Abstract
A role for the telomerase reverse transcriptase subunit (Tert) in tumorigenesis independent of telomere length is emerging. K5-Tert mice, which overexpress Tert in the skin, show increased tumorigenesis and faster wound healing than wild-type controls. Here, we demonstrate that the telomerase RNA component Terc is necessary to mediate these effects of Tert overexpression. In contrast to K5-Tert mice, K5-Tert mice in a Terc-deficient background, K5-Tert/Terc−/−, do not show increased tumorigenesis or increased wound healing compared with wild-type controls. Indeed, K5-Tert/Terc−/− mice show a reduction in tumour growth compared with Terc−/− controls, indicating an inhibitory effect of Tert overexpression in the absence of Terc. These results indicate that the tumour-promoting effects of Tert overexpression require the formation of Tert–Terc complexes. In addition, we show that the increased expression of Tert in the absence of Terc has an inhibitory effect on tumorigenesis, independently of telomere length and telomerase activity. These findings highlight Terc as a target for telomerase-based anticancer therapies.
Keywords: Tert, Terc, mouse models, cancer, wound healing
Introduction
Recent evidence suggests that telomerase can promote tumorigenesis in mice independently of net telomere elongation (Blasco, 2002). It has been long reported that telomerase is upregulated during mouse tumorigenesis, despite the fact that mice have very long telomeres (Blasco et al, 1996; Broccoli et al, 1996). Moreover, mice with transgenic expression of the telomerase reverse transcriptase subunit (Tert) have a higher susceptibility to develop tumours compared with wild-type controls in the absence of changes in telomere length (Gonzálezsuarez et al, 2001; Artandi et al, 2002; Canela et al, 2004). Data generated with cultured cells also support a role of Tert in promoting growth independently of telomere length (Smith et al, 2003; Stewart et al, 2003).
We had previously generated K5-Tert mice that express Tert in basal keratinocytes from stratified epithelia (Gonzálezsuarez et al, 2001). K5-Tert transgenic mice were described as being about twofold more susceptible to skin tumorigenesis than wild-type mice, and as showing an increased rate of would healing (Gonzálezsuarez et al, 2001). To study the mechanisms by which Tert overexpression mediates these telomere-length-independent effects, we have generated K5-Tert in a Terc−/− genetic background (Blasco et al, 1997): K5-Tert/Terc−/− mice. This genetic approach makes it possible to address whether the formation of Tert–Terc complexes is required to mediate these extracurricular effects of increased expression of Tert.
The results presented here show that both the increased tumorigenesis and the faster wound healing associated with Tert overexpression in K5-Tert mice are dependent on the telomerase RNA component Terc. In addition, we make the unexpected finding that the increased expression of Tert per se, in the absence of its RNA partner (K5-Tert/Terc−/− mice), has an inhibitory effect on both wound-healing and tumorigenesis assays. Importantly, this effect of Tert overexpression in the absence of Terc is independent of telomerase activity, as well as of changes in telomere length. Altogether, these findings highlight the importance of Terc in the effects associated with Tert overexpression, as well as pinpoint Terc as an optimal target for telomerase-based cancer therapies.
Results and Discussion
Telomere length in telomerase-mutant keratinocytes
We generated K5-Tert/Terc−/−, Terc−/−, K5-Tert and wild-type (wt) littermates by intercrossing K5-Tert (het)/Terc+/− and Wt Tert/Terc+/− mice (see Methods). Both Terc−/− and K5-Tert/Terc−/− mice lack telomerase and correspond to a first generation of telomerase deficiency, whereas K5-Tert mice show increased telomerase activity in the skin compared with wild-type littermates (not shown), in agreement with previous reports (Gonzálezsuarez et al, 2001). Finally, both K5-Tert and K5-Tert/Terc−/− mice express similar levels of Tert messenger RNA as determined by real-time PCR (supplementary Fig 1 online).
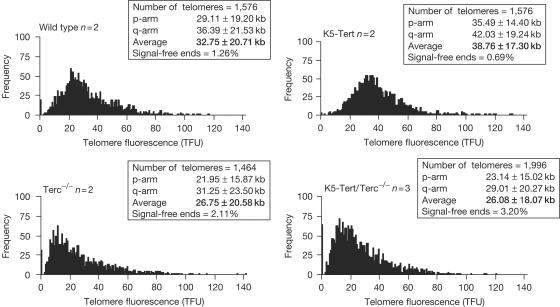
To study the effect of these telomerase mutants on telomere length, we first established primary keratinocyte cultures from newborn mice (see Methods), and measured telomere length on metaphase spreads using quantitative fluorescence in situ hybridization (Q-FISH) with a PNA-telomere probe (see Methods; Fig 1). We quantified the fluorescence intensity of more than 1,400 individual telomeres per keratinocyte culture, and at least two independent cultures from each genotype were analysed (Fig 1). Wild-type and K5-Tert keratinocytes showed the characteristic long telomeres of mouse cells with average lengths of 32.75±20.71 and 38.76±17.30 kb, respectively (Fig 1). Both wild-type and K5-Tert keratinocytes showed a low percentage (1.26 and 0.69%, respectively) of chromosome ends with undetectable telomeres (signal-free ends). These results are in agreement with the fact that both genotypes have telomerase activity, as well as with previous findings showing that Tert overexpression does not alter significantly the normal distribution of telomere lengths in mouse cells (Gonzálezsuarez et al, 2001; Canela et al, 2004). In contrast, Terc−/− and K5-Tert/Terc−/− keratinocytes showed a significant decrease in average telomere length compared with wild-type cells, as well as a shift in the distribution of individual telomere length values towards shorter telomeres (Fig 1). In particular, average telomere lengths were 26.75±20.58 and 26.08±18.07 kb for Terc−/− and K5-Tert/Terc−/− keratinocytes, respectively, compared with 32.75±20.71 kb in wild-type cells (Fig 1). Similarly, the percentage of signal-free ends was slightly increased in Terc−/− and K5-Tert/Terc−/− cells (2.11 and 3.20%, respectively), compared with wild-type cells (1.26%). The shorter telomeres shown by Terc−/− and K5-Tert/Terc−/− cells are in agreement with the fact that both genotypes lacked telomerase activity during one mouse generation (Blasco et al, 1997). A similar decrease in average telomere length was previously described for first-generation Terc−/− keratinocytes using independent FISH techniques (Gonzálezsuárez et al, 2000). Importantly, we did not detect significant differences in telomere length or in the percentage of signal-free ends when comparing Terc−/− and K5-Tert/Terc−/− cells, indicating that Tert overexpression in the absence of Terc does not affect telomere length.
Figure 1.
Histograms showing distribution of individual telomere lengths in keratinocytes. The total number of telomeres analysed and the percentage of chromosome ends without TTAGGG signal (signal-free ends) are indicated. n, number of keratinocyte cultures.
Terc is required for the tumour-promoting effects of Tert
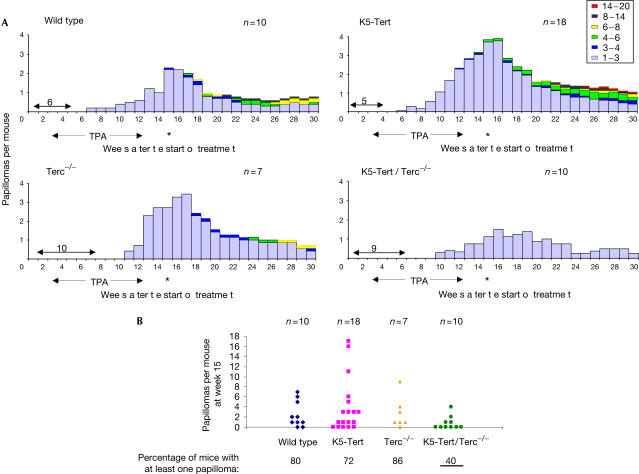
We next studied the susceptibility of all four genotypes to a classical 7,12-dimethylbenz(a)anthracene (DMBA)+12-O-tetradecanoylphorbol-13-acetate (TPA) chemical carcinogenesis protocol of the skin, which induces the formation of papillomas (Balmain et al, 1984; see Methods). First, we confirmed our previous observations that K5-Tert mice are more susceptible to skin tumorigenesis than wild-type controls (Fig 2A,B; Gonzálezsuarez et al, 2001). In particular, K5-Tert mice developed about 1.5-fold more papillomas per mouse than wild-type mice at week 15, and these papillomas progressed to bigger lesions than in wild-type mice (Fig 2A,B). It is important to note that this increased papilloma formation associated with Tert overexpression occurred in the absence of significant differences in the distribution of individual telomere lengths, or in the percentage of signal-free ends, between wild-type and K5-Tert keratinocytes (Fig 1). We also confirmed our previous observation that first-generation Terc−/− mice are slightly less susceptible to skin tumorigenesis than wild-type controls (Gonzálezsuárez et al, 2000). In particular, although the total number of papillomas in Terc−/− mice at week 15 was similar to that of wild-type mice, papilloma formation was delayed in Terc−/− mice compared with wild-type mice: the first papillomas appeared at week 10 in Terc−/− mice and at week 6 in wild-type mice (Fig 2A,B). Furthermore, Terc−/− papillomas never progressed to lesions bigger than 8 mm in contrast to those of wild-type mice (Fig 2A).
Figure 2.
Impact of various telomerase mutants on skin tumorigenesis. (A) Total number of papillomas is plotted versus number of weeks after the start of treatment. An asterisk indicates termination of TPA treatment (week 15). Different colours in the bars refer to papilloma size (in millimetres). The papilloma latency period for each genotype is indicated with arrows. (B) Average number of papillomas per mouse at week 15. The percentage of treated mice of each genotype that showed at least one papilloma is also indicated. Note that 60% of the treated K5-Tert/Terc−/− mice never developed papillomas.
Importantly, K5-Tert mice in a Terc−/− background, K5-Tert/Terc−/− mice, showed impaired skin tumorigenesis compared with all other genotypes, and in particular compared with single Terc−/− mice, which also lack telomerase activity and show a similar telomere length (Figs 1, 2A). Only 40% of the K5-Tert/Terc−/− mice developed papillomas compared with 80%, 72% and 86% of the wild-type, K5-Tert and Terc−/− mice, respectively (Fig 2B). In addition, the number of papillomas per mouse at week 15 was significantly reduced in K5-Tert/Terc−/− mice compared with both single K5-Tert and Terc−/− controls (Fig 2A,B; Student's t-test P<0.05). Finally, papillomas in K5-Tert/Terc−/− mice never progressed to lesions bigger than 3 mm, in contrast with the other genotypes (Fig 2A). These results indicate that Tert overexpression in the absence of Terc, thus in the absence of formation of Tert–Terc complexes, fails to promote papilloma formation, in contrast to that observed for single K5-Tert mice. This different behaviour of K5-Tert and K5-Tert/Terc−/− mice cannot be explained by changes in Tert expression levels, as Tert is similarly overexpressed in both genotypes (supplementary Fig 1 online). Importantly, the fact that tumorigenesis is also significantly impaired in K5-Tert/Terc−/− mice relative to single Terc−/− controls (Fig 2A,B) suggests that Tert overexpression inhibits tumorigenesis when Terc is not present. It is particularly relevant to note that this inhibitory effect of Tert overexpression in the absence of Terc is independent of both telomerase activity and telomere length, as both Terc−/− and K5-Tert/Terc−/− mice lack telomerase activity and show a similar average telomere length and a similar percentage of signal-free ends (Fig 1).
Delayed wound healing in skin of K5-Tert/Terc−/− mice
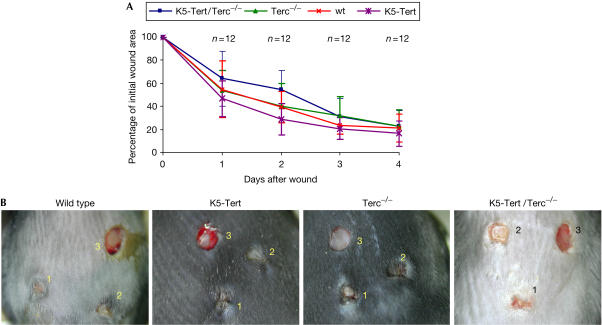
We have previously described (Gonzálezsuarez et al, 2001) that K5-Tert mice show a higher rate of wound healing in skin compared with wild-type controls. To study whether Terc was also required for this short-term effect of Tert overexpression, we carried out wound-healing experiments in the skin of wild-type, Terc−/−, K5-Tert and K5-Tert/Terc−/− littermates (see Methods). The rate of wound healing was monitored as the percentage of the initial wound area left open at different times after the wound was created (see Methods). As previously reported, single K5-Tert transgenics showed a faster rate of wound healing than their wild-type littermates (Fig 3A,B), with average wound area values at day 2 of 40±14 mm2 and 29±14 mm2 for wild-type and K5-Tert mice, respectively (Student's t-test P<0.05). No significant differences in the rate of wound healing were detected between Terc−/− and wild-type mice, with wound area values at day 2 of 40±19 mm2 and 40±14 mm2 for Terc−/− and wild-type mice, respectively (Fig 3A,B). Importantly, K5-Tert/Terc−/− mice showed a significantly delayed rate of wound healing compared with single K5-Tert controls, with average wound area values at day 2 of 54±17 mm2 and 29±14 mm2 for K5-Tert/Terc−/− and K5-Tert mice, respectively (Student's t-test P=0.0005). At later time points, these differences disappeared due to the fact that the wounds were largely healed (Fig 3A,B). These results indicate that Terc is necessary for the higher wound-healing rate associated with Tert overexpression. Moreover, the fact that K5-Tert/Terc−/− mice showed a delayed rate of wound healing relative to Terc−/− controls, which have a similar telomere length (Fig 1), further supports an inhibitory effect of Tert overexpression in the absence of Terc that is independent of both telomerase activity and telomere length.
Figure 3.
Wound healing in skin from a range of genotypes. (A) Percentage of initial wound area left at the indicated days after the punch was created. Three wounds were created per mouse, and the total number of wounds per genotype is indicated in the graph (n). wt, wild type. (B) Representative images of wounds 4 days after the first wound was made (1, 2 and 3 in the figure correspond to the first, second and third wound at 4, 2 and 0 days, respectively, after the first wound was created).
Terc is required for TPA response
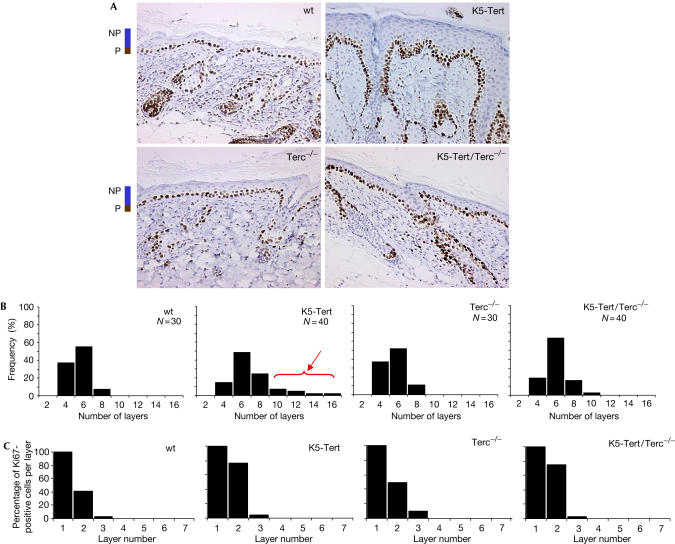
To understand the mechanisms by which Tert transgenic expression results in faster wound healing and increased tumorigenesis, we first studied the skin response to TPA treatment in wild-type, Terc−/−, K5-Tert and K5-Tert/Terc−/− mice (see Methods). Reproducibly, TPA-treated K5-Tert mice showed a high number of skin keratinocyte layers (up to 16 layers), which were never present in the other genotypes (see Fig 4A for representative examples of skin sections and Fig 4B for quantification of keratinocyte layers). The number of actively proliferating keratinocyte layers (with Ki67-positive staining) ranged between one and three layers and was similar for all genotypes (Fig 4C). Apoptosis, as measured by caspase-3-positive staining, was undetectable in all genotypes after TPA treatment (not shown). These results demonstrate a more sustained proliferative response of K5-Tert skin to TPA treatment, which results in a more acute skin hyperplasia in TPA-treated K5-Tert mice compared with the other genotypes (Fig 4A). As the number of actively proliferating keratinocyte layers was similar for all genotypes (Fig 4C), these findings may suggest an impact of Tert expression in the mobilization of skin stem cells.
Figure 4.
Response to TPA treatment. (A) Representative histopathology images of skin hyperplasia in TPA-treated mice. Notice the more acute hyperplasia in K5-Tert mice compared with K5-Tert/Terc−/− mice. Ki67 labelling (brown staining) to visualize proliferating keratinocytes is shown. NP, nonproliferating keratinocyte layers; P, proliferating keratinocyte layers; wt, wild type. (B) Frequency of number of keratinocyte layers after TPA treatment. N, number of skin sections that were analysed for the indicated genotype. (C) Percentage of actively proliferating cells (Ki67-positive) per keratinocyte layer.
Chromosomal instability in K5-Tert/Terc−/− cells
Short and/or dysfunctional telomeres in the context of the telomerase-deficient mouse have been previously shown to lead to increased chromosomal instability, as well as loss of both cellular and organismal viability (Blasco et al, 1997; Goytisolo & Blasco, 2002). In addition, telomerase-deficient mice with dysfunctional telomeres show an impaired tumorigenesis except when in a p53-deficient background, suggesting that telomere dysfunction can act as a tumour suppressor mechanism (Chin et al, 1999; Greenberg et al, 1999; Gonzálezsuárez et al, 2000; Goytisolo & Blasco, 2002; Wong et al, 2003; Espejel et al, 2004). As a possible explanation for the differences in skin tumour growth and wound healing between the different genotypes studied here, we determined whether telomere capping was normal in skin keratinocytes derived from these mice (see Methods). Uncapped or critically short telomeres have been associated with the formation of end-to-end telomere fusions, a hallmark of telomeric dysfunction (Blasco et al, 1997). To this end, we measured the frequencies of spontaneous chromosomal aberrations involving telomeric sequences in metaphases from primary keratinocyte cultures using telomeric Q-FISH (see Methods). As shown in Table 1 (see supplementary Fig 2 online for examples), we did not detect a significant increase in telomere end-to-end fusions or telomere associations in K5-Tert or Terc−/− cells compared with the wild-type controls, suggesting that telomere capping was not affected by Tert overexpression alone or by single telomerase deficiency. These results agree with the recent findings from our group showing that Tert overexpression in a different Tert transgenic mouse model, the Lck-Tert mice, which overexpress Tert in the thymus, does not result in increased end-to-end telomere fusions (Canela et al, 2004). Interestingly, we detected an overall increase in the frequency of chromosome aberrations and, in particular, of end-to-end fusions, breaks and telomere associations in K5-Tert/Terc−/− cells compared with the other genotypes (Table 1). This finding suggests that increased Tert expression in the absence of Terc may interfere with proper telomere capping or proper DNA damage repair, which in turn can negatively affect tumorigenesis. A similar negative impact of chromosomal instability on tumorigenesis has been previously described for telomerase-deficient mice with dysfunctional telomeres, as well as for mice with mutations in several DNA repair proteins such as Ku86 and Mre11, which are not prone to cancer despite showing a rampant chromosomal instability (Gonzálezsuárez et al, 2000; Goytisolo & Blasco, 2002; Theunissen et al, 2003; Wong et al, 2003; Espejel et al, 2004).
Table 1.
Frequency of chromosomal aberrations in primary keratinocytes of the indicated genotypes as determined by Q-FISH
| Wild type | Terc−/− | K5-Tert | K5-Tert/Terc−/− | |
|---|---|---|---|---|
| No. of metaphases |
93 |
91 |
90 |
87 |
| Total end-to-end fusionsa |
0.043 (4) |
0.021 (2) |
0.033 (3) |
0.066 (6) |
| (−) TTAGGG |
|
|
|
|
| Chromatid fusionsa |
0.010 (1) |
0 |
0.022 (2) |
0 |
| Chromosome fusionsa (RL)b |
0.010 (1) |
0.021 (2) |
0 |
0.045 (4) |
| (+) TTAGGG |
|
|
|
|
| Chromatid fusionsa |
0 |
0 |
0.011 (1) |
0.022 (2) |
| Chromosome fusionsa(RL, p–q)c |
0.021 (2) |
0 |
0 |
0 |
| Total breaks+fragmentsa |
0.204 (19) |
0.109 (10) |
0.088 (8) |
0.298 (26) |
| Breaksa |
0.021 (2) |
0.021 (2) |
0.033 (3) |
0.034 (3) |
| Fragmentsa |
0.184 (17) |
0.087 (8) |
0.055 (5) |
0.264 (23) |
| Telomeric associationsa |
0.204 (19) |
0.164 (15) |
0.166 (15) |
0.425 (37) |
| Complex aberrationsa | 0 | 0.022 (2) | 0.066 (6) | 0.034 (3) |
aFrequency of each type of aberration per metaphase. (total no. of aberrations). (−) TTAGGG and (+) TTAGGG refer to the absence or presence of telomeric repeats at the fusion point, respectively.
bIncludes Robertsonian-like (RL) fusions only.
cIncludes fusions between p-arms and q-arms as well as Robertsonian-like (RL) fusions.
Conclusions
Here we show that the RNA component of telomerase, Terc, is required for the tumour-promoting effects of Tert overexpression in the mouse. In particular, we demonstrate that Tert overexpression in K5-Tert mice does not promote increased wound healing or increased tumorigenesis in the absence of Terc. The findings imply that the formation of Tert–Terc complexes is required for these effects of Tert overexpression, and predict that Terc may be required for full tumorigenesis even at stages when telomeres are long, in agreement with the fact that Terc is frequently amplified in human tumours (Yokoi et al, 2003).
Finally, the study of K5-Tert/Terc−/− mice has uncovered the fact that Tert overexpression in the absence of Terc has an inhibitory effect on tumorigenesis and wound-healing assays, which is independent of both telomerase activity and telomere length. Altogether, these results highlight Terc as an optimal target for telomerase-based cancer therapies. In particular, Terc inhibition may be effective in limiting the growth of tumours that overexpress Tert (most human tumours), even at stages when telomeres are long.
Methods
Mice. Terc+/– and K5-Tert mice were generated as described (Blasco et al, 1997; Gonzálezsuarez et al, 2001). All mice used are littermates generated from K5-Tert (het)/Terc+/− and Wt Tert/Terc+/− intercrosses in a C57Bl/6 genetic background.
Isolation of primary keratinocytes. Newborn mice were killed, soaked in Betadine (5 min), in a PBS antibiotics solution (5 min), in 70% ethanol (5 min) and in a PBS antibiotics solution again (5 min). Limbs and tail were amputated, and the skin peeled using forceps. Skins were then soaked in PBS (2 min), PBS antibiotics solution (2 min), 70% ethanol (1 min) and in PBS antibiotics solution again (2 min). Using forceps skins were floated on 1 × trypsin (Sigma) solution (4 ml on 60 mm cell culture plate) for 16 h at 4°C. Skins were transferred to a sterile surface and the epidermis separated from the dermis, minced and stirred at 37°C for 30 min in serum-free Cnt-02 medium (CELLnTEC Advanced Cell Systems AG, Bern, Switzerland). The cell suspension was filtered using a sterile Teflon mesh (Cell Strainer 0.7 m, Falcon, Bedford, MA, USA) to remove cornified sheets. Keratinocytes were collected by centrifugation (160g) for 10 min and seeded on collagen I precoated plates (BD Biosciences, San Diego, CA, USA).
Quantitative fluorescence in situ hybridization Q-FISH on keratinocyte metaphases. For Q-FISH, first-passage keratinocytes were treated with colcemid (0.1 μg/ml) for 6 h and telomere length was determined on at least ten metaphases from each genotype as described (Canela et al, 2004). For chromosomal aberrations, more than 90 metaphases of each genotype were analysed.
Tumour-induction experiments. Ten wild-type, seven single Terc−/−, 18 single K5-Tert and ten K5-Tert/Terc−/− mice were shaved and treated with DMBA (20 μg in 200 μl of acetone; Sigma, Cambridge, UK) 48 h after shaving. Mice were subsequently treated twice weekly with TPA (10−4 M in acetone; 200 μl; Sigma) for 15 weeks. Two control mice of each genotype were treated with acetone only.
For TPA-only experiments, mice were treated twice a week with TPA (12.5 μg in acetone; 200 μl; Sigma) for four weeks and killed 24 h after the last TPA treatment. One control mouse of each genotype was treated with acetone only.
Wound-healing experiments. Three full-thickness punch biopsies extending through the epidermis and dermis (punch diameter 4 mm; PFM, Köln, Germany) were performed in three wild-type, four single Terc−/−, four single K5-Tert and four K5-Tert/Terc−/− mice. All mice used were seven-week-old littermates. Mice were anaesthetized before wound creation. Three consecutive wounds were made and each wound was created 2 days after the other. The rate of wound healing was calculated as the percentage of initial wound area with increasing days. Wound areas were calculated with the formula area=πr2, where r is the radius of the wound.
Real-time quantitative reverse transcription–PCR. Total RNA was extracted from the skin of three mice of each genotype (K5-Tert and K5-Tert/Terc−/−) using Trizol (Life Technologies Inc., Rockville, MD, USA). Reverse transcription was performed using 1 μg of total RNA, random hexamers and Superscript II reverse transcriptase (Life Technologies) according to the manufacturer. Real-time PCR was performed with an ABI PRISM 7700 instrument (Applied Biosystems, Foster City, CA, USA) using SYBR Green PCR Core Reagents (Applied Biosystems). The final MgCl2 concentration was 4.5 μM and primers were used at 0.4 μM. Reaction mixtures were incubated for 10 min at 95°C followed by 40 PCR cycles (15 s at 95°C, 90 s at 67°C). Relative Tert expression was determined using β-actin mRNA-normalized standard curves or by calculating ΔΔC1 values, which express the difference between the cycle threshold of the Tert primer pair and that of the β-actin mRNA primer pair. Primers used were TERT-F (5′-GGA TTG CCA CTG GCT TCC G-3′) and TERT-R (5′-TGC CTG ACC TCC TCT TGT GAC-3′). Parallel reverse transcription–PCR analyses were carried out for actin using primers ACTIN-F (5′-GGC ACC ACA CCT TCT ACA ATG-3′) and ACTIN-R (5′-GTG GTG GTG AAG CTG TAG-3′). In all cases, each PCR was repeated at least twice.
Telomerase assays. Telomerase activity was measured with a modified telomeric repeat amplification protocol, as described (Blasco et al, 1997).
Histopathological analyses. Tissue sections were fixed in 10% buffered formalin and stained with haematoxylin–eosin. Images were captured with an Olympus-Vanox microscope.
Immunohistochemistry for Ki67 was performed on deparaffinized sections (Vectabond slides) pretreated with peroxidase-blocking reagent (DAKO, A/S Denmark). For Ki67 detection, a monoclonal rabbit anti-human Ki67 (Master Diagnóstica, Granada, Spain) was used followed by incubation with an anti-rabbit IgG–HRP (DAKO, 1:5 dilution). The chromogen was developed with diaminobenzidine, and slides were counterstained with haematoxylin.
Immunohistochemistry to detect active caspase 3 was performed on deparaffinized sections (Vectabond slides) pretreated with peroxidase-blocking reagent (DAKO). For detection of active caspase 3, a monoclonal rabbit anti-human active Caspase-3 (Pharmigen) was used, followed by incubation with a biotinylated goat anti-rabbit IgG (DAKO, 1:800 dilution) and by streptavidin–HRP. The chromogen was developed with diaminobenzidine, and slides were counterstained with haematoxylin.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400359s1.pdf).
Supplementary Material
Supplementary Figures
Acknowledgments
We thank J.M. Caballero, E. Santos, J. Freire and R. Serrano for mouse work. M.L.C. is a postdoctoral fellow from the Spanish Ministry of Education and Culture (MECD). The laboratory of M.A.B. is funded by MCyT (SAF2001-1869, GEN2001-4856-C13-08), the Regional Government of Madrid (08.1/0054/01), the European Union (TELOSENS FIGH-CT-2002-00217, INTACT LSHC-CT-2003-506803, ZINCAGE FOOD-CT-2003-506850, RISC-RAD FI6R-CT-2003-508842) and the Josef Steiner Award 2003.
References
- Artandi SE et al. (2002) Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc Natl Acad Sci USA 99: 8191–8196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain A, Ramsden M, Bowden GT, Smith J (1984) Activation of the mouse Harvey-ras gene in chemically induced benign skin papillomas. Nature 307: 658–660 [DOI] [PubMed] [Google Scholar]
- Blasco MA (2002) Telomerase beyond telomeres. Nat Cancer Rev 2: 627–632 [DOI] [PubMed] [Google Scholar]
- Blasco MA, Rizen M, Greider CW, Hanahan D (1996) Differential regulation of telomerase activity and telomerase RNA during multistage tumorigenesis. Nat Genet 12: 200–204 [DOI] [PubMed] [Google Scholar]
- Blasco MA et al. (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34 [DOI] [PubMed] [Google Scholar]
- Broccoli D, Godley LA, Donehower LA, Varmus HE, de Lange T (1996) Telomerase activation in mouse mammary tumors: lack of detectable telomere shortening and evidence for regulation of telomerase RNA with cell proliferation. Mol Cell Biol 16: 3765–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela A, Martín-Caballero J, Flores JM, Blasco MA (2004) Constitutive expression of Tert in thymocytes leads to increased incidence and dissemination of T-cell lymphoma in Lck-Tert mice. Mol Cell Biol 24: 4275–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L et al. (1999) p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97: 527–538 [DOI] [PubMed] [Google Scholar]
- Espejel S et al. (2004) Impact of telomerase ablation on organismal viability, aging, and tumorigenesis in mice lacking the DNA repair proteins PARP-1, Ku86 or DNA-PKcs. J Cell Biol 167: 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzálezsuárez E, Samper E, Flores JM, Blasco MA (2000) Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet 26: 114–117 [DOI] [PubMed] [Google Scholar]
- Gonzálezsuarez E et al. (2001) Increased epidermal tumors and increased skin, wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J 20: 2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goytisolo FA, Blasco MA (2002) Many ways to telomere dysfunction: in vivo studies using mouse models. Oncogene 21: 584–591 [DOI] [PubMed] [Google Scholar]
- Greenberg RA et al. (1999) Short dysfunctional telomeres impair tumorigenesis in the INK4a(δ2/3) cancer-prone mouse. Cell 97: 515–525 [DOI] [PubMed] [Google Scholar]
- Smith LL, Coller HA, Roberts JM (2003) Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 5: 474–479 [DOI] [PubMed] [Google Scholar]
- Stewart SA et al. (2003) Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA 99: 12606–12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen JU et al. (2003) Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell 12: 1511–1523 [DOI] [PubMed] [Google Scholar]
- Wong KK et al. (2003) Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421: 643–648 [DOI] [PubMed] [Google Scholar]
- Yokoi S et al. (2003) TERC identified as a probable target within the 3q26 amplicon that is detected frequently in nonsmall lung cancers. Clin Cancer Res 9: 4705–4713 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures