Abstract
In mammals, the master circadian pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN is thought to drive peripheral oscillators by controlling neuronal and humoral signals that can entrain the peripheral clocks. Here, we show that prostaglandin E2 (PGE2), a proinflammatory compound known to have diverse biological effects, is able to act as an in vivo clock-resetting agent. We find that in cultured NIH3T3 fibroblasts, PGE2 is able to induce transient expression of Period 1 messenger RNA and the following circadian oscillation of clock gene expression. Furthermore, we demonstrate that intraperitoneal administration of PGE2 results in the phase shift of circadian gene expression in mouse peripheral tissues in a time-dependent manner. This phase shift is also induced by the EP1/EP3 agonist sulprostone but not by the EP2 agonist butaprost. The PGE2-induced phase shift is inhibited by the EP1 antagonist SC-51322. These results suggest that PGE2 acts as an in vivo clock-resetting factor by means of the EP1 subtype of PGE receptors.
Keywords: circadian rhythm, clock, periphery, PGE2, phase shift
Introduction
In mammals, the master circadian pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, but circadian oscillators also exist in most peripheral tissues (Reppert & Weaver, 2001). The SCN receives light information by means of the visual pathway from the retina to entrain the clock to the light–dark cycles of the environment, and it is thought to drive peripheral oscillators by controlling neuronal and humoral signals that can entrain the peripheral clocks (Yamazaki et al, 2000). Circadian gene expression can also be observed in cultured cell lines in vitro (Balsalobre et al, 1998; Akashi & Nishida, 2000). In these cell lines, various stimuli are known to induce the immediate expression of Period 1 (Per1) messenger RNA and the following circadian gene expression of clock and clock-controlled genes (Balsalobre et al, 2000a). However, in vivo clock-resetting factors that synchronize peripheral oscillators have not yet been fully clarified. It has been reported that glucocorticoid hormones, which are secreted in daily cycles, are able to reset the peripheral oscillators without affecting the SCN pacemaker (Balsalobre et al, 2000b). The glucocorticoid receptor, however, is dispensable for the entrainment of circadian gene expression in liver under steadystate conditions (Balsalobre et al, 2000b), indicating the existence of other intrinsic timing cues for the circadian oscillators in vivo. In the present study, we show that prostaglandin E2 (PGE2) is able to reset the rhythm-generating core feedback loops of circadian gene expression in murine peripheral tissues. PGE2 is a proinflammatory compound known to have diverse biological effects, such as hyperthermia and waking effect (Narumiya et al, 1999; Yoshida et al, 2000; Bos et al, 2004), and PGE2 secretion levels show diurnal variations in several tissues (Pandey et al, 1995; Yosipovitch et al, 1995). Our finding demonstrates that PGE2 acts as an in vivo clock-resetting agent.
Results And Discussion
Circadian rhythms induced by PGE2 in NIH3T3 cells
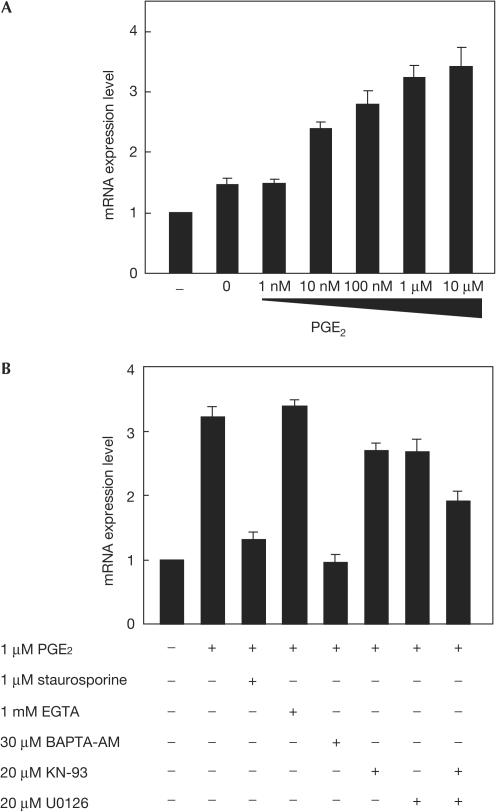
We searched for a new candidate for a clock-resetting agent and found that PGE2 treatment of NIH3T3 cells is able to induce the acute and transient expression of mPer1 mRNA in a dose-dependent manner (Fig 1A). The immediate and acute induction of Per1 mRNA expression is strongly associated with the entrainment of circadian rhythms both in vivo and in vitro (Shigeyoshi et al, 1997; Balsalobre et al, 1998). To investigate the downstream signalling pathway participating in this transient expression of mPer1, we tested a wide range of inhibitors for their ability to prevent the PGE2-induced mPer1 expression. As shown in Fig 1B, mPer1 expression was significantly inhibited by pretreatment of cells with the widespectrum kinase inhibitor staurosporine (P<0.0001 by Student's t-test), indicating that some kinase-dependent pathways contribute to the induction. Moreover, this mPer1 expression was strongly inhibited by the intracellular Ca2+ chelator BAPTA-AM (P<0.001 by Student's t-test), but not by the extracellular Ca2+ chelator EGTA, suggesting that intracellular Ca2+ store is responsible for the activation of mPer1 expression. We also found that both the Ca2+/calmodulin-dependent kinase II (CaMK II) inhibitor KN-93 and the MEK inhibitor U0126 slightly but significantly inhibited the mPer1 induction (P<0.05 by Student's t-test), and cotreatment with KN-93 and U0126 resulted in more effective inhibition of this mPer1 induction by PGE2 (P<0.01 by Student's t-test). In contrast, the PKA inhibitor H-89 or Rp-8-Br-cAMPS, the PKC inhibitor bisindolylmaleimide I, the PKG inhibitor KT5823, the epidermal growth factor (EGF) receptor kinase inhibitor AG1478, the casein kinase I inhibitor CKI-7, the PI3K inhibitor LY294002, the p38 inhibitor SB203580 and the JNK inhibitor SP600125 all failed to inhibit the induction of mPer1 expression by PGE2 (data not shown). These results suggest that PGE2 induces transient expression of mPer1 mRNA in NIH3T3 cells by increasing an intracellular Ca2+ level and activating relevant protein kinases including CaMK II and ERK MAP kinase. Both kinases are known to be activated downstream of Ca2+ signalling (Egea et al, 1999; Hudmon & Schulman, 2002). Among four PGE2 receptor subtypes (EP1, EP2, EP3 and EP4), the EP1 and the EP3 subtypes are thought to be responsible for stimulation of intracellular Ca2+ mobilization (Negishi et al, 1995), although signalling pathways downstream of the EP receptors are fairly complicated and also cell typespecific (Bos et al, 2004). Thus, it is likely that PGE2 induces the transient mPer1 expression mainly by means of the EP1 and/or the EP3 receptor.
Figure 1.
Induction of mPer1 mRNA expression by prostaglandin E2 (PGE2) in NIH3T3 cells. (A) Dose-dependent induction of mPer1 mRNA by PGE2 treatment of NIH3T3 cells. Relative levels of mRNA expression 1 h after the indicated treatment are evaluated by the real-time quantitative PCR method. Each value was normalized to mG3PDH. Values are mean±s.e.m. from three experiments. (B) Effect of inhibitors on mPer1 induction by PGE2 treatment. NIH3T3 cells are treated with the indicated agents 30 min before PGE2 treatment. Values are mean±s.e.m. from three experiments.
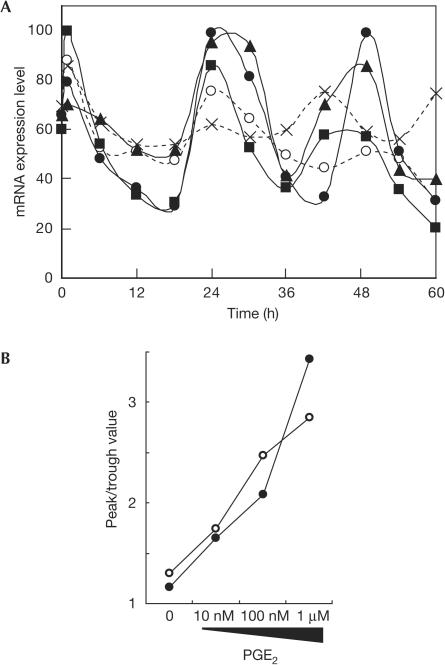
To examine whether circadian oscillation of clock gene expression follows the acute and transient induction of mPer1 expression, we monitored the expression level of clock genes after PGE2 treatment of NIH3T3 cells. As a result, circadian oscillation of mPer2 mRNA expression was clearly observed after PGE2 treatment (Fig 2A). To verify the robustness of circadian oscillation of the mRNA expression level, the extent of amplitude (the difference between the second peak level in the graph and the second trough level) was evaluated. As shown in Fig 2B, the robustness of circadian expression of mPer2 is dose-dependently enhanced by PGE2 treatment. Essentially similar results were obtained for mDBP, another clock-controlled transcription factor (data not shown; Fig 2B). Taken together, these results indicate that PGE2 has the ability to induce robust circadian oscillation of clock gene expression in NIH3T3 fibroblasts.
Figure 2.
Circadian gene expression induced by prostaglandin E2 (PGE2) in NIH3T3 cells. (A) Circadian oscillation of the expression level of mPer2 mRNA induced by varying concentrations of PGE2. At time 0, NIH3T3 cells were treated with no stimulus (crosses), PBS (open circles) and 10 nM (squares), 100 nM (triangles) and 1 μM (filled circles) of PGE2, and the mRNA expression levels were monitored by the real-time quantitative PCR method. Each value was normalized to mG3PDH. Data shown are representative of three independent experiments. (B) Robustness of circadian gene expression induced by PGE2 in NIH3T3 cells. The peak and trough values of mRNA expression levels in the second cycle of oscillation were read from the graph and the extent of amplitude was plotted. Graphs shown are data for mPer2 (filled circles) and mDBP (open circles).
PGE2 induces the phase shift of peripheral clocks
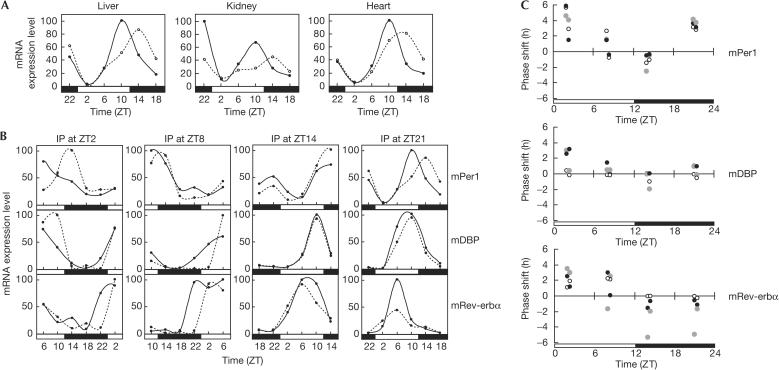
We tested whether PGE2 is able to reset the circadian clocks in peripheral tissues in vivo. Intraperitoneal administration of mice, which were maintained under 12:12 light:dark cycles, with PGE2 at ZT21 (zeitgeber time; light-on at ZT0 and light-off at ZT12) resulted in marked phase shifts of mPer1 expression rhythms in liver, kidney and heart (Fig 3A). The marked immediate induction of mPer1 by PGE2 was observed in kidney but not in liver or heart (Fig 3A). This may be due to the much higher expression level of the EP1 and the EP3 receptors in kidney than in liver or heart (Sugimoto et al, 1992; Watabe et al, 1993). We have also found that the phase shifts of the mPer1 expression rhythm induced by PGE2 show significant variances that depend on the circadian time of the injection (Fig 3B). To enhance reliability, expression profiles of mDBP and mRev-erbα genes were also analysed (Fig 3B). These two genes are known to show robust and high-amplitude circadian oscillations in their mRNA expression levels (Yamaguchi et al, 2000; Preitner et al, 2002). All these results indicate that PGE2 is able to shift the phase of the circadian rhythm in peripheral tissues in a time-dependent manner. The amplitudes of the phase advance or delay obtained from these data were plotted so as to clarify the dependency of phase shifts on the circadian time of PGE2 injection (Fig 3C). The characteristics of PGE2-induced phase shifts are similar among three types of tissue, that is, the phases of the rhythm advance in the early day and delay in the early night. The late day and the late night are thought to be transition phases of the shift direction. Again, the remarkable phase delays of mRev-erbα expression in kidney may reflect high expression levels of the EP1 and the EP3 receptors. The phase shifts throughout the 24-h day seem to be characteristic of peripheral oscillators, whereas the phase shifts of central clock by light are shown to be limited to the night time (Daan & Pittendrigh, 1976; Balsalobre et al, 2000b). Next, we investigated whether the PGE2-induced phase shift is sustained over the second cycle after the injection at ZT21. As a result, the phase shift induced by intraperitoneal injection of PGE2 was kept for only one cycle under light–dark cycles and disappeared in the second cycle (supplementary information online). This result indicates that the PGE2-induced phase shift is an apparent phase shift as observed in the case of dexamethasone (Balsalobre et al, 2000b).
Figure 3.
Phase shifts induced by prostaglandin E2 (PGE2) in mouse peripheral tissues. (A) Phase shifts of circadian rhythm of mPer1 mRNA expression in peripheral tissues by PGE2. Mice were entrained under a 12:12 light:dark cycle for 2 weeks and intraperitoneal administration of PGE2 dissolved in PBS (solid line), or PBS alone (dotted line) was performed at ZT21. Two mice (one PGE2 injected and the other PBS injected) were killed and mRNA was collected from tissues at each time point. The mRNA expression levels were evaluated by real-time quantitative PCR. Each value was normalized to mG3PDH. Data shown are representative of two independent experiments. (B) Variations of the phase shifts of circadian gene expression in liver induced by PGE2 at different time points. Intraperitoneal (i.p.) administration of PGE2 (solid line) and PBS (dotted line) was performed at different circadian time points. The mRNA expression levels of mPer1, mDBP and mRev-erbα were monitored. Data shown are representative of two independent experiments. (C) The amplitudes of phase shifts by PGE2 at each circadian time point (ZT2, ZT8, ZT14 and ZT21). The amplitudes of phase shifts of mPer1, mDBP and mRev-erbα were evaluated in liver (black circles), kidney (grey circles) and heart (open circles). Data from two independent experiments were plotted.
EP1 contribution to the phase shifts by PGE2
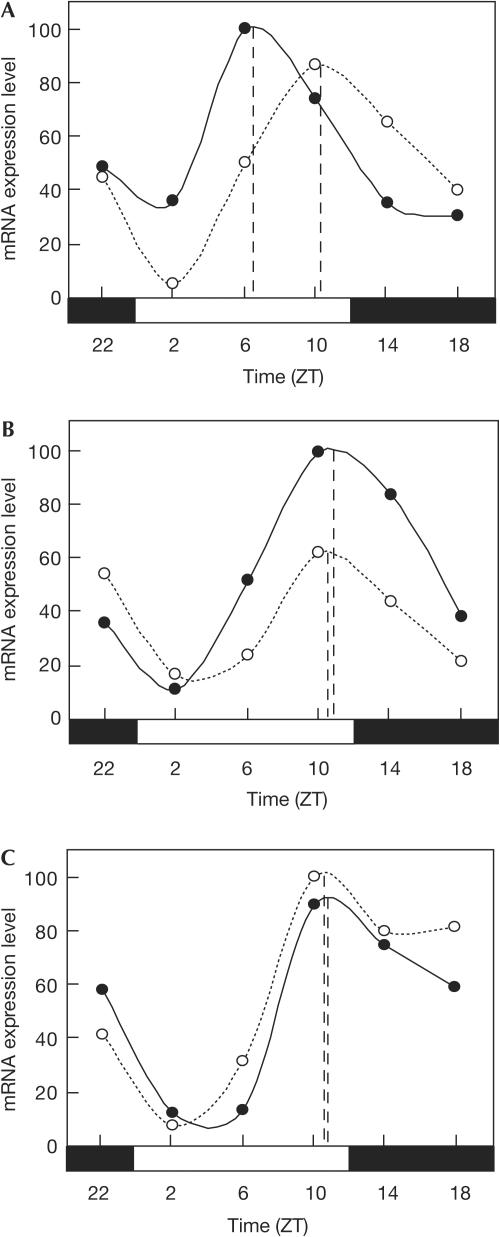
We then examined which subtype of PGE2 receptors is responsible for the phase shifts by PGE2 in vivo. Intraperitoneal administration of an agonist of both the EP1 and the EP3 receptors, sulprostone, was able to induce the phase shift of the mPer1 expression rhythm in liver (Fig 4A). In contrast, an agonist of the EP2 receptors, butaprost, failed to shift the phase of the mPer1 expression rhythm (Fig 4B). Furthermore, an antagonist of the EP1 receptors, SC-51322, markedly inhibited the phaseshifting effect of PGE2 in liver (Fig 4C). These results strongly suggest that the EP1 subtype of PGE2 receptors is responsible for the entrainment of circadian clocks in peripheral tissues. Given the contribution of the EP1 receptors to Ca2+ signalling, the EP1 may have an important role in both transient upregulation of mPer1 mRNA expression and circadian phase shifts in peripheral tissues.
Figure 4.
The EP1 receptor is responsible for the phase shifts by prostaglandin E2 (PGE2). (A) Intraperitoneal administration of sulprostone (solid line) or PBS alone (dotted line) was performed at ZT21. mRNA was collected from liver at each time point and the expression levels of mPer1 mRNA were evaluated by real-time quantitative PCR. Each value of mRNA expression levels was normalized to mG3PDH. Data shown are representative of two independent experiments. (B) Intraperitoneal administration of butaprost (solid line) or PBS alone (dotted line) was performed at ZT21. The expression levels of mPer1 mRNA in liver were monitored as in (A). (C) Intraperitoneal administration of PGE2 together with SC-51322 (solid line), or PBS alone (dotted line) was performed at ZT21. The expression levels of mPer1 mRNA in liver were monitored as in (A).
Locomotor activity rhythm is unaffected by PGE2
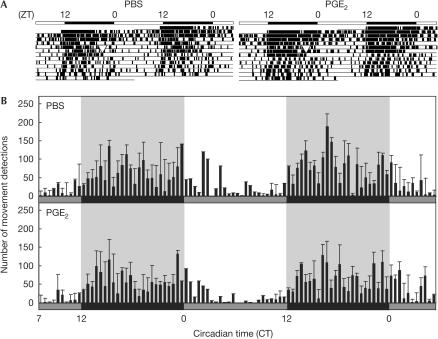
To assess whether PGE2 phase shifts the behavioural rhythm of mice, we monitored their locomotor activity before and after intraperitoneal injection of PGE2. In this experiment, mice were maintained in constant darkness after the injection so as to eliminate the effect of light on their activity rhythm. As a result, no significant difference was observed in the activity onset time between PGE2-injected and phosphate-buffered saline (PBS)-injected mice (Fig 5A), whereas a marked phase advance was observed in the mRNA expression rhythm of mPer1 in peripheral tissues (Fig 3A). To see detailed locomotor activity after PGE2 injection, an averaged actogram for the 2 days following the injection was determined (Fig 5B). There was no significant effect of PGE2 injection on the following activity onset time. This result suggests that PGE2 does not affect the central circadian pacemaker that controls circadian behavioural rhythms. It is likely that PGE2, similar to glucocorticoid hormones, functions as a transducer of the clock-resetting signal from the SCN to the peripheral clocks rather than as a direct resetting agent for the core pacemaker (Balsalobre et al, 2000b).
Figure 5.
Prostaglandin E2 (PGE2) induces no significant alteration in circadian locomotor activity. (A) Representative double-plotted actograms show locomotor activity records of PBS alone (left) or PGE2-injected (right) mouse at ZT21 (arrowheads). Mice were housed in a light–dark cycle for a week and injected with PBS or PGE2 intraperitoneally at ZT21. Then, mice were maintained in constant darkness. (B) Activity profile over 2 days from 10 h after intraperitoneal injection of PBS or PGE2 at ZT21. Bars represent mean (±s.e.m., n=4) summed number of movement detections during 30 min of recording. Grey and black bars represent the subjective day and night, respectively. Shaded background also represents the subjective night. CT0 corresponds to ZT0 in constant dark conditions.
Several agents including glucocorticoid hormones are reported to be able to reset the internal circadian oscillators (Balsalobre et al, 2000b; McNamara et al, 2001). Our results suggest that PGE2 also contributes to the synchronization of circadian oscillators in vivo. Detailed mechanisms showing how PGE2 induces the phase shifts are yet to be shown. It is likely that PGE2 acts directly on circadian oscillators via cell autonomous mechanisms, because PGE2 is able to induce circadian gene expression in cultured NIH3T3 cells (Fig 2). It is also possible, however, that PGE2 acts indirectly by inducing production of other factors. As PGE2 is known to induce recruitment of a variety of cytokines (Hinson et al, 1996), these cytokines may participate in the resetting of the circadian rhythm in vivo. This study has shown a hitherto unidentified function of PGE2, that is, resetting of the rhythm-generating core feedback loops of circadian gene expression in the periphery. The influence of PGE2 on the master circadian pacemaker of the brain remains to be elucidated. Previous studies have suggested a role of PGE2 in the regulation of body temperature and the sleep–wake cycle—two events that are closely related to an output pathway in circadian physiology and behaviour. Thus, PGE2 may have a dual role in the circadian clock: a clock-resetting function in an input pathway and a circadian physiology-regulating function in an output pathway.
Methods
Cell culture and time course experiment. NIH3T3 mouse fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum and antibiotics (100 U penicillin and 0.2 mg kanamycine/ml) at 37°C under 5% CO2. Cells were plated at a density of 2.5 × 105 cells per 35 mm dish. The cells reached confluence 2 days after the plating, and were kept for 2 days in medium containing 1% calf serum before the start of experiments. At time 0, PGE2 (1 mM in PBS, Cayman Chemical, Ann Arbor, MI, USA) was added to the culture medium and then the cells were frozen in liquid nitrogen at indicated time points. Preparation of RNA samples and reverse transcriptase–PCR (RT–PCR) analysis were performed as described previously (Tsuchiya et al, 2003).
Mice. C57BL/6CrSlc wild-type mice were maintained under a 12:12 light:dark cycle for 2 weeks before the start of experiments. Male mice aged 11–13 weeks were used. Animal care was in accordance with institutional guidelines. Intraperitoneal injection of 3 μg/g body weight of PGE2, sulprostone (Cayman), butaprost (Cayman) or SC-51322 (Biomol International LP, Plymouth meeting, PA, USA), all dissolved in PBS or ethanol, was performed under dim red light (during the night) or under ambient light (during the day). At indicated time points, mice were killed by cervical dislocation and tissues were excised and immediately frozen in liquid nitrogen and stored at −80°C. Frozen tissues were homogenized by using QIAshredder (Qiagen, Japan) and then total RNA was extracted by using the RNeasy kit (QIAGEN). RT–PCR analysis was performed as described previously (Tsuchiya et al, 2003). Mouse locomotor activity was measured by using an infrared sensor. The number of movement detections was counted and summed over the subsequent 3 min by the counting processor.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400356s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank T. Yoshino, H. Hayashi and Y. Nakayama for their technical assistance and Dr M. Ikeda for the locomotor activity analysis software. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to E.N.).
References
- Akashi M, Nishida E (2000) Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev 14: 645–649 [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Marcacci L, Schibler U (2000a) Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 10: 1291–1294 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U (2000b) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347 [DOI] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH (2004) Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 36: 1187–1205 [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS (1976) A functional analysis of circadian pacemakers in nocturnal rodents: II. The variability of phase response curves. J Comp Physiol A 106: 253–266 [Google Scholar]
- Egea J, Espinet C, Comella JX (1999) Calcium influx activates extracellular-regulated kinase/mitogen-activated protein kinase pathway through a calmodulinsensitive mechanism in PC12 cells. J Biol Chem 274: 75–85 [DOI] [PubMed] [Google Scholar]
- Hinson RM, Williams JA, Shacter E (1996) Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proc Natl Acad Sci USA 93: 4885–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H (2002) Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71: 473–510 [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA (2001) Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105: 877–889 [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F (1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79: 1193–1226 [DOI] [PubMed] [Google Scholar]
- Negishi M, Sugimoto Y, Ichikawa A (1995) Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta 1259: 109–119 [DOI] [PubMed] [Google Scholar]
- Pandey HP, Ram A, Matsumura H, Satoh S, Hayaishi O (1995) Circadian variations of prostaglandins D2, E2, and F2α in the cerebrospinal fluid of anesthetized rats. Biochem Biophys Res Commun 213: 625–629 [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U (2002) The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2001) Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647–676 [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y et al. (1997) Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Honda A, Hayashi Y, Negishi M, Ichikawa A, Narumiya S (1992) Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem 267: 6463–6466 [PubMed] [Google Scholar]
- Tsuchiya Y, Akashi M, Nishida E (2003) Temperature compensation and temperature resetting of circadian rhythms in mammalian cultured fibroblasts. Genes Cells 8: 713–720 [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugimoto Y, Honda A, Irie A, Namba T, Negishi M, Ito S, Narumiya S, Ichikawa A (1993) Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem 268: 20175–20178 [PubMed] [Google Scholar]
- Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H (2000) Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol 20: 4773–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H (2000) Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Matsumura H, Nakajima T, Mandai M, Urakami T, Kuroda K, Yoneda H (2000) Prostaglandin E (EP) receptor subtypes and sleep: promotion by EP4 and inhibition by EP1/EP2. Neuroreport 11: 2127–2131 [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Yosipovitch Z, Harell D, Ashkenazi I, Erman A (1995) Diurnal rhythm of prostanoid secretion from bone/marrow organ in the rat. Bone 17: 79–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information