpp overexpression phenotype at all (compare Fig 4T with 4Q). Cv and Tsg also differ in their effect on Gbb overexpression. Although Cv or Sog alone has no effect on Gbb overexpression (Fig 4N,P), Cv together with Sog re-establishes the intervein tissue and the longitudinal veins with a series of expanded crossveins between L2 and L3 (Fig 4R). In contrast, Tsg alone suppresses fairly effectively the excess Gbb effect (Fig 4U), whereas Tsg together with Sog leads to an intermediate level of rescue (Fig 4V). These observations indicate that Cv and Tsg have distinct activities with respect to the Dpp and Gbb ligands and also different requirements for Sog to inhibit BMP.
Discussion
The Tsg and Cv subfamilies
Comparing the Cv protein with Tsg proteins from other insect species reveals two protein groups (Fig 1B; supplementary Figs S2,S3 online) that may reflect the distinct functions of Cv and Tsg. Supporting this inference, cv fails to rescue tsg mutant embryos (Fig 4B) despite the fact that the Drosophila virilis tsg gene can rescue D. melanogaster tsg mutants (J.C. Becerra, 1998, PhD thesis, University of California, Irvine), indicating that the tsg-like genes may share a common function that is distinct from the cv-like genes.
Cv is required for vein refinement
Vein and crossvein development in the fly wing depends on tightly regulated BMP signalling and is sensitive to changes of BMP activity (Posakony et al, 1990; Yu et al, 1996; Ray & Wharton, 2001). Key proteins involved in vein development include the two ligands, Dpp and Gbb, Sog, Cv-2 and the metalloproteases Tld and Tlr-2 (Nguyen et al, 1994; Marques et al, 1997; Piccolo et al, 1997; Conley et al, 2000).
The meandering and widened veins seen in cv mutants (Fig 2E) are similar to those caused by loss of Sog (Yu et al, 1996). Further, the effects of cv on crossvein development mimic those seen with loss of the Gbb or Dpp ligands (Ray & Wharton, 2001), mutants of tlr-2 (Nguyen et al, 1994; Finelli et al, 1995) or overexpression of sog (Yu et al, 2000). Both the development of wing veins and dorsal patterning in the embryo require the formation of sharp stripes of BMP signalling by processes that involve extracellular modulators of activity (Eldar et al, 2002; Kao et al, 2003). In the embryo, Tsg facilitates the binding of ventrally produced Sog to Dpp and Scw (Ferguson & Anderson, 1992; Arora et al, 1994; Ross et al, 2001) until ligand is released by the cleavage of Sog by dorsally located Tld. This process concentrates Dpp activity in a narrow stripe along the dorsal midline. In the wing, Cv, together with Sog, may have a similar role by facilitating the binding of Sog to Dpp/Gbb ligands until released by Tlr and establishing a sharp stripe of BMP signalling between the gbb- and sog-expressing intervein regions (Fig 3H; Yu et al, 1996; de Celis, 1997, 1998; Conley et al, 2000).
Tsg-like molecules have a dual role in BMP signalling (Larrain et al, 2001; Oelgeschlager et al, 2003). When complexed with Sog-like molecules, they can bind BMP ligands and prevent receptor activation (Harland, 2001). However, when levels of Tsg-like proteins are high relative to Sog or Chd, they can promote BMP activity by catalysing the release of a Tld-generated inhibitory fragment of Sog/Chd (Oelgeschlager et al, 2000; Larrain et al, 2001). The appearance of small ectopic veins with excess Cv alone (Fig 4C) may reflect this process (Larrain et al, 2001; Oelgeschlager et al, 2003). Similarly, the ability of Tsg alone to rescue the A9>gbb phenotype can be due either to Tsg acting as a sole inhibitor of Gbb at high concentrations or to its restoring a more favourable Gbb to Dpp ratio by augmenting Dpp activity. Notably, we could find no evidence that either Cv or Tsg could act together with the sog-like Cv-2. Our data are consistent with a model in which Cv retains the dual activities of Tsg-like molecules albeit with distinct substrate specificity; for example, Cv synergizes with Sog to block Gbb but not Dpp signalling (compare Fig 4Q with 4R).
Interestingly, despite the presence of several BMP ligands involved in many developmental events, only a few Tsg/Cv-like genes are present in vertebrates and invertebrates and their activity is highly restricted. Perhaps Tsg-like proteins are only required where it is necessary to generate a straight and narrow band of BMP signalling such as along the dorsal axis of the embryo or the veins of the wing.
Methods
Protocols are described in supplementary information online.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400347s1.pdf).
Supplementary Material
Supplementary Online Material
Acknowledgments
We apologize to our colleagues whose work could not be cited, or was cited indirectly, due to space limitations. We thank M. O'Connor, K. Arora and E. Bier for sharing fly stocks, M. O'Connor, R. Warrior and A. Lander for helpful discussions and K. Gaudenz for technical assistance. Z. Vilmos's help and support is acknowledged by P.V. This study was supported by National Institutes of Health grants HD36049 and HD36081 to J.L.M. This work was made possible, in part, through access to the National Drosophila Stock Center in Bloomington, IN, USA.
References
- Arora K, Levine MS, O'Connor MB (1994) The screw gene encodes a ubiquitously expressed member of the TGF-β family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev 8: 2588–2601 [DOI] [PubMed] [Google Scholar]
- Bridges CB (1920) The mutant crossveinless in Drosophila melanogaster. Proc Natl Acad Sci USA 6: 660–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH (2001) Twisted gastrulation can function as a BMP antagonist. Nature 410: 483–487 [DOI] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS (2000) Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127: 3947–3959 [DOI] [PubMed] [Google Scholar]
- de Celis JF (1997) Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development 124: 1007–1018 [DOI] [PubMed] [Google Scholar]
- de Celis JF (1998) Positioning and differentiation of veins in the Drosophila wing. Int J Dev Biol 42: 335–343 [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N (2002) Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419: 304–308 [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV (1992) Localized enhancement and repression of the activity of the TGF-β family member, decapentaplegic, is necessary for dorsal–ventral pattern formation in the Drosophila embryo. Development 114: 583–597 [DOI] [PubMed] [Google Scholar]
- Finelli AL, Xie T, Bossie CA, Blackman RK, Padgett RW (1995) The tolkin gene is a tolloid/BMP-1 homologue that is essential for Drosophila development. Genetics 141: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H (2003) Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 130: 1515–1522 [DOI] [PubMed] [Google Scholar]
- Garcia Abreu J, Coffinier C, Larrain J, Oelgeschlager M, De Robertis EM (2002) Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene 287: 39–47 [DOI] [PubMed] [Google Scholar]
- Harland RM (2001) Developmental biology. A twist on embryonic signalling. Nature 410: 423–424 [DOI] [PubMed] [Google Scholar]
- Kao J, Nie Q, Teng A, Wan F, Lander A, Marsh JL (2003) Can morphogen activity be enhanced by its inhibitors? Proc Second MIT Conf Computational Fluid and Solid Mechanics, Vol 2 pp 1729–1733 [Google Scholar]
- Khalsa O, Yoon JW, Torresschumann S, Wharton KA (1998) TGF-β/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing. Development 125: 2723–2734 [DOI] [PubMed] [Google Scholar]
- Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM (2001) Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development 128: 4439–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques G, Musacchio M, Shimell MJ, Wunnenbergstapleton K, Cho KW, O'Connor MB (1997) Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91: 417–426 [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, Marsh JL (1994) Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev 8: 1489–1501 [DOI] [PubMed] [Google Scholar]
- Mason ED, Williams S, Grotendorst GR, Marsh JL (1997) Combinatorial signaling by Twisted gastrulation and Decapentaplegic. Mech Dev 64: 61–75 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Jamal J, Shimell MJ, Arora K, O'Connor MB (1994) Characterization of tolloid-related-1: a BMP-1-like product that is required during larval and pupal stages of Drosophila development. Dev Biol 166: 569–586 [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM (2000) The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405: 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager M, Reversade B, Larrain J, Little S, Mullins MC, De Robertis EM (2003) The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development 130: 4047–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PW, Reizes O, Bernfield M (2000) Cell surface heparan sulfate proteoglycans: selective regulators of ligand–receptor encounters. J Biol Chem 275: 29923–29926 [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM (1996) Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM (1997) Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 91: 407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony LG, Raftery LA, Gelbart WM (1990) Wing formation in Drosophila melanogaster requires decapentaplegic gene function along the anterior–posterior compartment boundary. Mech Dev 33: 69–82 [DOI] [PubMed] [Google Scholar]
- Ray RP, Wharton KA (2001) Context-dependent relationships between the BMPs Gbb and Dpp during development of the Drosophila wing imaginal disk. Development 128: 3913–3925 [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O'Connor MB, Marsh JL (2001) Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410: 479–483 [DOI] [PubMed] [Google Scholar]
- Scott IC et al. (1999) Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol 213: 283–300 [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS (2001) Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature 410: 475–478 [DOI] [PubMed] [Google Scholar]
- Shimmi O, O'Connor MB (2003) Physical properties of Tld, Sog, Tsg and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development 130: 4673–4682 [DOI] [PubMed] [Google Scholar]
- Strobel E, Dunsmuir P, Rubin GM (1979) Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell 17: 429–439 [DOI] [PubMed] [Google Scholar]
- Vilmos P, Gaudenz K, Hegedus Z, Marsh JL (2001) The Twisted gastrulation family of proteins, together with the IGFBP and CCN families, comprise the TIC superfamily of cysteine rich secreted factors. Mol Pathol 54: 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH (1940) The genetic control of wing development in Drosophila. J Genet 41: 75–139 [Google Scholar]
- Wharton KA, Cook JM, Torresschumann S, de Castro K, Borod E, Phillips DA (1999) Genetic analysis of the bone morphogenetic protein-related gene, gbb, identifies multiple requirements during Drosophila development. Genetics 152: 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will BM, Bayev AA, Finnegan DJ (1981) Nucleotide sequence of terminal repeats of 412 transposable elements of Drosophila melanogaster. A similarity to proviral long terminal repeats and its implications for the mechanism of transposition. J Mol Biol 153: 897–915 [DOI] [PubMed] [Google Scholar]
- Yu K, Sturtevant MA, Biehs B, Francois V, Padgett RW, Blackman RK, Bier E (1996) The Drosophila decapentaplegic and short gastrulation genes function antagonistically during adult wing vein development. Development 122: 4033–4044 [DOI] [PubMed] [Google Scholar]
- Yu K, Srinivasan S, Shimmi O, Biehs B, Rashka KE, Kimelman D, O'Connor MB, Bier E (2000) Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development 127: 2143–2154 [DOI] [PubMed] [Google Scholar]
- Zusman SB, Wieschaus EF (1985) Requirements for zygotic gene activity during gastrulation in Drosophila melanogaster. Dev Biol 111: 359–371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Online Material