Abstract
The components and pathways that regulate and execute developmental cell death programmes in plants remain largely unknown. We have found that the PROMOTION OF CELL SURVIVAL 1 (PCS1) gene in Arabidopsis, which encodes an aspartic protease, has an important role in determining the fate of cells in embryonic development and in reproduction processes. The loss-of-function mutation of PCS1 causes degeneration of both male and female gametophytes and excessive cell death of developing embryos. Conversely, ectopic expression of PCS1 causes the septum and stomium cells that normally die in the anther wall to survive instead, leading to a failure in anther dehiscence and male sterility. PCS1 provides a new avenue for understanding the mechanisms of the programmed cell death processes that are associated with developmental pathways in plants and makes available a useful tool for engineering the male sterility trait for hybrid seed production.
Introduction
A nucleated cell generally carries all the components required for programmed cell death (PCD), and its fate is determined by external signals and internal milieu (Jacobson et al, 1997). Exposure to death-promoting factors and deprivation of survival/growth factors are two primary inputs that lead to cell death (Morita & Tilly, 1999). In animals, PCD is regulated and executed by components and pathways conserved from worms to humans (Lawen, 2003). Death signals are relayed and integrated through intracellular apoptotic networks in which the Bcl-2 family members and the caspases are the main players.
PCD has essential roles in the formation of many types of tissue and organ during embryogenesis in animals, and a failure in regulating normal PCD often causes embryonic lethality (Jacobson et al, 1997). Although PCD is not implicated in shaping body patterns in a plant as extensively as in animals, several invariant cell death patterns are associated with normal seed development, gametogenesis and other developmental processes (Greenberg, 1996; Pennell & Lamb, 1997; Gray & Johal, 1998). However, the components and pathways involved in plant PCD remain largely unknown. Mutations of key components involved in these PCD processes will probably cause embryonic lethality and/or sterility, making it difficult to recover such mutants for detailed characterization or for identification of important genes in regulating and executing PCD by forward genetic approaches. Using a reverse genetic approach, we have identified an Arabiodopsis aspartic protease that functions as an anti-cell-death component.
Results
PCS1 functions in gametogenesis and embryogenesis
The Arabidopsis genome encodes at least 60 putative aspartic proteases (AtASPs). In an effort towards functional characterization of this gene family, we have obtained putative knockout lines for more than 40 AtASP genes from the two large T-DNA tagging populations (Sessions et al, 2002; Alonso et al, 2003). One of them (441-H06) from the Syngenta collection has an insertion in AtASP38 (AGI# At5g02190), which we named PROMOTION OF CELL SURVIVAL 1 (PCS1) because this mutation causes excessive cell death (see below).
PCS1 contains a single exon encoding a 50 kDa polypeptide. Genomic DNA blotting and the sequence analysis of the T-DNA flanking genomic fragments showed that the mutant line carries a single copy of T-DNA inserted in the exon at ∼380 base pairs (bp) 3′ to the start codon of PCS1. This insertion probably results in a null mutation. We were unable to obtain any progeny homozygous for the insertion allele, indicating that the homozygous pcs1 mutant is lethal. As germination of mature seeds from self-pollinated pcs1/+ heterozygous plants is normal, the lethality is probably embryonic. In addition to embryonic lethality, the mutant allele is transmitted to progeny at a lower frequency than the wild-type (wt) allele through both the male and the female. When pollen from the heterozygous plants was used to pollinate wt flowers (in a pi/pi background, which lacks male organs to avoid self-pollination), 36% (310 of 856 plants) of the resulting progeny were resistant to the herbicide BASTA, which is conferred by the BAR gene in T-DNA carried by the mutant allele. Similarly, when wt plants were used to pollinate plants heterozygous for the pcs1 insertion, only 34% (261 of 775 plants) of the progeny were BASTA resistant. PCR analysis for presence of the insertion allele showed that the reduced segregation ratio is indeed due to reduced transmission of the mutant allele and does not result from silencing of the BAR gene in progeny. All of the above mutant phenotypes were rescued in a complementation test using a genomic clone of PCS1 (PCS1t), demonstrating that the mutation of this single gene causes defects in both gametogenesis and embryogenesis. Consistent with the pcs1 phenotype, RNA blotting and the histochemical GUS activity assays of the PCS1 promoter:GUS (PCS1p:GUS) reporter lines indicate that PSC1 is expressed specifically in developing gametophytes and in developing seeds (Fig 1A–D).
Figure 1.
PCS1 functions in gametogenesis and embryogenesis. (A) RNA blot showing that PCS1 is expressed in developing flowers and young siliques (1–8 days post-pollination (DPP)). (B) Arabidopsis inflorescence of a PCS1p:GUS line stained for GUS activity. GUS activity is evident in developing anthers and in pollen (inset). (C,D) Relatively weaker GUS activity was detected in developing ovules (C) and young seeds (∼5 DPP) (D). No GUS activity was detected in other organs. (E) Mature pollen grains from a pcs1/+ qrt1/qrt1 plant (qrt1, quartet1). (F) Viability staining of pollen from a pcs1/+ qrt1/qrt1 plant showed that the pcs1 mutation causes pollen degeneration. The inset shows stained pollen from wt plants. The size difference is due to difference in magnification. (G) Portion of an ovary from a pcs1/+ plant at the flower-opening stage showing two aborted ovules (arrows). (H) Portion of a silique (∼6 DDP) from a pcs1/+ plant showing a degenerated ovule (red arrow) and two immature mutant seeds (white arrows) with abnormal morphology. Scale bars in (G,H), 0.2 mm. (I,J) Confocal laser scanning microscopy images of a wt embryo (I) and a mutant embryo (J) from the same silique of a pcs1/+ plant. (K) Genomic DNA from developing pcs1 mutation seeds showing oligonucleosomal DNA ladders. Genomic DNA was isolated from 4- to 8-day-old mutant and wt seeds, separated on an agarose gel and probed with total genomic DNA. Magnifications: B, × 7; C, × 5; D, × 6; E, × 200; F, × 140.
pcs1 mutation causes excessive cell death
The original T-DNA insertion line was coupled with the quartet1 (qrt1) mutation (Preuss et al, 1994), which keeps the four products of male meiosis (tetrad) attached together, making it easier to examine the fate of pollen. In qrt1/qrt1 pcs1/+ plants, two pollen grains of each tetrad carry the wt PCS1 allele, whereas the other two carry the pcs1 allele. Mutant pollen grains did not show obvious morphological differences from wt pollen (Fig 1E). However, pollen viability staining showed that around 35% of pcs1 pollen grains from qrt1/qrt1 pcs1/+ plants were degenerated (Fig 1F). Observation of ovules from pcs1/+ plants showed that around one-third of the ovules carrying pcs1 mega-gametophytes showed abnormal morphology at the stage of flower opening (Fig 1G, arrows), which turned brown and degenerated a few days post-pollination (DPP; Fig 1H, red arrow). The partial penetrance of the mutant allele through male and female gametophytes may be due to partial compensation by homologues of PCS1 encoded by the genome.
In addition to aborted ovules, around 10%–15% of developing seeds from a self-pollinated pcs1/+ plant are homozygous for the mutant allele, and all pcs1/pcs1 seeds were aborted at early stages (Fig 1H,J). Examination of developing embryos using confocal laser scanning microscopy showed no obvious differences between wt and pcs1 embryos before the heart stage. However, pcs1 embryos underwent degeneration when wt embryos from the same siliques were at the torpedo stage (Fig 1I,J). Genomic DNA isolated from the 4–8 DPP mutant seeds of self-pollinated pcs1/+ plants showed oligonucleosomal (i.e. 180 bp) DNA ladders (Fig 1K), a hallmark of apoptotic cells, which indicates that the pcs1 mutation causes excessive cell death of embryonic tissues.
Ectopic expression of PCS1 blocks anther dehiscence
We have generated more than 50 independent transgenic Arabidopsis lines that express PCS1 or epitope-tagged PCS1 under the control of the cauliflower mosaic virus 35S promoter (35S). The epitope-tagged constructs carry PCS1 fused at its 3′ terminus with a tandem tag (PCS1–FAST, see below) or a 6 × polyhistidine tag (PCS1–HIS). The transgenic lines showed no obvious phenotype during the vegetative stage; however, more than one-third of these lines carrying either of the constructs was found to be partially to almost completely sterile. Northern blotting and/or western blotting indicated that the lines showing higher sterility expressed higher levels of the transgenes (Fig 2). Further examination showed that PCS1 ectopic expression does not affect female fertility or seed development, but causes male sterility by blocking and delaying anther dehiscence (Fig 3A,B). Normal pollen grains were observed when the anther wall was torn apart, and they were fully able to fertilize egg cells when hand-pollinated. Therefore, the sterility of the 35Sp:PCS1 lines is caused solely by the defect in anther dehiscence.
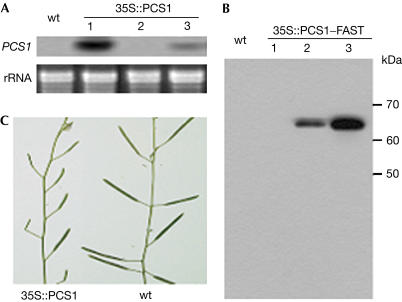
Figure 2.
Ectopic overexpression of PCS1 leads to sterility. (A) RNA gel analysis of some transgenic lines expressing 35S:PCS1. Total RNA was extracted from the inflorescence and probed with the labelled PCS1 fragment. The endogenous PCS1 transcript was not detected due to a short exposure time. (B) Western blot analysis of some transgenic lines expressing 35S:PCS1–FAST. Total protein was extracted from the inflorescence and probed with the anti-FLAG-M2 monoclonal antibody. The difference in the expression levels of the transgenes in the independent transgenic lines is probably due to positional effects. The lines 35S:PCS1-1 and 35S:PCS1–FAST-3 are highly sterile, 35S:PCS1-3 and 35S:PCS1–FAST-2 are partially sterile, and 35S:PCS1-2 and 35S:PCS1–FAST-1 are fully fertile. (C) Portion of a highly sterile 35S:PCS1-1 plant and a wt plant. Magnification: C, × 0.7.
Figure 3.
Overexpression of PCS1 prevents normal cell death associated with anther dehiscence. (A,B) An open wt flower (A) showed normal anther dehiscence, whereas the one from a 35S:PCS1–FAST plant (B) failed to dehisce. (C) Anther section from a wt flower before the anther dehiscence process. Septum (SP) and stomium (ST) were not degenerated. (D–F) Anther sections of a wt flower (D) and a 35S:PCS1–HIS flower (E,F) at the flower-opening stage. (F) Close-up view. (G–J) Exogenous ethephon treatments accelerated anther dehiscence of wt flowers (H) but not that of the 35S:PCS1 flowers (I,J). (G) Water-treated wt flower used as a negative control. The flowers in (G–I) were at the stage of 2–3 days before flower opening. The flower shown in (J) was at the stage 2–3 days after flower opening. Magnifications: A,B, × 30; C, × 120; D, × 40; E, × 50; F, × 200; G–I, × 40; J, × 30.
Anther dehiscence requires PCD of specified cells of the anther wall including stomium (ST) and septum (SP) cells (Fig 3C,D) cells that are derived from the L1 layer and the L2 layer, respectively (Goldberg et al, 1993). SP degenerates first to create a bilocular anther, and the stomial cells then degenerate to break the anther wall (Fig 3D). In the 35S:PCS1 lines, both ST and SP remained at the stages when they are normally degenerated (Fig 3E,F). Ethylene and jasmonate have important roles in controlling anther dehiscence (Sanders et al, 2000; Ishiguro et al, 2001; Rieu et al, 2003). Exogenous application of jasmonate did not rescue the anther dehiscence phenotype of the 35S:PCS1 plants (data not shown). Treatment with ethephon accelerated anther dehiscence of wt flowers (Fig 3G,H); however, PCS1 ectopic expression blocked the ethephon-mediated acceleration of anther dehiscence (Fig 3I,J).
The 35S:PCS1 lines also showed delayed leaf senescence and enhanced resistance to silique dehiscence (data not shown). However, it remains to be determined whether PCS1 ectopic expression directly affects leaf senescence and pod shattering, or whether the phenotypes are indirectly caused by the reduced seed set of those plants. We have also generated transgenic lines (35S:PCS1*) that overexpress a mutant form of PCS1 in which the predicted active-site aspartic acid residue is changed to asparagine. None of the 35S:PCS1* lines showed any alteration in anther dehiscence or the other phenotypes associated with the 35S:PCS1 lines (data not shown), indicating that PCS1 protease activity is responsible for PCS1 function.
PCS1 has protease activity with high substrate specificity
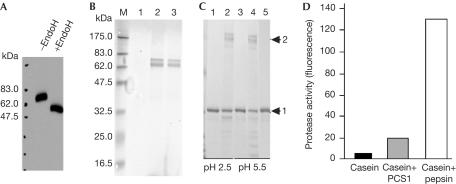
We developed an epitope-tagging approach for purifying PCS1 from transgenic plants. We generated transgenic Arabidopsis lines expressing PCS1 fused at its carboxyl terminus with the FLAG tag (Einhauer & Jungbauer, 2001) and the StrepII tag (Skerra & Schmidt, 1999) in tandem. The tandem tag was named FAST. Western blot analysis showed that the fusion protein (PCS1–FAST) expressed in transgenic plants under 35S control has a molecular size of ∼67 kDa, which is bigger than the calculated size of 53 kDa (Fig 4A). Digestion of the protein extract with EndoH reduced its size by ∼15 kDa, indicating that PCS1 is glycosylated (Fig 4A). Singlestep purification using anti-FLAG-M2 affinity gel resulted in a major and a minor protein band (around 67 and 70 kDa, respectively) detected by Coomassie staining (Fig 4B, lane 2). A third band of 60 kDa was visible in some preparations (Fig 4C). These three proteins were again co-purified after secondstep purification using a Strep-Tactin affinity column (Fig 4B, lane 3, and Fig 4C). Peptide mass fingerprinting analysis using MALDI-TOF mass spectrometry showed that the 67 kDa band is PCS1–FAST, the 70 kDa protein is luminal binding protein (BiP; AGI #At5g42020) and the 60 kDa protein is calnexin 1 (At5g61790). Both BiP and calnexin 1 are protein chaperones localized in the endoplasmic reticulum (ER), which were probably co-purified by their association with newly synthesized PCS1–FAST. As chaperones generally do not bind to fully folded proteins, we assume their presence does not affect PCS1 activity.
Figure 4.
PCS1 has proteolytic activity. (A) PCS1 is a glycosylated protein. The western blot containing protein extracts with or without EndoH digestion from the 35S:PCS1–FAST transgenic plants was probed with the anti-FLAG-M2 antibody. (B) SDS–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of purified PCS1–FAST. Lanes 2 and 3 contain proteins eluted after single-step purification with the anti-FLAG-M2 affinity gel and after second-step purification with a Strep-tactin column, respectively. Lane 1 is the protein elute from nontransgenic plants subjected to M2 affinity purification. (C) SDS–PAGE analysis of fragmented casein catalysed by the purified PCS1–FAST at the two pH conditions. Lanes 1, 3: casein only; lanes 2, 4: casein+PCS1–FAST. The elute from transgenic plants expressing only the FAST tag (lane 5) did not have protease activity. Arrows 1 and 2 point to casein and PCS1–FAST, respectively. (D) Semiquantitative assay on proteolytic activity of PCS1–FAST. Reaction was carried out at pH 5.5.
The purified PCS1–FAST was found to partially hydrolyse casein, but was not active against three other substrates tested (BSA, haemoglobin and ovalbumin; Fig 4C; data not shown). The two co-purified chaperones were not hydrolysed in the reactions; and we did not detect self-degradation of PCS1–FAST. PCS1–FAST was found to be more active at pH 5.5–6.5 in the assays, although it had activity at a wide range of the tested pH conditions (pH 2.5–7.5; Fig 4C; data not shown). A semiquantitative assay using fluorescein isothiocyanate (FITC)-labelled casein as a substrate showed that the activity of PCS1–FAST was around 14% of that of bovine pepsin, a positive control (Fig 4D).
PCS1 is localized to the endoplasmic reticulum
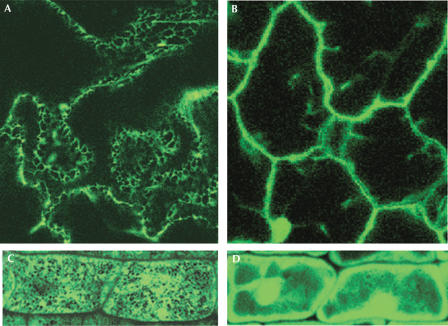
Glycosylation of PCS1 and co-purification of the two ER chaperones indicate that PCS1 is synthesized on the ER. The removal of the bulk of oligosaccharides from PCS1 by EndoH, which digests N-glycans of a high-mannose type, suggests that PCS1 does not move from the ER to the Golgi because a glycoprotein moved to the Golgi will be further modified to form complex oligosaccharides that are resistant to EndoH digestion. Therefore, PCS1 probably resides in the ER. To further determine the subcellular localization of PCS1, we generated transgenic lines expressing a translational fusion of PCS1 with enhanced green fluorescent protein (eGFP) under the 35S control. Examination of GFP activity in the roots and leaves of the transgenic Arabidopsis plants expressing PCS1–eGFP showed that the GFP signal was predominantly found in the ER (Fig 5).
Figure 5.
GFP-fused PCS1 is localized to the endoplasmic reticulum. Fluorescence signal in leaf epidermal cells (A) and root cells (C) of seedlings expressing 35S:PCS1–GFP. PCS1–GFP is associated with network-like structures of the ER. (B) and (D) show fluorescence signal in leaf epidermal cells (B) and root cells (D) of seedlings expressing free GFP under the 35S control.
Discussion
Several proteases such as caspases have been implicated in regulating and executing cell death processes. We previously reported that hyperactivation of another aspartic protease in Arabidopsis constitutive disease resistance 1 (CDR1) causes spontaneous cell death, as determined by microscopy (Xia et al, 2004). In contrast, we found that PCS1 functions to promote cell survival during embryogenesis and gametogenesis, and its ectopic overexpression blocks normal PCD processes associated with anther dehiscence. PCS1 is apparently not a housekeeping gene but functions in specific developmental processes. Therefore, it is unlikely that loss of PCS1 function causes gametophytes and embryonic cells to die because PCS1 is required for general cellular functioning and metabolism. Although the precise function of PCS1 remains to be shown, our results indicate that PCS1 has an important role in determining the fate of cells associated with reproductive processes and embryogenesis.
Several well-known PCD processes are associated with seed development, including degeneration of suspensor cells and the death of endosperm cells and specific embryonic tissues that form the vascular system (Gray & Johal, 1998). In many plant species, several embryos develop from a single zygote (termed monozygotic polyembryony); however, only one embryo will survive, others being eliminated by means of PCD (Filonova et al, 2002). It is also thought that the endosperm might originate from a supernumerary embryo reprogrammed to have nutritive functions (Berger, 1999). These phenomena suggest that embryonic tissues are equipped with the PCD machinery, and both anti- and pro-cell-death pathways coordinate to ensure development of a functional embryo. It is tempting to speculate that PCS1 may function as an anti-cell-death component by processing and activating a polypeptide that functions as a survival factor. Alternatively, PCS1 may process and inactivate a pro-PCD component in the ER, which has recently emerged as a key organelle in regulating PCD (Breckenridge et al, 2003).
In conclusion, using loss-of-function and gain-of-function mutagenesis approaches, we have identified an aspartic protease that has an important role in determining the fate of cells in important developmental PCD processes. Future identification of physiological substrates of PCS1 will shed light on the precise role of PCS1 and on the molecular mechanisms involved in regulating cell survival and cell death in plants.
Methods
Plant materials. The Arabidopsis plants were grown at 22°C under long-day conditions (15 h 150 μE light and 9 h dark) and 50% humidity.
Nucleic acid analysis. Total RNA was isolated from different organs (except siliques) using the Trizol reagent, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). RNA from siliques was extracted according to Carpenter & Simon (1998).
PCR analysis of T-DNA insertion tags. T-DNA flanking sequence of the pcs1 allele was amplified by using a T-DNA left border primer LB2 (GCTTCCTATTATATCTTCCCAAATTACC) and a PCS1-specific primer (GAGTCGCCGAGGAGAAGAAACCCCG).
Construction of pPCS1t, pPCS1p:GUS and p35S:PCS1–eGFP. To construct pPCS1t, a 3 kb fragment of the PCS1 gene including a 1.4 kb promoter region was obtained by PCR from Col-0 genomic DNA with the primer set CAGTCGACGCTGCAATCATCAATAAAACTC and GAGCTCAAAAGTCCAAAACTGATGACG and subcloned into the binary vector pPZP212.
To construct pPCS1p:GUS, a 1.2 kb promoter fragment was PCR amplified from Col-0 genomic DNA using the primer set CTGCAGAGACGAACCCGGCTCG and GTCGACAAAGAACTGAGTTTGTGGCTA and cloned into pCR-BluntII-TOPO, dropped out by cutting with PstI and SalI and placed upstream of the GUS gene by ligating into the PstI/BamHI-digested binary vector pBAR-GUS687.
To construct p35Sp:PCS1–eGFP, the PCS1 gene amplified from Col-0 genomic DNA using the primer set TACGTATGTTCTCAAGATTCCATGCTC and GAGACTCGGAATCGGATCGGCGCGCC was cloned into pCR-BluntII-TOPO and subcloned into SnaB1/AscI-digested pBI-35S:eGFP.
Purification of PCS1–FAST. p35S:PCS1–FAST was made by PCR amplification of the PCS1 gene from Col-0 genomic DNA using the primer set GAGCTCAGTTCTTTGCAAGAAACACAAAC and AGATCTTCGATCCGATTCCGAGTCTCTGAC, which was cloned into pCR-BluntII-TOPO, cut out using SacI and BglII and ligated into the SacI/BamHI-digested binary vector p35S:FAST.
Up to 50 g of rosette leaves was ground into powder in liquid nitrogen and mixed with 100 ml of lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, pH 7.4). The extract was centrifuged at 14,000 r.p.m. for 15 min at 4°C, and the supernatant was filtered through filter papers. The flow-through (50–100 ml) was incubated with anti-FLAG-M2 affinity gel (0.5–1 ml; Sigma, F2426) at 4°C with gentle agitation for several hours. The resin was washed three to four times with TBS (50 mM Tris–HCl, 150 mM NaCl, pH 7.4). Elution was carried out with 150 μg/ml 3 × Flag peptide. Purification was carried out on the basis of the StrepII tag using a Superflow Strep-Tactin column and the Strep tag purification kit (IBA GmbH, Gottingen, Germany, #2-1102) according to the manufacturer's instructions.
Protease activity assays. A 2 μl portion of each substrate (1 mg/ml) was added to 13 μl of purified PCS1–FAST (0.05 mg/ml), and the final volume was increased to 25 μl with pH buffers. The hydrolysis reaction was carried out at 22°C. Different pH buffers were used as follows: 0.1 M glycine–HCl buffer (pH 2.5 and 3.5), 0.1 M sodium acetate buffer (pH 4.5 and 5.5) and 0.1 M sodium phosphate buffer (pH 6.5 and 7.5). For the semiquantitative protease assay, FITC-labelled casein (Sigma, St Louis, MO, USA; C-3777) was used as a substrate according to a previously described method (Twinging, 1984).
Microscopy. For thin sectioning, tissues were fixed with 4% paraformaldehyde overnight at 4°C, dehydrated through an ethanol series and embedded in paraplast. Sections of 7 μm thickness were cleared with Histoclear, rehydrated with water and stained with 0.5% toluidine blue. For pollen viability staining, pollen was collected at anthesis, placed in droplets of the staining solution (Alexander, 1969) on glass slides and covered with coverslips. Slides were incubated for 24 h at 50°C before pollen was observed. Embryogenesis and GFP fluorescence were observed using a Nikon C1-Eclipse 800 Confocal microscope (488 nm argon laser line, 500–530 nm BP detector). Fixation, clearing and visualization of developing siliques were performed by a previously described method (Christensen et al, 1997).
Ethylene and jasmonate treatments. For the ethylene treatment, flowering plants were sprayed to runoff with 1 mg/ml ethephon (Sigma, C-0143) and then enclosed in plastic bags. Flowers were observed 2 days after the treatment. The jasmonate treatment of flower buds was carried out according to a previous report (Sanders et al, 2000).
Acknowledgments
We thank S. Chen for assistance in protein identification using MALDI-TOF MS and C. Ehret for editing the manuscript.
References
- Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Alonso JM et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Berger F (1999) Endosperm development. Curr Opin Plant Biol 2: 28–32 [DOI] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22: 8608–8618 [DOI] [PubMed] [Google Scholar]
- Carpenter CD, Simon AE (1998) Preparation of RNA. In Arabidopsis Protocols, Martínez-Zapater JM, Salinas J (eds) pp 85–89. Totowa, NJ, USA: Humana [Google Scholar]
- Christensen CA, King EJ, Jordoan JR, Drews GN (1997) Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex Plant Reprod 10: 49–64 [Google Scholar]
- Einhauer A, Jungbauer A (2001) The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. J Biochem Biophys Methods 49: 455–465 [DOI] [PubMed] [Google Scholar]
- Filonova LH, von Arnold S, Daniel G, Zozhkov PV (2002) Programmed cell death eliminates all but one embryo in a polyembryonic plant seed. Cell Death Differ 9: 1057–1062 [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5: 1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Johal GS (1998) Programmed cell death in plants. In Arabidopsis, Anderson M, Roberts J (eds) pp 360–394. Sheffield, UK: Sheffield Academic [Google Scholar]
- Greenberg JT (1996) Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA 93: 12094–12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC (1997) Programmed cell death in animal development. Cell 88: 347–354 [DOI] [PubMed] [Google Scholar]
- Lawen A (2003) Apoptosis—an introduction. Bioessays 25: 888–896 [DOI] [PubMed] [Google Scholar]
- Morita Y, Tilly JL (1999) Oocyte apoptosis: like sand through an hourglass. Dev Biol 213: 1–17 [DOI] [PubMed] [Google Scholar]
- Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9: 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264: 1458–1460 [DOI] [PubMed] [Google Scholar]
- Rieu I, Wolters-Arts M, Derksen J, Mariani C, Weterings K (2003) Ethylene regulates the timing of anther dehiscence in tobacco. Planta 217: 131–137 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12: 1041–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A et al. (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A, Schmidt TG (1999) Applications of a peptide ligand for streptavidin: the Strep-tag. Biomol Eng 16: 79–86 [DOI] [PubMed] [Google Scholar]
- Twinging SS (1984) Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem 143: 30–34 [DOI] [PubMed] [Google Scholar]
- Xia Y, Suzuki H, Borevitz J, Blount J, Guo Z, Patel K, Dixon RA, Lamb C (2004) An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. EMBO J 23: 980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]