Abstract
The farnesylated SNARE (N-ethylmaleimide-sensitive factor attachment protein receptor) Ykt6 mediates protein palmitoylation at the yeast vacuole by means of its amino-terminal longin domain. Ykt6 is localized equally to membranes and the cytosol, although it is unclear how this distribution is mediated. We now show that Ykt6 is released efficiently from vacuoles during an early stage of yeast vacuole fusion. This release is dependent on the disassembly of vacuolar SNAREs (priming). In recent literature, it had been demonstrated for mammalian Ykt6 that the membrane-bound form is both palmitoylated and farnesylated at its carboxy-terminal CAAX box, whereas soluble Ykt6 is only farnesylated. In agreement with this, we find that yeast Ykt6 becomes palmitoylated in vitro at its C-terminal CAAX motif. Mutagenesis of the potential palmitoylation site in yeast Ykt6 prevents stable membrane association and is lethal. On the basis of these and other findings, we speculate that Ykt6 is released from membranes by depalmitoylation. Such a mechanism could enable recycling of this lipid-anchored SNARE from the vacuole independent of retrograde transport.
Keywords: Ykt6, palmitoylation, vacuole, acylation, CAAX box
Introduction
Membrane fusion along the endocytic and secretory pathways is tightly regulated. Rab guanosine triphosphate hydrolases (GTPases) and tethering complexes control membrane docking before N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) from opposing membranes form tight four-helix bundles (trans-SNARE complexes) that drive fusion (Bonifacino & Glick, 2004). Most SNAREs are permanently anchored to membranes by means of carboxy-terminal transmembrane domains, although some exceptions exist, suggesting that regulation of SNARE association to membranes may impart an additional level of fusion control (Dietrich et al, 2003).
The farnesylated SNARE Ykt6 has been found in both cytosolic and membrane-associated pools (McNew et al, 1997), implicating that it cycles in a regulated manner. In contrast to many other SNAREs, Ykt6 is involved in multiple intracellular fusion reactions (reviewed by Rossi et al, 2004). On the yeast vacuole, Ykt6 is needed for homotypic fusion (Ungermann et al, 1999), but does not seem to be present in the transsNARE complex that forms during vacuole docking (Dietrich et al, unpublished data). Recently, it was shown that Ykt6 is required for palmitoylation of the fusion factor Vac8 (Dietrich et al, 2004). Ykt6 binds palmitoyl-CoA (Pal-CoA) by means of its conserved amino-terminal longin domain and mediates Vac8 acylation in an early stage of vacuole fusion.
Ykt6 has a C-terminal CAAX motif that contains two conserved cysteines (CCIIM; Tochio et al, 2001), and the more C-terminal cysteine is farnesylated (Yalovsky et al, 1999; Long et al, 2002). As farnesylation alone is not sufficient to anchor proteins firmly to membranes (Yalovsky et al, 1999), we suggested that palmitoylation of the second cysteine could contribute to stable membrane localization, similar to Ras GTPase isoforms (Hancock, 2003). Here, we show that yeast Ykt6 is palmitoylated at the CAAX box cysteines as also observed for mammalian Ykt6 (Fukasawa et al, 2004; Veit, 2004), and this in combination with farnesylation confers tight membrane binding. Strikingly, the stable membrane association of Ykt6 gets lost during the vacuole fusion reaction, as Ykt6 gets efficiently released from vacuoles in a priming-dependant manner. This indicates that a palmitoylation/depalmitoylation cycle might dynamically control the localization of the widely distributed SNARE protein, thereby regulating the intracellular membrane flow.
Results and Discussion
Ykt6 is palmitoylated in vitro
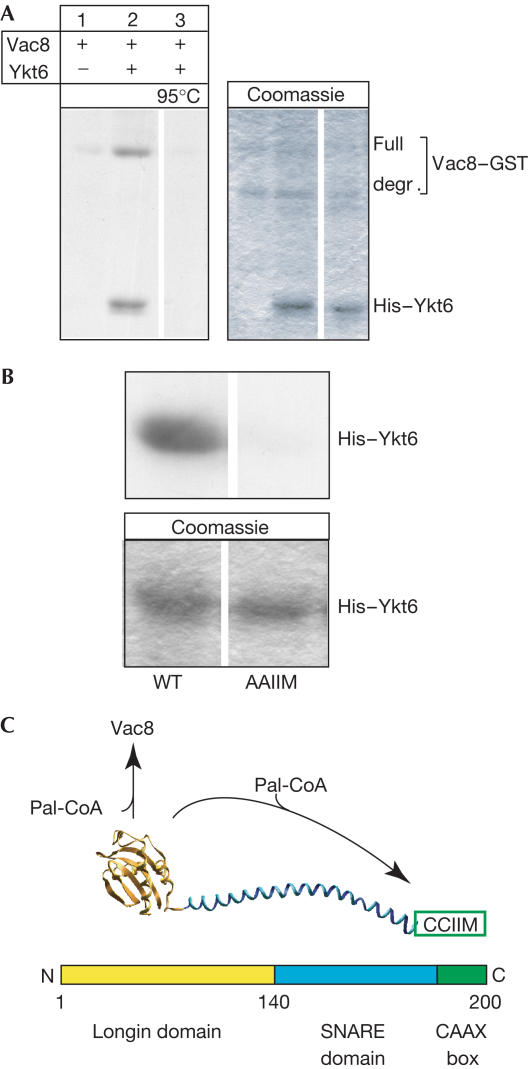
When analysing in vitro acylation of Vac8 in the presence of Ykt6, both purified from Escherichia coli, we observed acylation of Ykt6 (Fig 1A; supplementary Fig S1 online). Importantly, whereas denatured Vac8 does not affect Ykt6 acylation (data not shown), denaturation of Ykt6 prevents palmitoylation of both proteins (Fig 1A). This verifies the specificity of the two acylation events and demonstrates that the proper folding of Ykt6 is of crucial importance for both reactions. Ykt6 contains three cysteines: one nonconserved cysteine within the longin domain, and two conserved cysteines within the C-terminal CAAX box (see Fig 1C). When the C-terminal cysteines were mutated to alanines, acylation of Ykt6 was completely abolished (Fig 1B), suggesting that they are the targets of palmitoylation. As the cysteine residue nearest the C-terminal is farnesylated in vivo (Long et al, 2002), our data point to the upstream cysteine as the relevant palmitoylated residue, although it is possible that both sites are used in vitro. Fukasawa et al (2004) reported that the equivalent cysteine in mammalian Ykt6 also gets palmitoylated in vivo, indicating that palmitoylation of the Ykt6 CAAX box is conserved in evolution.
Figure 1.
Palmitoylation of Ykt6. (A) In vitro palmitoylation of Ykt6. Recombinant proteins (His–Ykt6 and Vac8–GST) were incubated in PSK buffer (PS buffer plus 125 mM KCl) and [3H]Pal-CoA for 30 min at 26°C, and then precipitated and analysed by SDS–PAGE and fluorography. Ykt6 in lane 3 had been preheated for 10 min at 95°C. The Coomassiestained gel is shown on the right. All lanes shown are taken from the same gel but have been regrouped for clarity. (B) Palmitoylation occurs at the C-terminal CAAX box. Incubations were as in (A) with His–Ykt6 (3 μg). Samples were processed as before. Only Ykt6 bands are shown. (C) Model of the Ykt6 domain structure. Arrows indicate the palmitoylation targets of Ykt6.
Analysis of purified yeast Ykt6
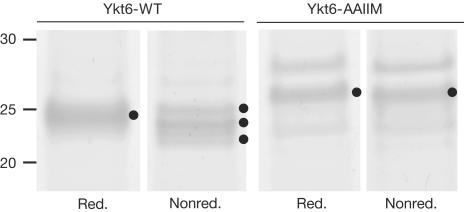
To address whether dual lipidation of yeast Ykt6 occurs in vivo, we purified wild-type protein and a mutant lacking both C-terminal cysteines (Ykt6-AAIIM) from yeast. The wild-type protein should receive several modifications: farnesylation at C197, which is followed by the removal of the IIM peptide and carboxymethylation (Schmidt et al, 1998), and potential palmitoylation. We expected these modifications to be reflected in the migration pattern on SDS–polyacrylamide gel electrophoresis (SDS–PAGE). For our analysis, we purified tagged Ykt6 from yeast and analysed its migration on gradient gels (Fig 2). Under reducing conditions, which trigger cleavage of thioester-bound palmitate (Veit et al, 2001), both wild-type and mutant Ykt6 ran as distinct bands, although with different mobilities (Ykt6-WT: 25 kDa; Ykt6-AAIIM: 27 kDa). This difference is most probably due to the farnesylation and proteolytic processing of the CCIIM motif of Ykt6-WT. Strikingly, nonreducing conditions, which should preserve a palmitate modification, yielded a very different picture: whereas Ykt6-AAIIM, which cannot get acylated, did not change its mobility, Ykt6-WT separated into three distinct bands. As prenyl anchors (which are attached to proteins by thioether bonds) are not affected by reducing conditions, we ascribe the enhanced mobility to palmitoylation of the CAAX box of Ykt6.
Figure 2.
Gel mobility of purified Ykt6 and the AAIIM mutant. His–Ykt6-WT and His–Ykt6-AAIIM were purified from Gal-overproducing strains as described in Methods, and analysed by reducing or nonreducing 4–10% gradient gels (Invitrogen, Karlsruhe, Germany). Indicated bands were identified by MALDI mass spectrometry.
Palmitoylation of Ykt6 is essential for viability
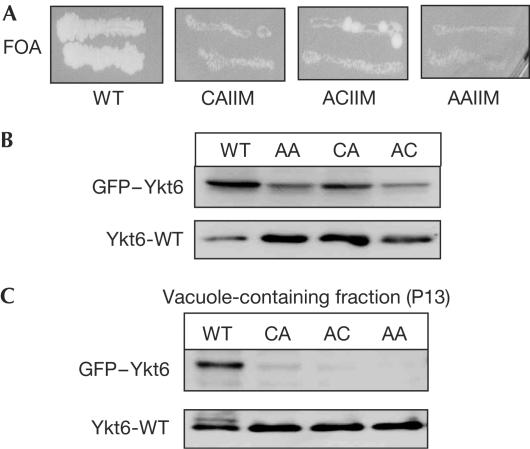
To evaluate the functional consequences of modifications in the CAAX box of Ykt6, we determined whether both C-terminal cysteines are essential for yeast survival. As shown in Fig 3A, wild-type Ykt6, but none of the CAAX box mutants, rescued growth of a ykt6 deletion strain. Thus, both the farnesylated cysteine (C197) and the presumably palmitoylated cysteine (C196) are essential for yeast viability and Ykt6 function in vivo. This indicates that dual lipidation is an essential feature of Ykt6 function.
Figure 3.
Analysis of Ykt6 CAAX box mutants. (A) In vivo analysis. CUY434 (BY4743 ykt6Δ; pRS416.YKT6Pr-YKT6; contains Ykt6-WT on a plasmid with a URA selection marker) was transformed with plasmids encoding Ykt6 with the respective mutations in the CAAX box and plated on 5-FOA to induce loss of the original plasmid. CAIIM, ACIIM and AAIIM indicate the C-terminal amino acids that were mutated (WT=CCIIM). (B,C) Expression and membrane localization of CAAX box mutants. CUY434 was transformed with vectors encoding Ykt6 wild type or mutants (all are internally GFP-tagged to distinguish them from the endogenous protein). (B) Cells were lysed in 0.25 N NaOH, 140 mM β-mercaptoethanol (β-ME), 3 mM PMSF, and then TCA-precipitated and analysed by SDS–PAGE and western blotting. (C) To obtain membranes, cells were lysed as for vacuole purifications, except that the lysis buffer was 200 mM sorbitol, 50 mM KOAc, 2 mM EDTA, 20 mM Hepes–KOH (pH 6.8) and 1 × PIC (Haas, 1995). The total lysate was cleared by centrifugation at 400g (5 min, 4°C) and the resulting supernatant was centrifuged for 15 min at 13,000g. The resulting ‘P13' pellets were collected and diluted to 0.3 mg/ml in PS buffer containing 0.1 × PIC. Proteins were analysed as above.
Farnesylation alone is not sufficient to anchor a protein to a membrane (Yalovsky et al, 1999). To test whether both CAAX box cysteines are required for membrane localization, we analysed membrane association of the mutants. For this, we expressed green fluorescent protein (GFP)-tagged wild-type or mutant Ykt6 in a wild-type background (Fig 3B), and found that removal of both cysteines abolished Ykt6 membrane localization (Fig 3C). Exchange of the single cysteines also interfered with membrane binding, indicating that the combination of palmitoylation and farnesylation stably anchors Ykt6 to membranes.
Ykt6 release at the yeast vacuole
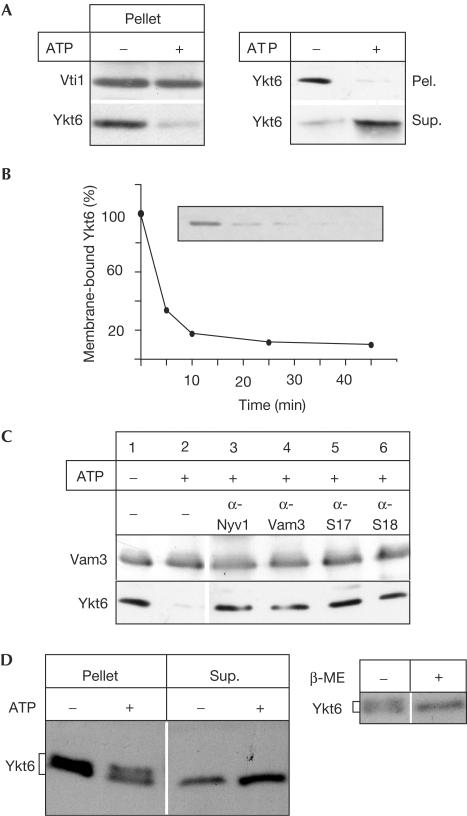
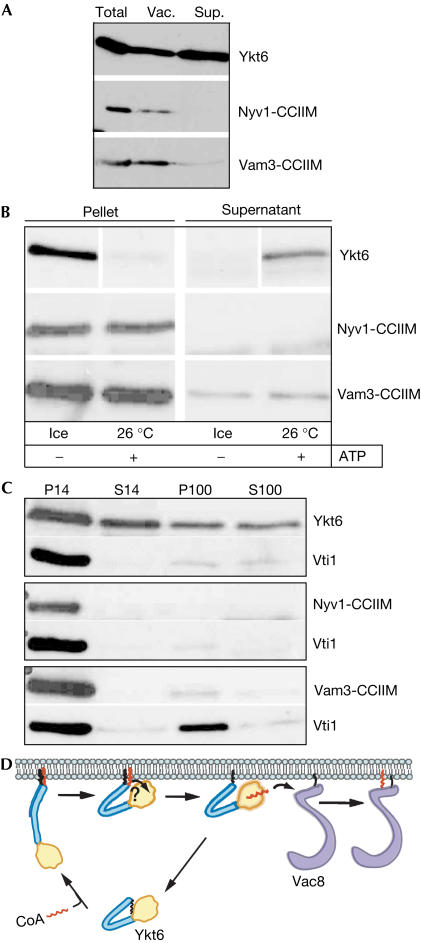
The SNARE Ykt6 is present in a soluble and a membrane-bound pool (McNew et al, 1997; Hasegawa et al, 2003; Fukasawa et al, 2004). The dynamics of Ykt6 that establish these pools are not understood. Here, we demonstrate that Ykt6 is released efficiently from yeast vacuoles on incubation under fusion conditions (Fig 4). This was not due to degradation, as we recovered the protein in the reaction supernatant (Fig 4A). Release was complete within the first 10 min of the fusion reaction (Fig 4B), which is coincident with Sec17 release, a marker for an early stage of the fusion reaction (priming; Mayer et al, 1996). As Ykt6 is localized to multiple membranes (McNew et al, 1997; Zhang & Hong, 2001; Hasegawa et al, 2003), and vacuole preparations are contaminated by endoplasmic reticulum (ER), endosome and Golgi membranes (our unpublished observations), we analysed specificity of the release. Antibodies that block vacuole SNARE complex disassembly and Sec17 release also inhibited Ykt6 release (Fig 4C), confirming simultaneously that this event is vacuole specific and dependent on vacuole priming. Before priming (that is, on freshly isolated vacuoles in the absence of ATP), vacuole-bound Ykt6 resisted carbonate extraction (not shown), suggesting that it is the dual lipid anchor and not protein–protein interactions that confers tight membrane binding. Next, we asked whether we could correlate the differences in Ykt6 mobility, shown in Fig 2, with the release reaction. Indeed, two forms of wild-type Ykt6 can be resolved when vacuoles are lysed in nonreducing sample buffer, and one form disappears when reducing conditions are used (Fig 4D, right). When vacuoles are incubated under fusion conditions, both forms are detected on the membrane, but, strikingly, only the lower form is released into the reaction supernatant (Fig 4D, left). This indicates that only the depalmitoylated form is released. These observations are in agreement with a recent report stating that palmitoylated Ykt6 is only found in membrane fractions (Fukasawa et al, 2004).
Figure 4.
Release of Ykt6 from yeast vacuoles. (A) ATP-dependent release of Ykt6. Vacuoles (30 μg), isolated from BJ3505, the vacuolar protease-deficient strain, were incubated for 10 min at 26°C in fusion buffer (20 mM PIPES, pH 6.8, 200 mM sorbitol, 125 mM KCl, 5 mM MgCl2), CoA (10 μM), with or without an ATP-regenerating system (0.5 mM ATP, 40 mM creatine phosphate, 0.1 mg/ml creatine kinase), and then pelleted (10 min, 12,000g, 4°C). Proteins of the supernatant (Sup.) were precipitated with 13% trichloroacetic acid, and the pellet fraction (Pel.) was washed in 500 μl of PSK buffer before analysis by SDS–PAGE and western blotting. (B) Time course of Ykt6 release. Incubations and analysis were as for the pellet fractions in (A). At the indicated time points, samples were removed and set on ice. (C) Inhibitor analysis of Ykt6 release. An experiment showing Sec17 release (Dietrich et al, 2004) was redecorated with Ykt6 and Vam3 antibodies. (D) Ykt6 migrates as a single band on treatment with reducing agent (right) or release from the membrane (right). Right: isolated vacuoles were heated in sample buffer in the absence or presence of β-mercaptoethanol (β-ME). Left: protease-deficient vacuoles were incubated in standard fusion reactions (45 μl), in the absence or presence of ATP for 30 min. Vacuole membranes were re-isolated (pellet) and supernatants were TCA-precipitated (Sup.). In both experiments, Ykt6 was analysed by nonreducing 12% SDS–PAGE (10-cm-long gel). Note that mobility of Ykt6 forms depends on the gel system used.
Would other vacuolar SNAREs undergo a similar release if equipped with the CCIIM-anchoring motif of Ykt6? To answer this question, we replaced the transmembrane domains of the second vacuolar R-SNARE, Nyv1, and the Q-SNARE Vam3 with CCIIM motifs. These CCIIM fusion proteins localized to vacuole membranes, as determined by cellular fractionation (Fig 5A). However, whereas Ykt6 was released from vacuoles during an ATP incubation, both Vam3-CCIIM and Nyv1-CCIIM remained membrane bound (Fig 5B). Thus, release from vacuoles is specific to Ykt6, and not due to the presence of a CCIIM anchor alone. A large portion of the released Ykt6 is soluble, as it can be recovered from the highspeed supernatant (Fig 5C).
Figure 5.
Release of CCIIM-anchored vacuolar SNAREs. (A) Fractionation of lipid-anchored SNARE. Cells expressing CCIIM-anchored Nyv1 or Vam3 were prepared for vacuole preparations, but before centrifugation, aliquots of total cell extract (total) were removed and placed into sample buffer or centrifuged (30 min, 100,000g, 4°C). The supernatant fraction was TCA-precipitated. Proteins were analysed as before. Total and vacuoles, 30 μg each; supernatant was obtained from 150 μg of total. (B) Ykt6 release assay, performed as in Fig 4A. (C) Solubility of released Ykt6. Following the ATP-dependent release, reaction samples were centrifuged (10 min, 14,000g, 4°C). Half of the supernatant was TCA-precipitated (S14), and the other half was centrifuged again (30 min, 100,000g, 4°C). The resulting supernatant (S100) was TCA-precipitated. Pellets from each centrifugation (P14 and P100) were resuspended in sample buffer and analysed as before. Vti1 served as a control for a membrane-bound protein. (D) Model of the Ykt6 cycle. The Ykt6 longin domain is shown in yellow, and the SNARE domain in blue. The prenyl (black) and the palmitate anchor (red) are indicated. Vac8 is shown in purple.
Taking into consideration that cytosolic mammalian Ykt6 is not palmitoylated (Fukasawa et al, 2004), mammalian Ykt6 can functionally replace yeast Ykt6 (McNew et al, 1997) and yeast Ykt6 localizes to the same compartments as neuronal Ykt6 (Hasegawa et al, 2003), our data suggest that partitioning between cytosol and membranes is specific for Ykt6. Depalmitoylation might be responsible for Ykt6 dissociation from membranes.
We therefore propose that the release of Ykt6 is coupled to depalmitoylation at the C-terminal CAAX box, completing a palmitoylation/depalmitoylation cycle of Ykt6. Importantly, Vac8 acylation on isolated vacuoles is restricted to early stages of the fusion reaction (Veit et al, 2001), suggesting a connection between Ykt6 release and Vac8 acylation. On the basis of this observation and our findings that (i) antibodies to Ykt6 do not inhibit fusion with the same kinetics as other SNARE antibodies (Dietrich et al, 2004) and (ii) Ykt6 is needed before but not during transsNARE complex formation (Dietrich et al, unpublished data), we propose the following model: contrary to its role at other organelles, Ykt6 does not function as a SNARE in vacuole fusion. Rather, it is specifically targeted to the vacuole by virtue of its SNARE domain, where it palmitoylates Vac8, and is released, facilitating its recycling (Fig 5D).
On the basis of these observations, we speculate that not only membrane binding, but also Ykt6 release are highly regulated events. In the cytosol, Ykt6 exists in a closed conformation (Hasegawa et al, 2003), potentially accommodating its farnesyl anchor within the N-terminal longin domain (Tochio et al, 2001; Hasegawa et al, 2004). This seems to be an essential intermediate of Ykt6, as mutations that block the interaction of N and C termini also affect yeast viability (Tochio et al, 2001). At the membrane, closed Ykt6 may be recognized by a receptor that triggers opening of the protein and membrane insertion of its farnesyl anchor. Subsequent palmitoylation would then ensure its stable anchoring. As Ykt6 can bind Pal-CoA by means of its N-terminal longin domain (Dietrich et al, 2004), is able to palmitoylate Vac8 and is efficiently acylated in vitro (Fig 1), we speculate that Ykt6 palmitoylates itself, as also suggested for human Ykt6 (Veit, 2004).
Release of Ykt6 from the vacuole is probably associated with its deacylation, and would be consistent with the observation that only membrane-bound Ykt6 is palmitoylated in mammalian cells (Fukasawa et al, 2004). So far, only one thioesterase, Apt1, has been identified in yeast. It is a nonessential cytosolic protein that specifically depalmitoylates Gα (Duncan & Gilman, 2002). An apt1 deletion does not affect any of the Vac8- and Ykt6-related functions we assayed for, and does not affect Ykt6 release (supplementary Fig S2 online). It is possible that another Ykt6-specific thioesterase resides on the vacuole or that deacylation of Ykt6 could directly be coupled to, for example, Vac8 acylation. In this context, it is noteworthy that Ykt6 release and Vac8 acylation both occur early in the vacuole fusion reaction, indicating a possible palmitate transfer from Ykt6 to Vac8 (Fig 5D; Veit et al, 2001). This, however, does not exclude the existence of other palmitate acceptors at the vacuole, as vacuoles deleted for vac8 still release Ykt6 (not shown).
Our data suggest that a dynamic palmitoylation/depalmitoylation cycle controls Ykt6 distribution. We propose two scenarios of how this cycle might be used in the endomembrane system: (i) Ykt6 might cycle on and off each of its target membranes (that is, Golgi, endosome, vacuole), resulting in multiple local cycles (reviewed by Rossi et al, 2004), and (ii) Ykt6 might enter specifically at the ER and eventually be released from vacuoles, constituting one cycle through the endomembrane system: farnesylated proteins are usually modified at their CAAX box by removal of their C-terminal IIM residues and carboxymethylation. The Caax protease Rce1 is an ER-localized enzyme (Schmidt et al, 1998), suggesting that newly synthesized farnesylated proteins, including Ykt6, initially associate with the ER. The ER could also be the site where subsequent Ykt6 acylation occurs to ensure stable membrane binding. From the ER, Ykt6 could move through the Golgi to the yeast vacuole as the terminal organelle, from which it is released (Fig 4). Release would complete the Ykt6 cycle through the endomembrane system, facilitating its recycling from the vacuole independent of degradation or retrograde transport (Bryant et al, 1998). This would be consistent with the behaviour of other prenylated proteins, such as Ras-like small GTPases, which cycle between membranes and cytosol to control their activation and localization (Segev, 2001). We speculate that the dynamic distribution of the most conserved SNARE, Ykt6, contributes to the control of the cellular membrane flux.
Methods
Biochemical reagents and antibodies. All biochemical reagents were purchased from Sigma (Steinheim, Germany) or Roth (Karlsruhe, Germany), unless indicated. All reagents added to vacuoles were prepared in, or dialysed into, PS (10–20 mM PIPES–KOH, pH 6.8, 200 mM sorbitol) buffer. Polyclonal antibodies were as described previously (Dietrich et al, 2004).
Yeast strains and molecular biology. Saccharomyces cerevisiae strains used are listed in supplementary Table S1 online. Yeast strains were cultured in YPD medium, except where indicated. Details on yeast strains and plasmids, and recombinant proteins are included in the supplementary information online.
Purification of His–Ykt6 from yeast. CUY437 (BY4743 pep4Δ:HIS3; pGALPatG1L-His-YKT6) or CUY569 (BY4743 pep4Δ:HIS3; pGALPatG1L-His-Ykt6AAIIM) strains were grown overnight in 2 l of SGC-ura-his to an OD600 of 2. Cells were collected by centrifugation (10 min, 5,000g), washed in lysis buffer (20 mM PIPES–KOH, pH 6.8, 125 mM KCl, 0.5 × PIC, 1 mM PMSF) and frozen as droplets in liquid nitrogen. Cells were then opened by grinding in a powder mill in liquid nitrogen. The cell powder was thawed, diluted in 2 ml of lysis buffer and centrifuged (30 min, 100,000g, 4°C). The soluble supernatant was loaded onto Ni-NTA agarose (Qiagen, Hilden, Germany) and incubated overnight at 4°C on a nutator. Beads were washed with lysis buffer containing 10 mM imidazole, and bound proteins were eluted in a step elution in 300 mM imidazole. The eluate was analysed by gradient nonreducing 4–10% gels and Coomassie staining.
In vitro palmitoylation. In vitro palmitoylation with [3H]Pal-CoA using recombinant Vac8–GST and His6–Ykt6 was as described previously (Dietrich et al, 2004).
Vacuole isolation. Vacuoles were isolated from the indicated yeast strains by DEAE–dextran lysis and Ficoll gradient centrifugation as described previously (Haas, 1995).
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400350s1.pdf).
Supplementary Material
Supplementary Materials
Acknowledgments
We thank M. Knop and R. Gurezka for plasmids, and G. Müller and N. Decker for expert technical assistance. This work was supported by a grant from the Deutsche Forschungsgemeinschaft, the SFB638, the EMBO Young Investigator Programme, the Fonds der Chemischen Industrie and by predoctoral fellowships of the Boehringer Ingelheim Fonds (to L.E.P.D.), the National Science Foundation NSFGRFP (to T.J.L.) and the Landesgraduiertenförderung Baden-Württemberg (to K.P.).
References
- Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116: 153–166 [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Weisman LS, Stevens TH (1998) Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol 142: 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LE, Boeddinghaus C, LaGrassa TJ, Ungermann C (2003) Control of eukaryotic membrane fusion by N-terminal domains of SNARE proteins. Biochim Biophys Acta 1641: 111–119 [DOI] [PubMed] [Google Scholar]
- Dietrich LE, Gurezka R, Veit M, Ungermann C (2004) The SNARE Ykt6 mediates protein palmitoylation during an early stage of homotypic vacuole fusion. EMBO J 23: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG (2002) Characterization of Saccharomyces cerevisiae acyl-protein thioesterase 1, the enzyme responsible for G protein α subunit deacylation in vivo. J Biol Chem 277: 31740–31752 [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Varlamov O, Eng WS, Söllner TH, Rothman JE (2004) Localization and activity of the SNARE Ykt6 determined by its regulatory domain and palmitoylation. Proc Natl Acad Sci USA 101: 4815–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A (1995) A quantitative assay to measure homotypic vacuole fusion in vitro. Methods Cell Sci 17: 283–294 [Google Scholar]
- Hancock JF (2003) Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol 4: 373–384 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Zinsser S, Rhee Y, Vik-Mo EO, Davanger S, Hay JC (2003) Mammalian Ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain. Mol Biol Cell 14: 698–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Yang Z, Oltedal L, Davanger S, Hay JC (2004) Intramolecular protein–protein and protein–lipid interactions control the conformation and subcellular targeting of neuronal Ykt6. J Cell Sci 117: 4495–4508 [DOI] [PubMed] [Google Scholar]
- Long SB, Casey PJ, Beese LS (2002) Reaction path of protein farnesyltransferase at atomic resolution. Nature 419: 645–650 [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A (1996) Sec18p (NSF)-driven release of Sec17p (αsNAP) can precede docking and fusion of yeast vacuoles. Cell 85: 83–94 [DOI] [PubMed] [Google Scholar]
- McNew JA, Sogaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Söllner TH (1997) Ykt6p, a prenylated SNARE essential for endoplasmic reticulum–Golgi transport. J Biol Chem 272: 17776–17783 [DOI] [PubMed] [Google Scholar]
- Rossi V, Banfield DK, Vacca M, Dietrich LEP, Ungermann C, D'Esposito M, Galli T, Filippini F (2004) Longins and their longin domains: regulated SNAREs and multifunctional SNARE regulators. Trends Biol Sci 29: 682–688 [DOI] [PubMed] [Google Scholar]
- Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S (1998) Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc Natl Acad Sci USA 95: 11175–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N (2001) Ypt/rab GTPases: regulators of protein trafficking. Sci STKE 100: 1–18 [DOI] [PubMed] [Google Scholar]
- Tochio H, Tsui MM, Banfield DK, Zhang M (2001) An autoinhibitory mechanism for nonsyntaxin SNARE proteins revealed by the structure of Ykt6p. Science 293: 698–702 [DOI] [PubMed] [Google Scholar]
- Ungermann C, von Mollard GF, Jensen ON, Margolis N, Stevens TH, Wickner W (1999) Three vsNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol 145: 1435–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M (2004) The human SNARE protein Ykt6 mediates its own palmitoylation at C-terminal cysteine residues. Biochem J 384: 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Laage R, Dietrich L, Wang L, Ungermann C (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J 20: 3145–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodr Guez-Concepcion M, Gruissem W (1999) Lipid modifications of proteins—slipping in and out of membranes. Trends Plant Sci 4: 439–445 [DOI] [PubMed] [Google Scholar]
- Zhang T, Hong W (2001) Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum–Golgi transport. J Biol Chem 276: 27480–27487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials